The study aimed to understand the associations between coinfections and HIV RNA shedding in the genital tract of men receiving antiretroviral therapy with suppressed blood plasma viral load.
Keywords: cytomegalovirus, HIV shedding, semen, antiretroviral therapy, HIV transmission
Abstract
Background. Current antiretroviral therapy (ART) suppresses human immunodeficiency virus (HIV) in blood to undetectable levels in most infected individuals; however, some men shed HIV in semen despite suppressed levels in blood.
Methods. This study included 114 chronically HIV type 1–infected men who have sex with men, who were receiving ART with blood plasma HIV <500 copies/mL. Asymptomatic participants were screened for bacterial sexually transmitted infections (STIs) and nonspecific genital inflammation. Levels of HIV and 7 human herpesviruses were measured by real-time polymerase chain reaction in seminal plasma. Predictors of HIV seminal shedding were determined for the entire cohort, and on the subset of 100 subjects with blood plasma HIV <50 copies/mL.
Results. Eleven subjects (9.6%) had detectable levels of seminal HIV (median, 2.1 log10 copies/mL), and 72 (63.2%) had at least 1 herpesvirus detected in their seminal plasma. Detectable levels of seminal HIV were present more often in persons with plasma HIV between 50 and 500 copies/mL compared to those <50 copies/mL (P values adjusted for false discovery rate [FDR] = 0.08). There was a trend for high-level cytomegalovirus (CMV; >4 log10 DNA copies/mL; FDR-adjusted P = .08), and presence of Epstein-Barr virus (FDR-adjusted P = .06) in semen to be associated with detectable seminal HIV levels. In a subanalysis of 100 subjects with blood plasma HIV <50 copies/mL, high levels of CMV in semen was the only significant predictor for seminal HIV shedding.
Conclusions. Low-level HIV replication in blood and high-level seminal CMV shedding, but not presence of asymptomatic STIs, is associated with seminal shedding of HIV in men receiving ART, conferring a potential risk for HIV transmission.
Current antiretroviral therapy (ART) suppresses human immunodeficiency virus (HIV) type 1 RNA in blood and semen to below the level of detection in most infected men, substantially decreasing the risk of sexual HIV transmission [1–4]. Recent studies reported a >90% reduction in HIV transmission among serodiscordant heterosexual couples after starting ART [2, 3], and a similar reduction in HIV transmission associated with anal intercourse was noted in men who have sex with men (MSM) receiving ART [5]. Nevertheless, sexual HIV transmission [6, 7], and intermittent seminal HIV shedding [8] can occur despite ART. The frequency of seminal HIV shedding in treated individuals ranges from 2% to 48% [9–12]. HIV shedding in the semen of ART-treated individuals might be a consequence of viral compartmentalization [13] with poor drug penetration within the genital tract [8, 14], or stimulation of virus replication by concurrent sexually transmitted infections (STIs) and genital inflammation [9, 10, 15–17]. Seminal cytomegalovirus (CMV) was previously associated with increased activation of CD4+ T cells in the genital tract [18], and several groups have described an association between CMV and Epstein-Barr virus (EBV) with HIV seminal shedding in individuals with detectable HIV in blood plasma [19–23]. From a public health perspective, it is crucial to understand if concurrent CMV and EBV shedding are associated with HIV shedding in semen of immunologically reconstituted and virologically controlled individuals.
MATERIALS AND METHODS
Participants, Samples, and Clinical Laboratory Tests
Semen samples from asymptomatic chronically HIV-infected high-risk sexually active MSM who were prospectively enrolled in the California Collaborative Treatment Group (CCTG) 592 were included in this study. CCTG 592 is a study of an Internet-based behavioral intervention of high-risk MSM that included baseline collection of semen and longitudinal follow-up on a total of 180 HIV-infected MSM who could be on or off ART.
For this study, we included baseline semen samples from a subset of 114 subjects who were receiving effective ART with blood plasma HIV <500 copies/mL within 3 months before the seminal sample collection. Semen was collected and processed, as previously described [18, 24]. For each subject, potential infection from Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis were tested from urine, rectal, and pharyngeal samples using transcription-mediated amplification (Aptima, Gen-Probe, San Diego, California). Active syphilis infection was evaluated by rapid plasma reagin titers, Treponema pallidum particle agglutination confirmatory testing, and clinical history. Urinalysis was performed with routine urine dipstick testing for leukocyte esterase; urine inflammation was defined as presence of leukocyte esterase of 1+ or greater. Blood CD4+ T-lymphocyte subsets were measured by flow cytometry (CLIA-certified local laboratories), and HIV levels were quantified by the Amplicor HIV Monitor Test (Roche Molecular Systems).
All subjects completed a baseline computer-assisted self-reported interview for sexual risk behavior, drug use, and adherence to ART in the past month. The studies were conducted with appropriate written subject consent and were approved by the Human Research Protections Program at the University of California, San Diego; the Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center; the University of Southern California; and the Feinstein Institute for Medical Research, North Shore-LIJ Health System, New York.
RNA Extraction From Seminal Plasma and HIV Quantification
HIV levels were measured in seminal plasma by first concentrating RNA from 500 μL of seminal plasma with high-speed centrifugation (23 500g at 4°C for 1 hour) after 1:1 dilution with phosphate-buffered saline, as previously described [18]. Concentrated RNA was extracted using a High Pure Viral RNA Kit (Roche), and complementary DNA was generated using the SuperScript III First-Strand Synthesis Kit (Invitrogen) with specific primer mf302 [18]. HIV in seminal plasma was quantified by real-time polymerase chain reaction (PCR) in an ABI 7900HT thermocycler (Applied Biosystems) [18,19]. HIV RNA quantification standard was obtained from the National Institutes of Health's DAIDS Virology Quality Assurance Program [25].
DNA Extraction From Seminal Plasma and Herpesvirus DNA Quantification
Viral DNA was extracted from 200 μL of seminal plasma using QIAamp DNA Mini Kit (Qiagen) per the manufacturer's protocol. Ethylenediaminetetraacetic acid (50 mM) was added to seminal plasma to inhibit DNase activity. Levels of different herpesviruses in semen were measured by real-time PCR in an ABI 7900HT thermocycler (Applied Biosystems) [18, 19]. Quantification standards for the different herpesviruses were obtained using plasmid preparations with known concentrations.
Statistics
Statistical analyses were performed using SAS software, version 9.2. Viral load variables were transformed to logarithm base-10 values. Nonnormal data were either dichotomized (undetectable/detectable) or ordinalized (undetectable/detectable; high vs low viral level with threshold at 4 log10 copies/mL) as described previously [19].
Comparisons between groups (seminal HIV shedding vs non shedding) were performed using Fisher exact test (for sparse categorical variables), t test (for continuous, normally distributed variables), or Mann-Whitney U test (for continuously, nonnormally distributed variables). Univariable P values were adjusted by false discovery rate (FDR) using Benjamini and Hochberg method to correct for multiple comparisons. A multivariable analysis was not performed, as the number of HIV-positive seminal samples was insufficient.
Analyses were performed on the total study population of 114 subjects with HIV levels in blood <500 copies/mL, and on a subset of 100 subjects with HIV levels in blood <50 copies/mL.
Associations between seminal shedding of CMV (high level) or EBV (any level) with the presence of bacterial STIs, other viral coinfections, CD4 count, time on ART, and ART regimen were evaluated.
RESULTS
Study Participants’ Demographics and Clinical Data
HIV-infected participants (N = 114) were high-risk MSM without clinical evidence of STIs who had a mean age of 44 years, were receiving ART, and with an HIV load of <500 copies/mL in blood plasma within 3 months from semen collection (median time between semen collection and blood HIV level determination, 33 days; interquartile range [IQR], 14–70 days). One hundred subjects (88%) had HIV levels <50 copies/mL. The median time on ART at the time of semen collection was 2.5 years (IQR, 13.5 months–4.8 years), and the median CD4 count was 580 cells/µL (IQR, 532–628 cells/µL). For 112 subjects with available specific ART information, 76.8% were on a regimen including tenofovir; 39.3% were on a regimen including a nonnucleoside reverse transcriptase inhibitor (NNRTI); 55.4% were on a regimen including a protease inhibitor (PI); and 18.8% were on a regimen including an integrase inhibitor. Of the 21 subjects who were taking a regimen including an integrase inhibitor, 10 also included a PI, and 4 included an NNRTI. Self-reported levels of ART adherence during the last month were >90% for 99 (87%) of the subjects. Self-reported substance abuse (including marijuana, cocaine, amphetamines, club drugs, and opiates) was high at 34.2%, and methamphetamine use within 1 month from sample collection was reported by 13.6% of participants. Characteristics and demographics of the subjects are summarized in Table 1.
Table 1.
Demographics and Bacterial Sexual Transmitted Diseases
| Characteristics | No. (%) |
|---|---|
| Participants | 114 |
| Age, y, mean (95% CI) | 44 (37–50) |
| Race/ethnicity | |
| Caucasian | 79 (70) |
| Black | 30 (26) |
| Other | 5 (4) |
| Time on ART, d, median (IQR) | 882 (406–1725) |
| HIV RNA <500 copies/mL | 114 (100) |
| HIV RNA <50 copies/mL | 100 (88) |
| ≥90% adherence to ART past month | 99 (87) |
| CD4+ cell counts/µL, mean (95% CI) | 580 (532–628) |
| Detectable HIV RNA in semen | 11 (9.6) |
| HIV in semen, log10 copies/mL, median (range) | 2.1 (1.7–2.5) |
| Bacterial sexually transmitted infections | |
| Any STI | 17 (14.9) |
| Urethral, any | 5 (4.4) |
| Chlamydia | 2 (1.8) |
| Gonorrhea | 0 (0) |
| Mycoplasma | 3 (2.6) |
| Trichomonas | 0 (0) |
| Nonspecifica | 8 (7.0) |
| Rectal, any | 6 (5.3) |
| Chlamydiab | 3 (2.6) |
| Gonorrheab | 4 (3.5) |
| Mycoplasma | NA |
| Trichomonas | 0 |
| Pharynx, any | 6 (5.3) |
| Chlamydia | 2 (1.8) |
| Gonorrhea | 3 (2.6) |
| Mycoplasma | NA |
| Trichomonas | 1 (0.9) |
| Syphilis | 1 (0.9) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not available; STI, sexually transmitted infection.
a Nonspecific urethritis defined as presence of leukocyte esterase of 1+ or greater in urine stick without any recognized associated infections,
b One case with dual gonorrhea/chlamydia rectal infection.
Bacterial and Viral Genital Infections
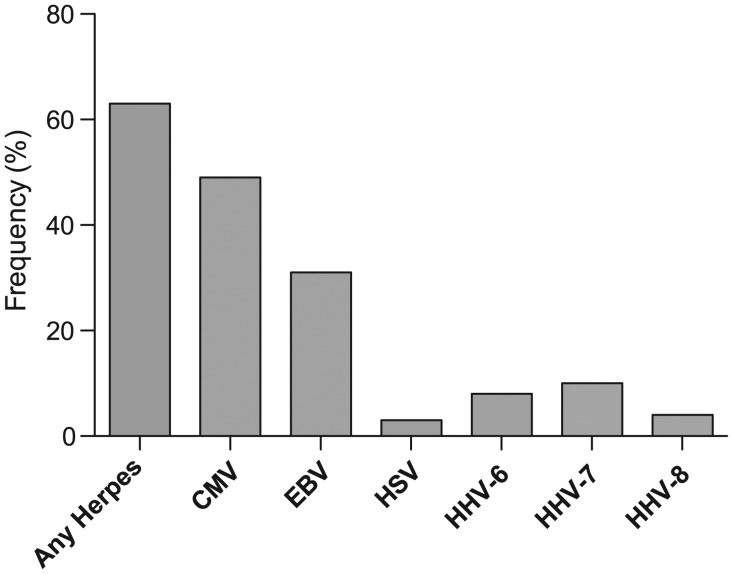
Eleven subjects (9.6% of the entire cohort) had detectable levels of HIV in semen, with a median of 2.1 log10 RNA copies/mL among detectable samples (range, 1.7–2.5), and 72 (63.2%) had at least 1 herpesvirus detected in their seminal plasma. These herpesviruses included CMV (frequency 49.1%, median detectable viral load of 4.3 log10 DNA copies/mL, IQR, 3.2–4.9 log10 DNA copies/mL), EBV (30.7%; 2.9 log10 copies/mL; IQR, 2.5–3.4 log10 copies/mL), herpes simplex virus (HSV) type 2 (2.6%; 5.1 log10 copies/mL), human herpesvirus type 6 (HHV-6; 7.0%; 3.3 log10 copies/mL), HHV type 7 (HHV-7; 8.8%; 3.2 log10 copies/mL), and HHV type 8 (HHV-8; 3.5%; 3.4 log10 copies/mL) (Figure 1). In contrast to our previous results on ART-naive subjects [19], no HSV type 1 shedding was found in the semen of this cohort. Urethral, rectal, or pharyngeal bacterial STIs were detected in 17 (14.9%) of the individuals, and nonspecific urethritis in an additional 8 (7.0%; Table 1). Specifically, 1 subject had syphilis, 5 subjects had urethral STIs (3 with M. genitalium and 2 with C. trachomatis), 6 patients had rectal STIs (2 with C. trachomatis, 3 with N. gonorrhoeae, and 1 subject coinfected with C. trachomatis and N. gonorrhoeae), and 6 patients had pharyngeal STIs (2 with C. trachomatis, 3 with N. gonorrhoeae, and 1 with trichomonas). All subjects were included in the study and treated for these infections after specimen collections. Subjects were all asymptomatic at the time of screening.
Figure 1.
Viral frequencies in semen for each human immunodeficiency virus type 1–infected subject (N = 114). Frequency (%) of patients having at least 1 semen sample positive for any herpesvirus DNA, and for each individual tested virus separately. Note that human herpesvirus (HSV) only refers to HSV type 2, as no HSV type 1 was detected in this cohort. Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus.
Key Predictors of Isolated HIV Seminal Shedding on ART
Subjects with evidence of HIV replication in plasma while on ART (ie, blood plasma HIV levels 50–500 copies/mL) had detectable levels of HIV in semen more frequently than subjects with <50 HIV copies/mL (35.7% vs 6.0%; FDR-adjusted P value = .08).
Neither the class of ART, the time on ART, or the self-reported pill adherence was significantly associated with HIV shedding in semen. In addition, self-reported substance abuse was not associated with HIV seminal shedding.
Similar to our previous findings in ART-naive HIV-infected MSM [19], there was a trend for high-level CMV (>4 log10 DNA copies/mL) and presence of EBV to be associated with detectable HIV seminal levels (Tables 2 and 3). Specifically, 7 subjects (21.9%) with high-level CMV replication in semen had concomitant HIV seminal shedding compared to 4 of those (4.9%) with no or low CMV shedding in semen (FDR-adjusted P value = .08), with a relative risk of 4.5 (95% confidence interval [CI], 1.4–14.2). A trend toward an increased frequency of detectable HIV seminal shedding was observed when comparing any level of CMV shedding to no CMV (14.3% vs 5.2%; P = .12). For those subjects with both CMV and HIV detectable in semen, the mean CMV DNA level was 1 log10 higher in semen compared to nonshedders (3.9 vs 2.9 log10 DNA copies/mL), but this difference was not statistically significant. Any detectable EBV shedding was also associated with HIV shedding in semen (22.9% HIV seminal shedding in subjects with concomitant EBV replication compared to 3.8% in those without EBV; FDR-adjusted P = .06), but there was no evidence that higher EBV levels were associated with greater HIV shedding. In fact, for patients with HIV and EBV coshedding, the levels of EBV DNA copies was lower in those shedding HIV in semen compared to those with no HIV shedding (2.6 vs 3.1 log10 DNA copies/mL; P = .06). High-level CMV shedding in semen was also significantly associated with EBV shedding.
Table 2.
Factors Associated With HIV Shedding in Semen During Antiretroviral Therapy
| Factor | HIV in Semen, Detectable | HIV in Semen, Not Detectable | Relative Risk | Univariate P Value | FDR, P Valuea |
|---|---|---|---|---|---|
| Total No. | 11 (100) | 103 (100) | |||
| Main effect variables | |||||
| Blood HIV RNA 50–500 copies/mL | 5 (45.5) | 9 (8.7) | 6.0 (2.1–17.0) | <.01 | .08 |
| ≥4 log10 CMV DNA copies/mL | 7 (63.6) | 25 (24.3) | 4.5 (1.4–14.2) | .01 | .08 |
| Any detectable EBV DNA in semen | 8 (72.7) | 27 (26.2) | 6.0 (1.7–21.3) | <.01 | .06 |
| Demographics | |||||
| Caucasian/non-Hispanic | 5 (45.5) | 39 (37.9) | .75 | 1.00 | |
| Age, y, mean (95% CI) | 41 (33–49) | 45 (43–47) | .31 | .96 | |
| CD4+ T-cells/µL, mean (95% CI) | 527 (354–700) | 586 (536–637) | .47 | 1.00 | |
| Time on ART, d, mean (95% CI) | 804 (190–1418) | 1265 (1037–1494) | .25 | .96 | |
| Additional variables | |||||
| Any detectable HSV-1 DNA | 0 (0.0) | 0 (0.0) | N/A | N/A | N/A |
| Any detectable HSV-2 DNA | 0 (0.0) | 3 (2.9) | N/A | 1 | 1.00 |
| Any detectable HHV-6 DNA | 0 (0) | 8 (7.8) | N/A | 1 | 1.00 |
| Any detectable HHV-7 DNA | 1 (9.1) | 9 (8.7) | 1.4 (0.6–3.2) | 1 | 1.00 |
| Any detectable HHV-8 DNA | 1 (9.1) | 3 (2.9) | 2.8 (0.5–16.6) | .34 | .96 |
| Bacterial STI in urine | 0 (0) | 5 (4.9) | N/A | 1 | 1.00 |
| Bacterial STI at rectum | 1 (9.1) | 5 (4.9) | 1.8 (0.3–11.8) | .46 | 1.00 |
| Bacterial STI at pharynx | 0 (0) | 6 (5.8) | N/A | 1 | 1.00 |
| Syphilis | 0 (0) | 1 (1.0) | N/A | 1 | 1.00 |
| Nonspecific urethritisb | 1 (9.1) | 7 (6.8) | 1.4 (0.2–12.3) | .57 | 1.00 |
Data are presented as No. (%) unless otherwise specified. P values in bold represent significant values.
Abbreviations: ART, antiretroviral therapy; CI, confidence intervals; CMV, cytomegalovirus; EBV, Epstein-Barr virus; FDR, false discovery rate; HHV, human herpesvirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; NA, not available; STI, sexually transmitted infection.
a P values were adjusted by false discovery rate using the Benjamini and Hochberg method to correct for multiple comparisons.
b Nonspecific urethritis defined as presence of white blood cells in urine stick without any recognized associated infections.
Table 3.
Coinfections in HIV-Infected Men on Antiretroviral Therapy Who Are Shedding HIV in Semen
| Subject | Blood HIV | Semen HIV | CMV | EBV | HSV-1 | HSV-2 | HHV-6 | HHV-7 | HHV-8 | Nonspecific Urethritisa | Any STI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.4 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | No |
| 2 | ND | 2.3 | 4.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | No |
| 3 | ND | 2.4 | 6.2 | 3.5 | 0 | 0 | 0 | 0 | 0 | 0 | No |
| 4 | ND | 2.0 | 4.8 | 2.6 | 0 | 0 | 0 | 0 | 0 | 0 | No |
| 5 | 2.4 | 2.2 | 5.2 | 2.9 | 0 | 0 | 0 | 2.7 | 3.5 | ++ | No |
| 6 | ND | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | No |
| 7 | ND | 1.7 | 0 | 2.6 | 0 | 0 | 0 | 0 | 0 | 0 | No |
| 8 | ND | 2.3 | 4.9 | 2.3 | 0 | 0 | 0 | 0 | 0 | 0 | No |
| 9 | 2.0 | 1.7 | 5.1 | 1.8 | 0 | 0 | 0 | 0 | 0 | 0 | No |
| 10 | 2.2 | 1.1 | 0.3 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | Yesb |
| 11 | 2.5 | 2.5 | 4.7 | 3.3 | 0 | 0 | 0 | 0 | 0 | 0 | No |
Plasma and semen HIV: log10 levels of HIV RNA in blood and seminal plasma.
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; ND, not detectable; STI, sexually transmitted infection.
a Nonspecific urethritis defined as presence of white blood cells in urine stick without any recognized associated infections.
b Rectal chlamydia/gonorrhea dual infection.
In a subanalysis of the 100 subjects with HIV in blood of <50 copies/mL, of whom 6 had detectable HIV seminal levels, high CMV levels remained the only factor associated with HIV shedding with a relative risk of 5.7 (95% CI, 1.1–29.3). Specifically, 15.4% of semen samples with high-level CMV also shed HIV compared to 2.7% in those without high levels of CMV. There was also a trend for an increased frequency of HIV shedding in semen being associated with EBV shedding (13.8% vs 2.8%) with a relative risk of 4.9 (95% CI, .9–25.3). The presence of bacterial STI (rectal, urethral, and/or pharyngeal) and nonspecific urethritis was not associated with HIV seminal shedding. Only the subject with rectal C. trachomatis and N. gonorrhoeae coinfection shed detectable HIV in the semen (Table 3).
Factors Associated With Seminal CMV and EBV Shedding on ART
High-level CMV seminal shedding was positively associated with presence of pharyngeal STIs, with seminal EBV and seminal HHV-8. Moreover, seminal EBV was associated with detectable HHV-7 in semen. Seminal CMV and EBV were both associated with lower CD4 counts. There was a trend for individuals receiving PI-based ART regimens to have higher CMV shedding compared to those receiving NNRTI-based regimens (33.8% vs 18.2%). There was also a trend for patients with seminal EBV shedding to have a shorter time on ART compared to people without seminal EBV shedding (974 vs 1357 days of ART). Time on ART, however, was not associated with presence of CMV seminal shedding.
DISCUSSION
Because HIV in male genital secretions accounts for the majority of HIV transmission in both women and MSM [26], it is crucial to understand the factors associated with increased seminal HIV replication in order to inform strategies to reduce sexual HIV transmission and the development of HIV drug resistance. Effective ART substantially reduces the HIV levels in blood and semen [1–4]; however, a significant number of subjects intermittently shed HIV in semen despite having suppressed levels in blood plasma [9–12].
In our study of sexually active, ART-treated MSM with blood plasma HIV levels <500 copies/mL, 11 (9.6%) had detectable HIV in seminal plasma at very low levels between 50 and 230 copies/mL. The prevalence of seminal HIV shedding falls within the range previously described in the literature (2%–48%) [9–12], depending on the characteristics of the observed population. Detectable levels of seminal HIV were present significantly more often in persons with plasma HIV between 50 and 500 copies/mL compared to those with levels of <50 copies/mL.
We previously described that seminal shedding of different herpesviruses (ie, high-level CMV, EBV, HHV-8) was associated with increased levels of HIV within the genital tract of ART-naive MSM. The present study aimed to investigate if this remains true as well in subjects receiving ART with suppressed HIV levels in blood plasma.
Consistent with previous studies [9], we found a high prevalence of herpesviruses shedding in our treated cohort. Compared to our previous results on a similar cohort of ART-naive HIV-infected MSM, we observed a lower frequency of seminal shedding for most herpesviruses, except for CMV and HHV-6. Specifically, in semen samples of ART-treated compared to untreated HIV-infected subjects we found 49.1% versus 51.3%, respectively, of seminal samples with detectable CMV DNA; 30.7% versus 40.9% with detectable EBV DNA; 2.6% versus 10.4% with detectable HSV DNA; 7.0% versus 7.0% with HHV-6 DNA; 8.8% versus 14.8% with HHV-7 DNA; and 3.5% versus 11.3% with HHV-8 DNA. These observations suggest that ART initiation may reduce seminal shedding of many herpesviruses, but CMV DNA seminal shedding, in particular, remains highly prevalent in treated HIV-infected subjects. This finding is in contrast with other reports showing decreased frequency of CMV replication after ART initiation [27, 28], but these previous studies included subjects with lower CD4 counts and evaluated CMV replication in blood and not in semen.
Consistent with data from untreated subjects, we found that high-level CMV replication (ie, >4 log10 DNA copies/mL) was associated with HIV seminal shedding even after the initiation of ART. We also found an association between EBV shedding and detectable HIV in semen, although no dose response was observed. Similar to our previous findings [19, 23], CMV and EBV seminal shedding were positively associated with each other, and both viruses were associated with lower CD4 counts. Higher level CMV seminal shedding was positively associated with presence of pharyngeal STIs, with seminal HHV-8, and with use of PI-based ART regimens.
Interestingly, HIV seminal shedding in our cohort was not associated with nonspecific genital inflammation or with any asymptomatic bacterial STIs. This may be a consequence of the relative low prevalence of urethral STIs in this asymptomatic cohort (3.5%). However, this also suggests that CMV may be a more common precipitant of HIV shedding in semen than bacterial STIs during ART even in this high-risk MSM population. It is also possible that HIV seminal shedding is derived from the proximal genital tract rather than distally and therefore asymptomatic (nonulcerative) STIs would not contribute directly to an increase in HIV seminal shedding. Of note, low levels of HIV in blood plasma between 50 and 500 copies/mL were associated with HIV seminal shedding, suggesting that a complete suppression of HIV blood levels may minimize the risk of sexual HIV transmission.
A recent study [9] observed that frequent, isolated seminal HIV shedding among 25 subjects was associated with increased compartmentalized immune activation within the genital tract, but could not find any association between detectable HIV levels and any of the evaluated factors, including CMV replication. We previously described that seminal CMV replication is positively associated with levels of immune activation in the male genital tract [18], and our present study on a larger cohort suggests that high-level CMV replication also likely plays a role in HIV seminal shedding in successfully treated HIV-infected individuals.
This study has several limitations. Most importantly, despite including seminal samples from 114 different subjects, the low frequency of seminal shedding for HIV limited our power to find additional associations. Moreover, in this observational study, the causality of the associations cannot be definitively determined. We did not assess pre- and posttreatment levels in semen to address directly the impact of treatment in the same subject. However, as successful treatment of HIV in this cohort did not influence the rate of CMV replication compared to untreated subjects in our previous cohort, CMV replication is likely driving HIV shedding and not vice versa. Last, because we only included MSM in this study, it needs to be determined if these associations remain true in genital secretion of heterosexual men and women, as this could have an impact on management of serodiscordant couples trying to conceive children.
In summary, the present study evaluated a large number of seminal samples (N = 114) from HIV-infected sexually active MSM treated with ART with an extensive battery of tests for viral and bacterial coinfections. The association between isolated seminal HIV shedding and high-level CMV replication and EBV replication in the genital tract suggests that the presence of these viruses could play a role in HIV transmission not only in ART-naive subjects, as previously described [19, 21], but also in individuals on ART. These findings have important implications for the development of strategies to reduce HIV transmission.
Notes
Acknowledgments. We are grateful to all the participants in the CCTG. We acknowledge all the nurses at all the enrollment sites, and Christy Anderson for her very helpful discussion. HIV RNA quantification standard was obtained through the National Institutes of Health AIDS Research and Reference Reagent Program, DAIDS, National Institute of Allergy and Infectious Diseases, and the HIV VQA RNA Quantification Standard from the DAIDS Virology Quality Assurance Program. Primer and probe for quantification of herpesviruses as well as the plasmids and quantification standards were kindly provided by Fred Lakeman.
Author contributions. S. G. participated in the study design, performed the laboratory experiments, participated in the data analyses for this study, and wrote the primary version of the manuscript. D. M. S. participated in the study design, participated in the data analyses and wrote the revised the manuscript. M. V. V. performed the laboratory experiments. S. R. M. participated in study design, performed statistical analysis, and wrote the primary version of the manuscript. D. D. R., R. H. H., C. C. G., E. S. D., and M. P. D. participated in study design and revised the manuscript. S. R. M., S. J. L., E. S. D., M. P. D., and R. H. H. enrolled participants. C. C. G. and F. Z. provided Aptima testing. All authors read and approved the final manuscript.
Financial support. This work was supported by the Department of Veterans Affairs; the James Pendleton Charitable Trust; the National Institutes of Health awards AI69432, AI043638, MH62512, MH083552, AI100665, AI077304, AI36214, AI047745, AI74621, GM093939AI080353, AI306214 (CFAR), AI27670 (ACTU), AI064086 (K24 to R. H. H.), and AI43638; the California HIV/AIDS Research Program RN07-SD-702, MC08-SD-700, and EI-11-SD-005; the National Center for Advancing Translational Sciences through UCLA CTSI Grant UL1TR000124; and the National Institute of General Medical Sciences grant GM093939. Aptima reagents were provided by Gen-Probe.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. D. D. R. has served as a consultant for Bristol-Myers Squibb, Gilead Sciences, Merck & Co, Monogram Biosciences, Biota, Chimerix, Gen-Probe, Tobira, and Idenix Pharmaceuticals. D. M. S. has received grant support from ViiV Pharmaceuticals and consultant fees from Gen-Probe and Testing Talent Services. M. P. D. has received grant support from Merck, Gilead, Serono, and ViiV and has served as a consultant to Serono. E. S. D. has received grant support from Abbott, Gilead, Merck, Pfizer, and ViiV and has acted as a consultant for Bristol-Myers Squibb, Gilead, Merck, and ViiV. C. C. G. has received consultant fees and serves on the scientific advisory board of Gen-Probe. R. H. H. reports having received honoraria or consultant fees from Bristol-Myers Squibb, Gilead Sciences, and Janssen (Tibotec) and grant support (to the University of California, San Diego) from Abbott, GlaxoSmithKline, Pfizer, and Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25:473–7. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu W, Zeng G, Luo J, et al. HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr. 2010;55:232–8. doi: 10.1097/QAI.0b013e3181e9b6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturmer M, Doerr HW, Berger A, Gute P. Is transmission of HIV-1 in non-viraemic serodiscordant couples possible? Antiviral Therapy. 2008;13:729–32. [PubMed] [Google Scholar]
- 8.Taylor S, Davies S. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:335–43. doi: 10.1097/COH.0b013e32833a0b69. [DOI] [PubMed] [Google Scholar]
- 9.Sheth PM, Yi TJ, Kovacs C, et al. Mucosal correlates of isolated HIV semen shedding during effective antiretroviral therapy. Mucosal Immunol. 2012;5:248–57. doi: 10.1038/mi.2012.1. [DOI] [PubMed] [Google Scholar]
- 10.Politch JA, Mayer KH, Welles SL, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS. 2012;26:1535–43. doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halfon P, Giorgetti C, Khiri H, et al. Semen may harbor HIV despite effective HAART: another piece in the puzzle. PLoS One. 2010;5:e10569. doi: 10.1371/journal.pone.0010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcelin AG, Tubiana R, Lambert-Niclot S, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS. 2008;22:1677–9. doi: 10.1097/QAD.0b013e32830abdc8. [DOI] [PubMed] [Google Scholar]
- 13.Craigo JK, Gupta P. HIV-1 in genital compartments: vexing viral reservoirs. Curr Opin HIV AIDS. 2006;1:97–102. doi: 10.1097/01.COH.0000200507.27578.26. [DOI] [PubMed] [Google Scholar]
- 14.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther. 2011;16:1149–67. doi: 10.3851/IMP1919. [DOI] [PubMed] [Google Scholar]
- 15.Sheth PM, Kovacs C, Kemal KS, et al. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS. 2009;23:2050–4. doi: 10.1097/QAD.0b013e3283303e04. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JA, Ping LH, Dibben O, et al. HIV-1 Populations in semen arise through multiple mechanisms. PLoS Pathog. 2010;6:e1001053. doi: 10.1371/journal.ppat.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351(suppl 3):5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 18.Gianella S, Strain MC, Rought SE, et al. Associations between the virologic and immunologic dynamics in blood and in the male genital tract. J Virol. 2012;86:1307–15. doi: 10.1128/JVI.06077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianella S, Morris SR, Anderson C, et al. Herpesviruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS. 2013;27:39–47. doi: 10.1097/QAD.0b013e3283573305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisco A, Munawwar A, Introini A, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheth PM, Danesh A, Sheung A, et al. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J Infect Dis. 2006;193:45–8. doi: 10.1086/498576. [DOI] [PubMed] [Google Scholar]
- 22.Speck CE, Coombs RW, Koutsky LA, et al. Risk factors for HIV-1 shedding in semen. Am J Epidemiol. 1999;150:622–31. doi: 10.1093/oxfordjournals.aje.a010061. [DOI] [PubMed] [Google Scholar]
- 23.Gianella S, Morris SR, Vargas MV, et al. The role of seminal shedding of herpesviruses in HIV-1 transmission. J Infect Dis. 2012;207:257–61. doi: 10.1093/infdis/jis683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler DM, Delport W, Kosakovsky Pond SL, et al. The origins of sexually transmitted HIV among men who have sex with men. Sci Transl Med. 2010;2:18re1. doi: 10.1126/scitranslmed.3000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yen-Lieberman B, Brambilla D, Jackson B, et al. Evaluation of a quality assurance program for quantitation of human immunodeficiency virus type 1 RNA in plasma by the AIDS Clinical Trials Group virology laboratories. J Clin Microbiol. 1996;34:2695–701. doi: 10.1128/jcm.34.11.2695-2701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people. 2011 Available at http://whqlibdocwhoint/publications/2011/9789241501750_engpdf . Accessed October 2012. [PubMed] [Google Scholar]
- 27.O'Sullivan CE, Drew WL, McMullen DJ, et al. Decrease of cytomegalovirus replication in human immunodeficiency virus infected-patients after treatment with highly active antiretroviral therapy. J Infect Dis. 1999;180:847–9. doi: 10.1086/314943. [DOI] [PubMed] [Google Scholar]
- 28.Deayton J, Mocroft A, Wilson P, Emery VC, Johnson MA, Griffiths PD. Loss of cytomegalovirus (CMV) viraemia following highly active antiretroviral therapy in the absence of specific anti-CMV therapy. AIDS. 1999;13:1203–6. doi: 10.1097/00002030-199907090-00008. [DOI] [PubMed] [Google Scholar]