Abstract
Experimental diabetes consistently reduces the concentration of free myo-inositol in peripheral nerve, which usually exceeds that of plasma by 90-100-fold. This phenomenon has been explicitly linked to the impairment of nerve conduction in the acutely diabetic streptozocin-treated rat. However, the mechanism by which acute experimental diabetes lowers nerve myo-inositol content and presumably alters nerve myo-inositol content and presumably alters nerve myo-inositol metabolism is unknown. Therefore, the effects of insulin and elevated medium glucose concentration of 2-[3H]myo-inositol uptake were studied in a metabolically-defined in vitro peripheral nerve tissue preparation derived from rabbit sciatic nerve, whose free myo-inositol content is reduced by experimental diabetes. The results demonstrate that myo-inositol uptake occurs by at least two distinct transport systems in the normal endoneurial preparation. A sodium- and energy-dependent saturable transport system is responsible for at least 94% of the measured uptake at medium myo-inositol concentrations approximating that present in plasma. This carrier-mediated transport system has a high affinity for myo-inositol (Kt = 63 microM), and is not influenced acutely by physiological concentrations of insulin; it is, however, inhibited by hyperglycemic concentrations of glucose added to the incubation medium in a primarily competitive fashion. Thus, competitive inhibition of peripheral nerve myo-inositol uptake by glucose may constitute a mechanism by which diabetes produces physiologically significant alterations in peripheral nerve myo-inositol metabolism.
Full text
PDF

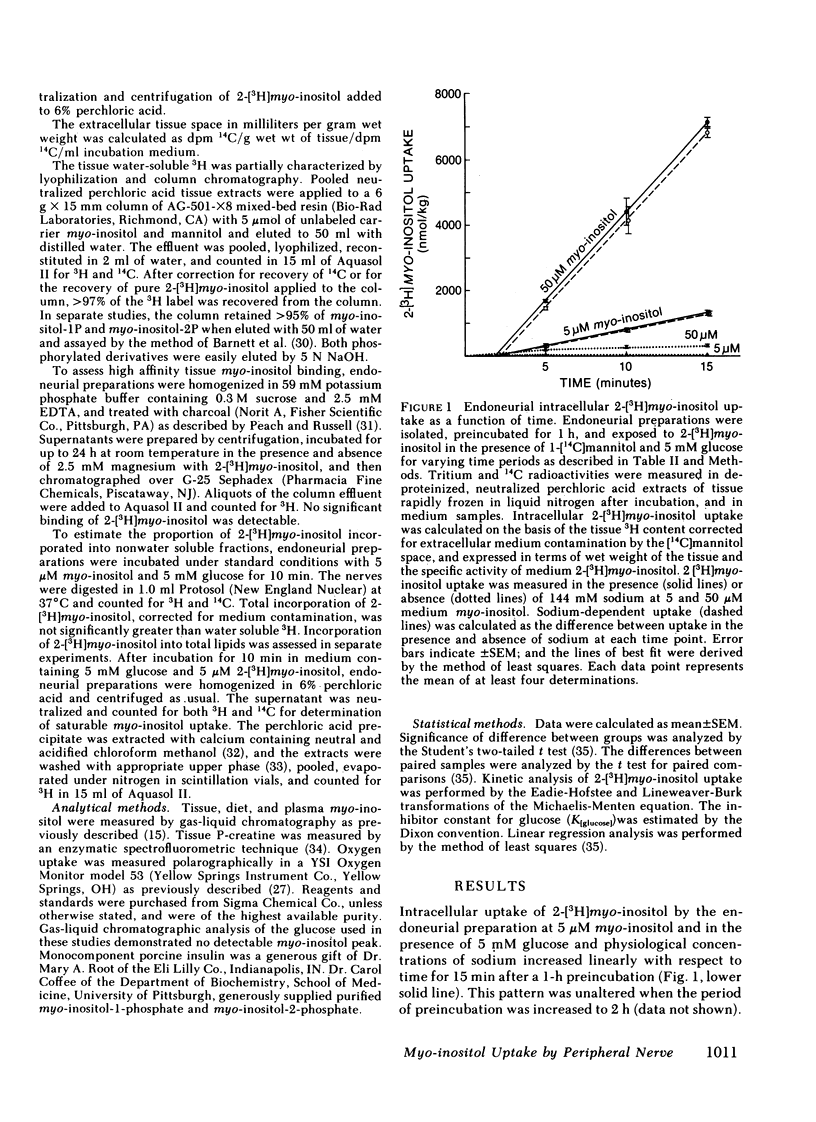







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Brice R. E., Corina D. L. A colorimetric determination of inositol monophosphates as an assay for D-glucose 6-phosphate-1L-myoinositol 1-phosphate cyclase. Biochem J. 1970 Sep;119(2):183–186. doi: 10.1042/bj1190183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. C., Sacktor B. Energetics of the Na+-dependent transport of D-glucose in renal brush border membrane vesicles. J Biol Chem. 1975 Nov 25;250(22):8674–8680. [PubMed] [Google Scholar]
- Caspary W. F., Crane R. K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim Biophys Acta. 1970 Apr 21;203(2):308–316. doi: 10.1016/0005-2736(70)90145-8. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr Diabetic neuropathy--new concepts of its etiology. Diabetes. 1979 Jun;28(6):604–611. doi: 10.2337/diab.28.6.604. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Stockard C. R. Abnormal sciatic nerve myo-inositol metabolism in the streptozotocin-diabetic rat: effect of insulin treatment. Diabetes. 1980 Mar;29(3):227–235. doi: 10.2337/diab.29.3.227. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Vourganti B., Kuba T., Oh S. J., Darnell B. Dietary myo-inositol intake and peripheral nerve function in diabetic neuropathy. Metabolism. 1979 Apr;28(4 Suppl 1):477–483. doi: 10.1016/0026-0495(79)90060-x. [DOI] [PubMed] [Google Scholar]
- Cotlier E. Myo-inositol: active transport by the crystalline lens. Invest Ophthalmol. 1970 Sep;9(9):681–691. [PubMed] [Google Scholar]
- DAWSON R. M., FREINKEL N. The distribution of free mesoinositol in mammalian tissues, including some observations on the lactating rat. Biochem J. 1961 Mar;78:606–610. doi: 10.1042/bj0780606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. F., Thorpe S. R., Baynes J. W. Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J Biol Chem. 1979 Feb 10;254(3):595–597. [PubMed] [Google Scholar]
- FIELD R. A., ADAMS L. C. INSULIN RESPONSE OF PERIPHERAL NERVE. I. EFFECTS ON GLUCOSE METABOLISM AND PERMEABILITY. Medicine (Baltimore) 1964 May;43:275–279. doi: 10.1097/00005792-196405000-00006. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Ferrendelli J. A., Rubin E. H., Orr H. T., Kinscherf D. A., Lowry O. H. Measurement of cyclic nucleotides in histologically defined samples of brain and retina. Anal Biochem. 1977 Mar;78(1):252–259. doi: 10.1016/0003-2697(77)90030-6. [DOI] [PubMed] [Google Scholar]
- Field R. A., Adams L. C. Insulin response of peripheral nerve. II. Effects on lipid metabolism. Biochim Biophys Acta. 1965 Dec 2;106(3):474–479. [PubMed] [Google Scholar]
- Gould R. M. Inositol lipid synthesis localized in axons unmyelinated fibers of peripheral nerve. Brain Res. 1976 Nov 19;117(1):168–174. doi: 10.1016/0006-8993(76)90569-2. [DOI] [PubMed] [Google Scholar]
- Graf R. J., Halter J. B., Halar E., Porte D., Jr Nerve conduction abnormalities in untreated maturity-onset diabetes: relation to levels of fasting plasma glucose and glycosylated hemoglobin. Ann Intern Med. 1979 Mar;90(3):298–303. doi: 10.7326/0003-4819-90-3-298. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Brown M. J., Braunstein S. N., Schwartz S. S., Asbury A. K., Winegrad A. I. Comparison of clinical couse and sequential electrophysiological tests in diabetics with symptomatic polyneuropathy and its implications for clinical trials. Diabetes. 1981 Feb;30(2):139–147. doi: 10.2337/diab.30.2.139. [DOI] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lewis R. A., Lattimer S. A., Brown M. J. Selective effects of myo-inositol administration on sciatic and tibial motor nerve conduction parameters in the streptozocin-diabetic rat. Diabetes. 1982 Jul;31(7):573–578. doi: 10.2337/diab.31.7.573. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Winegrad A. I., Carpentier J. L., Brown M. J., Fukuma M., Orci L. Rabbit sciatic nerve fascicle and 'endoneurial' preparations for in vitro studies of peripheral nerve glucose metabolism. J Neurochem. 1979 Nov;33(5):1007–1018. doi: 10.1111/j.1471-4159.1979.tb05237.x. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Winegrad A. I. Effects of acute experimental diabetes on composite energy metabolism in peripheral nerve axons and Schwann cells. Diabetes. 1981 Nov;30(11):967–974. doi: 10.2337/diab.30.11.967. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Winegrad A. I. In vitro studies of the substrates for energy production and the effects of insulin on glucose utilization in the neural components of peripheral nerve. Diabetes. 1979 Oct;28(10):878–887. doi: 10.2337/diab.28.10.878. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Sacktor B., Daughaday W. H. myo-Inositol transport in renal brush border vesicles and it inhibition by D-glucose. Am J Physiol. 1980 Aug;239(2):F113–F120. doi: 10.1152/ajprenal.1980.239.2.F113. [DOI] [PubMed] [Google Scholar]
- Hauser G., Eichberg J. Improved conditions for the preservation and extraction of polyphosphoinositides. Biochim Biophys Acta. 1973 Nov 29;326(2):201–209. doi: 10.1016/0005-2760(73)90246-4. [DOI] [PubMed] [Google Scholar]
- Hothersall J. S., McLean P. Effect of experimental diabetes and insulin onphosphatidyl-inositol synthesis in rat sciatic nerve. Biochem Biophys Res Commun. 1979 May 28;88(2):477–484. doi: 10.1016/0006-291x(79)92073-4. [DOI] [PubMed] [Google Scholar]
- Kusama H., Stewart M. A. Levels of myo-inositol in normal and degenerating peripheral nerve. J Neurochem. 1970 Mar;17(3):317–323. doi: 10.1111/j.1471-4159.1970.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Kyte J. Molecular considerations relevant to the mechanism of active transport. Nature. 1981 Jul 16;292(5820):201–204. doi: 10.1038/292201a0. [DOI] [PubMed] [Google Scholar]
- Mauck L. A., Wong Y. H., Sherman W. R. L-myo-Inositol-1-phosphate synthase from bovine testis: purification to homogeneity and partial characterization. Biochemistry. 1980 Jul 22;19(15):3623–3629. doi: 10.1021/bi00556a031. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Gravallese E., Bunn H. F. Nonenzymatic glycosylation of erythrocyte membrane proteins. Relevance to diabetes. J Clin Invest. 1980 Apr;65(4):896–901. doi: 10.1172/JCI109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris B. A., Karl I. E., Daughaday W. H. Concentration of myo-inositol in skeletal muscle of the rat occurs without active transport. J Clin Invest. 1980 Apr;65(4):783–788. doi: 10.1172/JCI109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmano K. P., Whiting P. H., Hawthorne J. N. Free and lipid myo-inositol in tissues from rats with acute and less severe streptozotocin-induced diabetes. Biochem J. 1977 Oct 1;167(1):229–235. doi: 10.1042/bj1670229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A., Garner W. H., Spector A. Glucosylation of human lens protein and cataractogenesis. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1260–1266. doi: 10.1016/0006-291x(79)92144-2. [DOI] [PubMed] [Google Scholar]
- Peach D. M., Russell J. C. A binding protein from rat nerve. Physiol Chem Phys. 1977;9(3):227–240. [PubMed] [Google Scholar]
- Reddy V. N., Varma S. D., Chakrapani B. Intraocular transport of myoinositol. I. Accumulation in the rabbit ciliary body. Invest Ophthalmol. 1970 Oct;9(10):785–793. [PubMed] [Google Scholar]
- Rosenberg H., Modrak J. B., Hassing J. M., Al-Turk W. A., Stohs S. J. Glycosylated collagen. Biochem Biophys Res Commun. 1979 Nov 28;91(2):498–501. doi: 10.1016/0006-291x(79)91549-3. [DOI] [PubMed] [Google Scholar]
- Salway J. G., Whitehead L., Finnegan J. A., Karunanayaka A., Barnett D., Payne R. B. Effect of myo-inositol on peripheral-nerve function in diabetes. Lancet. 1978 Dec 16;2(8103):1282–1284. doi: 10.1016/s0140-6736(78)92043-3. [DOI] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Glucosylation of human collagen in aging and diabetes mellitus. J Clin Invest. 1980 Nov;66(5):1179–1181. doi: 10.1172/JCI109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., McManus M. J., Zalut C., Bunn H. F. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem. 1980 Apr 10;255(7):3120–3127. [PubMed] [Google Scholar]
- Sherman W. R., Packman P. M., Laird M. H., Boshans R. L. Measurement of myo-inositol in single cells and defined areas of the nervous system by selected ion monitoring. Anal Biochem. 1977 Mar;78(1):119–131. doi: 10.1016/0003-2697(77)90015-x. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Stewart M. A., Kurien M. M., Goodwin S. L. The measurement of myo-inositol, myo-inosose-2 and scyllo-inositol in mammalian tissues. Biochim Biophys Acta. 1968 May;158(2):197–205. doi: 10.1016/0304-4165(68)90131-1. [DOI] [PubMed] [Google Scholar]
- Spector R. Inositol accumulation by brain slices in vitro. J Neurochem. 1976 Nov;27(5):1273–1276. doi: 10.1111/j.1471-4159.1976.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. Myo-inositol transport in the central nervous system. Am J Physiol. 1975 May;228(5):1510–1518. doi: 10.1152/ajplegacy.1975.228.5.1510. [DOI] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. The origin of myo-inositol in brain, cerebrospinal fluid and choroid plexus. J Neurochem. 1975 Sep;25(3):353–354. doi: 10.1111/j.1471-4159.1975.tb06980.x. [DOI] [PubMed] [Google Scholar]
- Spector R. The specificity and sulfhydryl sensitivity of the inositol transport system of the central nervous system. J Neurochem. 1976 Jul;27(1):229–236. doi: 10.1111/j.1471-4159.1976.tb01569.x. [DOI] [PubMed] [Google Scholar]
- Spritz N., Singh H., Marinan B. Metabolism of peripheral nerve myelin in experimental diabetes. J Clin Invest. 1975 May;55(5):1049–1056. doi: 10.1172/JCI108005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. A., Sherman W. R., Harris J. T. Effects of galactose on levels of free myo-inositol in rat tissues. Ann N Y Acad Sci. 1969 Oct 17;165(2):609–614. [PubMed] [Google Scholar]
- Stewart M. A., Sherman W. R., Kurien M. M., Moonsammy G. I., Wisgerhof M. Polyol accumulations in nervous tissue of rats with experimental diabetes and galactosaemia. J Neurochem. 1967 Nov;14(11):1057–1066. doi: 10.1111/j.1471-4159.1967.tb09516.x. [DOI] [PubMed] [Google Scholar]
- Varma S. D., Chakrapani B., Reddy V. N. Intraocular transport of myoinositol. II. Accumulation in the rabbit lens in vitro. Invest Ophthalmol. 1970 Oct;9(10):794–800. [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Nonenzymatic glycosylation of peripheral nerve protein in diabetes mellitus. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5190–5192. doi: 10.1073/pnas.78.8.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P. H., Palmano K. P., Hawthorne J. N. Enzymes of myo-inositol and inositol lipid metabolism in rats with streptozotocin-induced diabetes. Biochem J. 1979 Jun 1;179(3):549–553. doi: 10.1042/bj1790549. [DOI] [PMC free article] [PubMed] [Google Scholar]



