Abstract
The objective of this study was to determine the effect of a Melissa officinalis and Passiflora caerulea infusion on the severity of physiological chronic stress induced by movement restriction in CF-1 mice. 40 CF-1 male mice, six weeks of age, were divided into 4 groups (n = 10 for each group): (1) Group RS/MP received two treatments, induced stress through movement restriction and a infusion of Melissa officinalis and Passiflora caerulea in a dose of 200 mg/kg, (2) RS group with induced stress using movement restriction, (3) MP group, which received only a infusion, and (4) a CONTROL group that received no treatment. The severity of the stress was obtained by analysis of the physical parameters of body weight, thymus and spleen, and associated biomarkers with stress, corticosterone, and glucose. Animals that consumed Melissa officinalis and Passiflora caerulea infusion had lower plasma corticosterone levels (Student’s t test, Welch, p = 0.05), which is the most important biomarker associated with physiological stress, demonstrating a phytotherapy effect.
Keywords: Aqueous extract, Melissa officinalis, Passiflora caerulea, physiological stress, CF-1 mice
Introduction
Stress is a physiological reaction of the organism in order to affront threatening or demanding situations [1] and involves appropriate responses to promote survival, altering the physiological and behavioral processes. The main component of the endocrine stress response involves activation of the hypothalamic-pituitary-adrenocortical (HPA), which triggers a neuroendocrine cascade which culminates with the synthesis and secretion of glucocorticoids (mainly cortisol in humans and corticosterone in rats, mice and other species) [2]. When physiological stress becomes chronic, it turns an adaptive response to a health problem, given that many systems are adversely affected by the prolonged exposure to glucocorticoids and catecholamines, causing memory problems, poor concentration, mood disturbances and immune system level changes [3,4].
The model used to study the physiological stress in experimental animals is movement restriction [5], which is a method to be used in different intervals of time and is susceptible to modification in order to prevent a habituation to the stressor stimulus [6]. In rodents, physiological stress chronicity could be reviewed by weight logs, measurements of serum corticosterone levels and glucose, among others [7].
Currently there are various therapies of traditional and complementary medicine, which seek to reduce the effects of chronic stress. Melissa officinalis, is one of the plants with attributed soothing properties, such as anxiolytic, antispasmodic and stress decrease, and is used in different medications and is popularly used on infusions [8,10]. Passiflora caerulea is another herb that is used for therapeutic purposes; mainly because it traditionally shows sedative, antispasmodic and anxiolytic properties. It is also used to sleep induction, when insomnia as a neurogenic reason [11]. Herbs as Melissa officinalis and Passiflora caerulea have no contraindications and are popularly used to ease the ravages of stress [8,12].
There is evidence that Melissa officinalis, through its ethanolic extract, (200 mg/kg) has the ability to increase cell proliferation, differentiation and integration of neuroblasts in granular cells, because it reduces serum levels of corticosterone and as well it increases gamma- aminobutyric acid (GABA) levels in the hippocampal dentate gyrus in male mice C57BL/6J [13]. In humans, Melissa officinalis is expended in 300 mg capsules. It improves cognitive performance, better mood and reduces acutely induced anxiety [3]; also in randomized controlled trials, when it was administered in capsules of 300 and 600 mg. it was shown to reduce the effects of acute stress and associated itself with Valeriana officinalis, in a computerized simulator acute stress [8,9]. There is no evidence linking Melissa officinalis with chronic stress.
In the Passiflora genus, it could be presented in capsules of 500 mg. Passiflora incarnata reduces anxiety in patients when is administered previously to an ambulatory surgery [14]. Passiflora caerulea has been isolated and identified unequivocally; chrysin, a monoflavoide present in this plant, administered intracerebroventricularly to mice prevents the expression of tonic-clonic seizures induced by pentylenetetrazol, supporting the hypothesis that chrysin acts via central benzodiazepine receptors, but to the date it has not been possible to isolate or identify these natural molecules [11,15]. It has been compared the effect of chrysin, extracted from Passiflora caerulea, with the drug diazepam concluding that chrysin induces anxiolytic effects without sedation and muscle relaxation [16]. There are no reports on the effects of stress in Passiflora caerulea.
Despite the evidence, there is no information that these herbal separate or together, prepared as an infusion, have medicinal effects on physical and physiological markers associated with chronic stress, which is why an infusion of Melissa officinalis and Passiflora caerulea will be administered experimentally on animals subjected to chronic stress induced by restricted movement.
Materials and methods
To conduct this research it was used a randomized experimental design. The explanatory variable was the exposure to an infusion of Melissa officinalis and Passiflora caerulea; the response variable was the severity of chronic stress. All procedures were approved according to the Committee on Bioethics of the University of Talca (folio 00170). The protocols followed the guidelines of the Guide for the Care and Use of Laboratory Animals (U.S. National Research Council, 1996).
Animals
40 CF-1 male mice were used, six weeks of age (± 22 g), no consanguineous, with their corresponding health certification, obtained from the Public Health Institute of Chile, which were kept under controlled conditions of temperature (22 ± 1 °C) and cycles of 12 hours (light-dark , beginning at 08:00 am), with freely available food and water, in groups of 5 mice, kept in polycarbonate cages enriched with tissue paper and PVC pipes ranging his orientation each week [17], in the animal facility of the University of Talca, Chile. The mice were randomly divided into 4 groups (Figure 1): (1) RS/MP group (n = 10) received two treatments, stress movement restriction and infusion of Melissa officinalis and Passiflora caerulea, (2) RS group (n = 10) stress by movement restriction, (3) MP group (n = 10) received only the infusion, and (4) CONTROL group (n = 10) no treatment received. The mice weight and food consumption was recorded 2 times a week.
Figure 1.
Distribution of the experimental units. Each group was divided into two cages of five mice and two shelves (one upper and one lower) in the same rack, on the maintenance room.
Procedures
Aqueous extract of Melissa officinalis and Passiflora caerulea (MP)
100 ml were daily prepared as a infusion from commercial standardized capsules of MELIPASS® (KNOP Laboratorios Ltda., Santiago, Chile), which contained the dried plant material (127.5 mg of c/u). The aqueous extract was obtained in the Institute of Chemical and Natural Resources, University of Talca, Chile, according to the previously described process [10]. Briefly, by maceration of 631.4 mg of MELIPASS® in 100 ml of hot distilled water (100 °C for 10 minutes), the suspension was filtered through filtered paper which resulted in 200 mg of MP in 100 ml water according to the effective dose of Melissa officinalis previously described [13]. The mice in groups MP and MP/RS received the infusion freely with drinking bottles orally. During these days of the investigation, both groups only drank the prepared infusion.
Induction of stress (RS)
From the first day of the pilot phase the animals were stressed out for five hours a day for 32 days, on properly ventilated acrylic cylinders (25 mm diameter), which were covered by dimming boxes. Each session was conducted between 12:30 am and 17:30 pm, in which time the mice had no access to food or water. To avoid habituation to the stressor stimulus, we designed a protocol that involved two additional procedures, Fragance/Ledge 1: between 9 and 23 days, during the period of restriction of animal were stood in their shelves, but day by half were exposed to different flavors (banana, oregano, lemon balm, black pepper, coriander, mint, cinnamon, basil, cloves, dill). Fragance/Ledge 2: between days 24 and 32 animals were placed in different shelves, receiving daily change scent (Figure 2). Once the stress cycle was finished, the animals returned to the maintenance room, were the unstressed groups were located.
Figure 2.
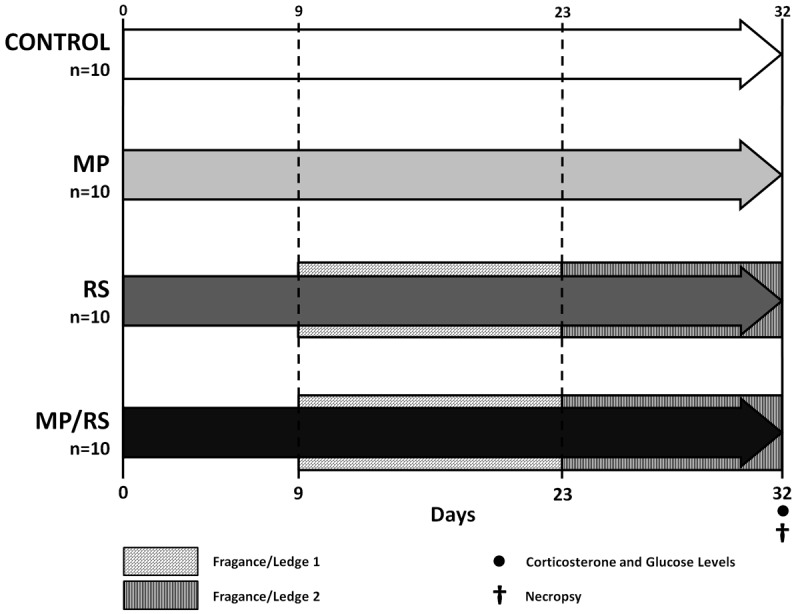
Key aspects of the experimental phase. It shows the distribution of animals according to received treatment and interventions.
Laboratory assays
Prior to sample collection, the animals were weighted and then anesthetized with ketamine/xylazine/acepromazine (Drag Pharma Invetec Chile SA, Santiago, Chile) in a ratio of 50/5/1 mg/kg intramuscularly (IM). Proven anesthesia, after the last cycle of stress, plasma samples were obtained by a cardiac puncture. Then, it was performed the euthanasia of animals by cervical dislocation. Samples were centrifuged and stored at -20 °C until the determination of plasma corticosterone levels (nmol/L) and glucose (mg/dL) in the Laboratory of Animal Physiology and Endocrinology, University of Concepción, Chillán, Chile. Corticosterone was quantified using a commercial ELISA kit (DRG International, Austin, TX, USA) validated for mouse corticosterone, with intra-assay coefficient of variation of 2.1%. Glucose was determined using a kit (Roche, Mannheim, Germany) based on the method GOD-POD (glucose oxidase and peroxidase) and measured at 505 nm using a spectrophotometer (Thermo Electron Co., Vantaa, Finland) with a interassay variation coefficient of 0.5-1.3% and intra-assay of 0 to 2.7%.
Tissue preparation
Immediately on euthanasia, thymus and spleen were removed and stored in 10% formalin with a pH of 7.4, and then were weighed on an analytical balance (AAA/LE, Adam Equipment, Danbury, USA). We calculated the ratio of each of these bodies according to the body weight of the animals.
Severity of stress
The severity of stress was measured by analyzing physical parameters (body weight, thymus and spleen) and biomarkers associated with stress (serum corticosterone and glucose).
Statistical analysis
Results are presented as mean + - standard deviation. They were tested for normality (Kolmogorov-Smirnov) and homogeneity of variance (Levene test). Quantitative data were compared in pairs, using the Student t test, (Welch). It was also used the one-way ANOVA test, adjusted for multiple comparisons (Bonferroni and Tukey). For statistical analysis, we used a 95% confidence level. To perform the statistical analysis was used the software SPSS® Statistics 17 and graphics were made with Microsoft® Office Excel®.
Results
The restriction of movement decreases body weight gain (g) of CF-1 mice
Measurements were made with food consumption and weight of the mice twice a week (Figure 3). Food consumption and body weight tended to increase throughout the experimental phase. The data was analyzed by one-way ANOVA with repeated measures, with an adjustment for multiple comparisons (Bonferroni), showing no significant difference for food consumption; the opposite situation was found for body weight between the CONTROL group versus RS and MP/RS (p = 0.05).
Figure 3.
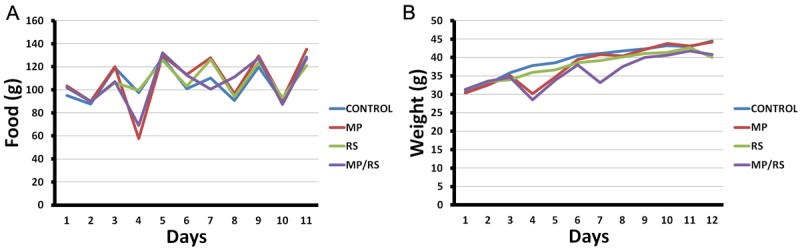
The restriction of movement decreases body weight gain. It could be appreciated irregular increase in food consumption (A) and the continuous increase in body weight in the study groups (B), which was statistically different when comparing the CONTROL group and the groups RS and RS/MP (p=0.05, one-way ANOVA with repeated measures and when it was adjusted for multiple comparisons by Bonferroni).
Movement restriction decreases the spleen weight (g) of CF-1 mice
Decreased weight of spleen and thymus are considered as adaptive effects in response to stress. The thymus weight (g) and spleen (g) can be seen in Table 1. The body weight and the lymphoid weights were distributed according to normal parameters; it was calculated with the relationship (thymus or spleen weight/body weight) for each mouse. Differences for thymus weights were not considered statistically significant using ANOVA and post-hoc Tukey. The same test was used to compare the weights of the spleen and it was found statistically significant differences for RS and CONTROL groups (p = 0.01), and for the group RS/MP and CONTROL (p = 0.001), being the CONTROL group the one with most weight.
Table 1.
Relative weight (g) of thymus and spleen (thymus or spleen weight/body weight; means ± DE)
| Thymus | Spleen | |
|---|---|---|
| g x 10-4 | g x 10-4 | |
| CONTROL | 16.1 ± 6.6 | 51.7 ± 11.1 |
| MP | 18.5 ± 5.6 | 43.2 ± 10.2 |
| RS | 23.6 ± 20.6 | 35.3 ± 11.1**,a |
| MP/RS | 34 ± 5.5 | 14.7 ± 4.2**,a |
MP Melissa officinalis y Passiflora caerulea, RS movement restriction. Tukey contrast, significativally difference
p ≤ 0.05,
p ≤ 0.01.
With CONTROL.
The CF-1 mice treated Melissa officinalis and Passiflora caerulea had lower corticosterone levels
Corticosterone is the most important biomarker associated with stress. Corticosterone levels (ng/mL) and glucose (mg/dL) are shown in Table 2. The Student t test, Welch, established significant differences between the groups treated with Melissa officinalis and Passiflora caerulea (MP and MP/RS); with the group receiving movement restriction (RS) for the measurement of corticosterone (p = 0.05). RS and CONTROL groups showed significant differences (p = 0.01). Interestingly, among the CONTROL, MP and MP/RS groups there was no statistically significant difference (p > 0.16).
Table 2.
Plasmatic levels of corticosterone and glucose (means ± DE) measured after stress cycles
| Corticosterone Levels | Glucose Levels | |
|---|---|---|
| ng/mL | mmol/L | |
| CONTROL | 356.9 ± 59.9**,a | 222,3 ± 38.5*,b |
| MP | 345 ± 60.1**,a | 217.0 ± 29.3 |
| RS | 418 ± 25.5*,b | 200.3 ± 46.7*,b |
| MP/RS | 373.4 ± 54.3 | 264.9 ± 52.7 |
MP Melissa officinalis y Passiflora caerulea, RS movement restriction. t - test Student-Welch, significativally difference
p ≤ 0.05,
p ≤ 0.01.
With RS.
With MP/RS.
Mice receiving Passiflora caerulea and Melissa officinalis with movement restriction (MP/RS) had higher levels of circulating glucose. Statistical analysis were performed using Student’s t test, Welch, establishing that there were significant differences between CONTROL and RS when compared with MP/RS (p = 0.05).
Discussion
To determine the induction of chronic stress for restricting movement throughout the experimental period it was recorded twice weekly, body weight and food consumption, and after the experimental phase, serum levels of corticosterone and glucose were measured and calculated the weight of spleen and thymus in relation to the body weight of all animals.
In the conducted stress model was considered a time of 5 hours per day [6], during which the animals were kept under a box of light attenuation, as during the dark phase is when they have the most activity; therefore immobilized during this period generates a higher stress [7]. To avoid additional stress on the environment it was enriched for all animals of all groups [17].
In this study, as well as in the literature, it was considered that the influence that could lead to the habituation on animals subjected to chronic stress, since exposure to a repetitively stimulus produces a less intense physiologic [18,19], for which a protocol was designed to avoid this problem, introduced from the second week a change in the smell of the microenvironment where the movement restriction going on and as of the last week began to change common position on the shelves of the procedure room who had until then mice. These changes during the experimental were able to adapt the model for animals.
The weight loss is considered as an adaptive effect in animals under stress [20]. In this study all animals tend to gain weight over time, considering the growth period in which they find themselves. However, mice subjected to movement restrictions have lower body weights, statistically significant differences between the CONTROL group versus RS and MP/RS, related with other studies that have found a direct relationship of weight loss stress [21]. This difference in the weight may be due to transient hypophagia while animals have restrictors, and are in registered feed consumption without any significant differences between groups; therefore, the change in body mass probably is due to endogenous factors altered by the restriction of movement.
Corticosterone is the most important biomarker associated with stress, studies examining chronic stress support this associating this to movement restriction [19,22,23]. By analyzing this biomarker, it was found that the animals treated with movement restriction present higher circulating levels of corticosterone. Between RS and CONTROL groups are significant differences, determining that they did reflect the induction of stress in the RS group. It should be noted that there are significant differences between the groups treated with Melissa officinalis and Passiflora caerulea (MP and MP/RS), with the group receiving movement restriction (RS) for the measurement of corticosterone. It is also interesting to mark that between CONTROL, MP and MP/RS there is no statistically significant difference. This evidence supports our hypothesis; mice that consumed Melissa officinalis and Passiflora caerulea had lower plasma corticosterone levels, and so it may be assumed, in our design, that mice presented no chronic stress.
One consequence of the release of glucocorticoids as a result of activation of the stress cascade is the elevation of blood glucose, stress-activated sympathetic nerve fibers of the autonomic nervous system, which innervate the tissues of the immune system, the release of catecholamines from the nerve cells resulting in hormonal secretion of norepinephrine and epinephrine from the adrenal medulla cells. Catecholamines released during stress conditions contribute to the development of hyperglycemia by direct stimulation of glucose output [20] which interferes with glucose disposal tissues as glucocorticoids inhibit insulin release [24]. Therefore we analyzed the levels of circulating glucose (mg/dL). Blood samples were taken after completion of the last cycle of stress established, there were significant differences between CONTROL and RS when compared with MP/RS (p = 0.05); paradoxically we found lower values of glucose in the RS group. These data differs from those reported previously, where are said that physiological markers are statistically significant in all groups of stress [20]. However, this drop in glucose can be caused by chronic stress and it causes decrease in body weight, and thus may induce lower blood glucose levels [24]. The MP group tends to have fewer circulating glucose than the CONTROL group, which is consistent with corticosterone levels found, reinforcing the idea of the protective role that could give the aqueous extract of Melissa officinalis and Passiflora caerulea in the body.
The involution of the thymus and spleen, are also seen as bioadaptative effects in animals under stress [20]. To investigate the relevance of in the central nervous system (CNS) and the endocrine system involves reciprocal interactions with the immune system. There are two primary mechanisms involved in the mediation of stress on the immune functions: adrenergic innervation of the lymphoid organs and production of a hormonal hyper secretion of HPA [20,23,25].
It was investigated the role of endogenous glucocorticoids in the involution of the thymus after stress inducing movement restriction [23]. Stress restriction, measured by increased glucocorticoid endogenous mice, resulted in rapid degradation of the genome of thymocytes and ultimately apoptosis of these cells, causing a diminution in the subpopulation of CD4+ and CD8 thymocytes, while that decreases thymus weight. Overall results indicate that the changes induced in the thymus during stress constraint probably were due to a specific process of cell death induced by glucocorticoids, and the possible migration of viable cells from the thymus into the circulation to other lymphoid organs.
It was concluded that various stressors are able adrenal hypertrophy, increasing the amount of glucocorticoids in mice [25], while it causes atrophy of thymus and decrease thymocytes. It also notes that the amount of plasma glucocorticoids was inversely proportional to the weight of the spleen and the number of B lymphocytes, which when adrenalectomy is performed in stressed mice, no changes were generated in the spleen and thymus. Moreover, the changes associated with the frequency of stress, began to be observed after 4 days of severe stress; otherwise observed changes were reversible once the stress conditions ceased, and system plasticity restore their normal functions.
In our research, the weight of the thymus and spleen were related to body weight of the mice and the weight of the thymus or spleen/body weight. Observing these values of thymus weight, differences were not statistically significant, probably, as explained Hori et al., that it requires a longer exposure to the stressor stimulus in order to show a morphological change in the atrophy of this organ, or may be due to the removal of the thymus, which is difficult and the sample was not harmless, which could alter the records. Measurement of spleen concords with previous evidence; statistically significant differences between groups RS and MP/RS, when compared with the CONTROL group (< 0.05), both with less weight than the CONTROL.
The results for physical and plasma biomarkers, in this research, support the chronic stress induced by movement restriction and taken actions to prevent habituation. Moreover, procedures have shown that an aqueous extract of Melissa officinalis and Passiflora caerulea reduces plasma corticosterone, the most important biomarker associated with stress, with no effects described above for the combination of these phytotherapeutic. Stress can cause several problems physiological and psychological; our results point to an alternative herbal medicine in order to combat and solve the disorders caused by chronic stress. Future research is still needed to verify the results, involving clinical trials with patients.
Acknowledgements
The authors wish to thank the Institute of Chemistry of Natural Resources and the Investigation Directorate (DI) from the University of Talca, Chile.
Disclosure of conflict of interest
It states that there are no conflicts of interest. There is no relationship with the manufacturer of the product tested here.
References
- 1.Dallman M. Modulation of stress responses: How we cope with excess glucocorticoids. Exp Neurol. 2007;206:179–182. doi: 10.1016/j.expneurol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cases J, Ibarra A, Feuillere N, Roller M, Sukkar SG. Pilot trial of Melissa officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Med J Nutrition Metab. 2011;4:211–218. doi: 10.1007/s12349-010-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard BE. The HPA and inmune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20:S302–306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 5.Patchev VK, Patchev AV. Experimental models of stress. Dialogues Clin Neurosci. 2006;8:417–32. doi: 10.31887/DCNS.2006.8.4/vpatchev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009;33:1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy DO, Little W, Scholey AB. Attenuation of laboratory-induced stress in humans after acute administration of Melissa officinalis (Lemon Balm) Psychosom Med. 2004;66:607–613. doi: 10.1097/01.psy.0000132877.72833.71. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy DO, Little W, Haskell CF, Scholey AB. Anxiolytic effects of a combination of Melissa officinalis and Valeriana officinalis during laboratory induced stress. Phytother Res. 2006;20:96–102. doi: 10.1002/ptr.1787. [DOI] [PubMed] [Google Scholar]
- 10.Martins EN, Pessano NT, Leal L, Roos DH, Folmer V, Puntel GO, Rocha JB, Aschner M, Avila DS, Puntel RL. Protective effect of Melissa officinalis aqueous extract against Mn-induced oxidative stress in chronically exposed mice. Brain Res Bull. 2012;87:74–79. doi: 10.1016/j.brainresbull.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Dhawan K, Dhawan S, Sharma A. Passiflora: a review update. J Ethnopharmacol. 2004;94:1–23. doi: 10.1016/j.jep.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Pardo K, Diaz M, Villegas LF, Bernabé E. Efecto del extracto etanólico de Melissa Officinalis (toronjil) en la modificación de la condiucta del niño ansioso en la consulta dental. Rev Estomatol Herediana. 2009;19:91–95. [Google Scholar]
- 13.Yoo DY, Choi JH, Kim W, Yoo KY, Lee CH, Yoon YS, Won MH, Hwang IK. Effects of Melissa officinalis L. (lemon balm) extract on neurogenesis associated with serum corticosterone and GABA in the mouse dentate gyrus. Neurochem Res. 2011;36:250–7. doi: 10.1007/s11064-010-0312-2. [DOI] [PubMed] [Google Scholar]
- 14.Movafegh A, Alizadeh R, Hajimohamadi F, Esfehani F, Nejatfar M. Preoperative oral Passiflora incarnata reduces anxiety in ambulatory surgery patients: a double-blind, placebo-controlled study. Anesth Analg. 2008;106:1728–32. doi: 10.1213/ane.0b013e318172c3f9. [DOI] [PubMed] [Google Scholar]
- 15.Medina JH, Paladini AC, Wolfman C, Levi de Stein M, Calvo D, Diaz LE, Peña C. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem Pharmacol. 1990;40:2227–31. doi: 10.1016/0006-2952(90)90716-x. [DOI] [PubMed] [Google Scholar]
- 16.Wolfman C, Viola H, Paladini A, Dajas F, Medina JH. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacol Biochem Behav. 1994;47:1–4. doi: 10.1016/0091-3057(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 17.Olsson IA, Dahlborn K. Improving housing conditions for laboratory mice: a review of “environmental enrichment”. Lab Anim. 2001;36:243–270. doi: 10.1258/002367702320162379. [DOI] [PubMed] [Google Scholar]
- 18.Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera C, Monsalve F, Suazo I, Becerra J. Stress increases periodontal inflammation. Exp Ther Med. 2012;4:883–888. doi: 10.3892/etm.2012.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takada T, Yoshinari N, Suglishi S, Kawase H, Yamane T, Toshihide N. Effect of restraint stress on the progression of experimental periodontitis in rats. J Periodontol. 2004;25:306–15. doi: 10.1902/jop.2004.75.2.306. [DOI] [PubMed] [Google Scholar]
- 21.Rivera C, Droguett D, Kemmerling U, Venegas B. Chronic restraint stress in oral squamous cell carcinoma. J Dent Res. 2011;90:799–803. doi: 10.1177/0022034511399911. [DOI] [PubMed] [Google Scholar]
- 22.Ibarra A, Feuillere N, Roller M, Lesburgere E, Beracochea D. Effects of chronic administraadministration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice. Phytomedicine. 2010;17:397–403. doi: 10.1016/j.phymed.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Tarcic N, Ovadia H, Weiss DW, Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol. 1998;82:40–46. doi: 10.1016/S0165-5728(97)00186-0. [DOI] [PubMed] [Google Scholar]
- 24.Bates HE, Kiraly MA, Yue JT, Goche Montes D, Elliott ME, Riddell MC, Matthews SG, Vranic M. Recurrent intermittent restraint delays fed and fasting hyperglycemia and improves glucose return to baseline levels during glucose tolerance tests in the Zucker diabetic fatty rat--role of food intake and corticosterone. Metabolism. 2007;56:1065–1075. doi: 10.1016/j.metabol.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Hori T, Fukuda M, Suzuki H, Yano S, Ono T. SART stress effects on lymphocytes in the thymus and spleen of normal, adrenalectomized, and sympathectomized mice. Clin Immunol Immunopathol. 1993;68:243–5. doi: 10.1006/clin.1993.1125. [DOI] [PubMed] [Google Scholar]


