Abstract
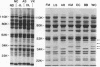
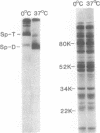
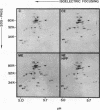
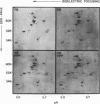
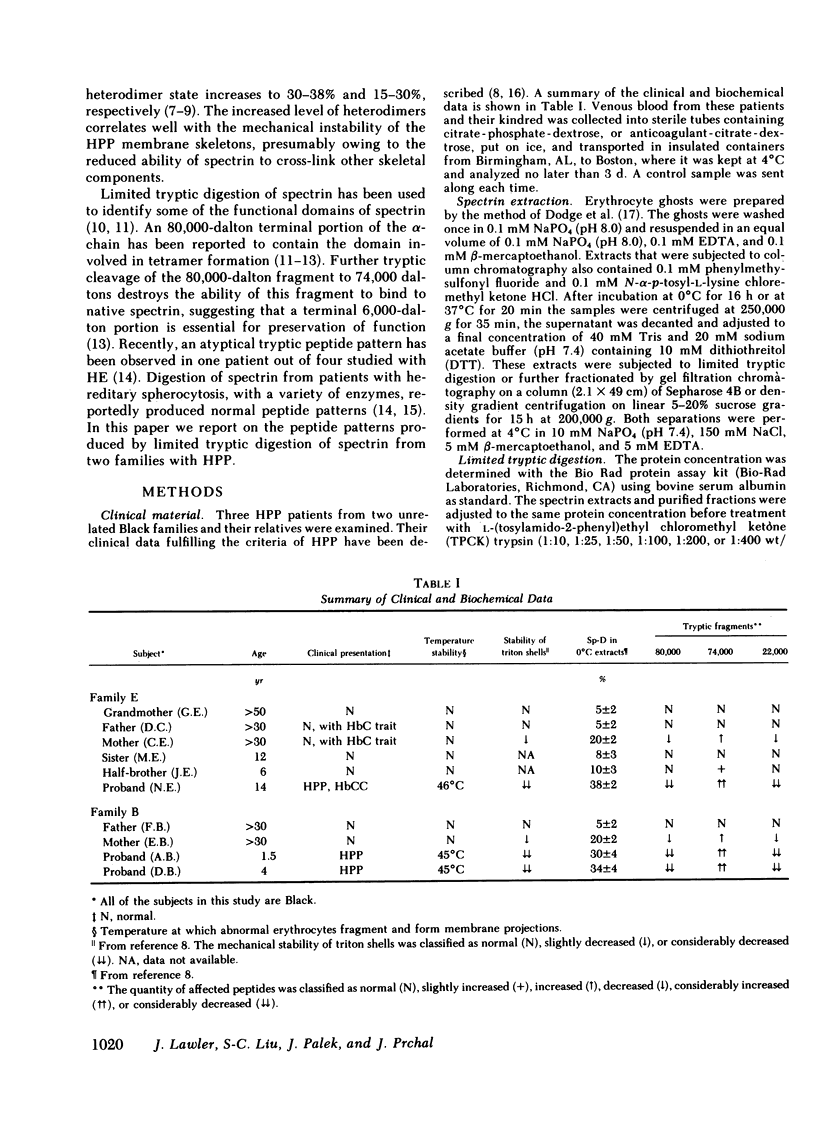
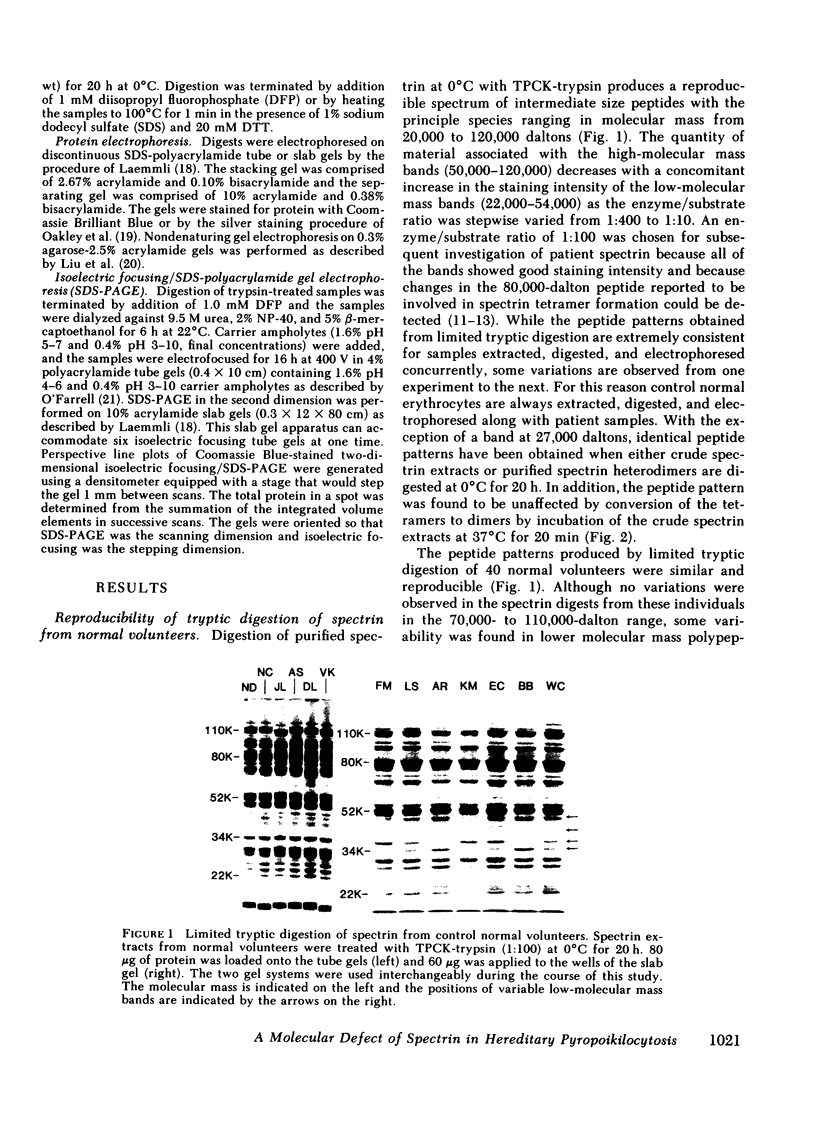
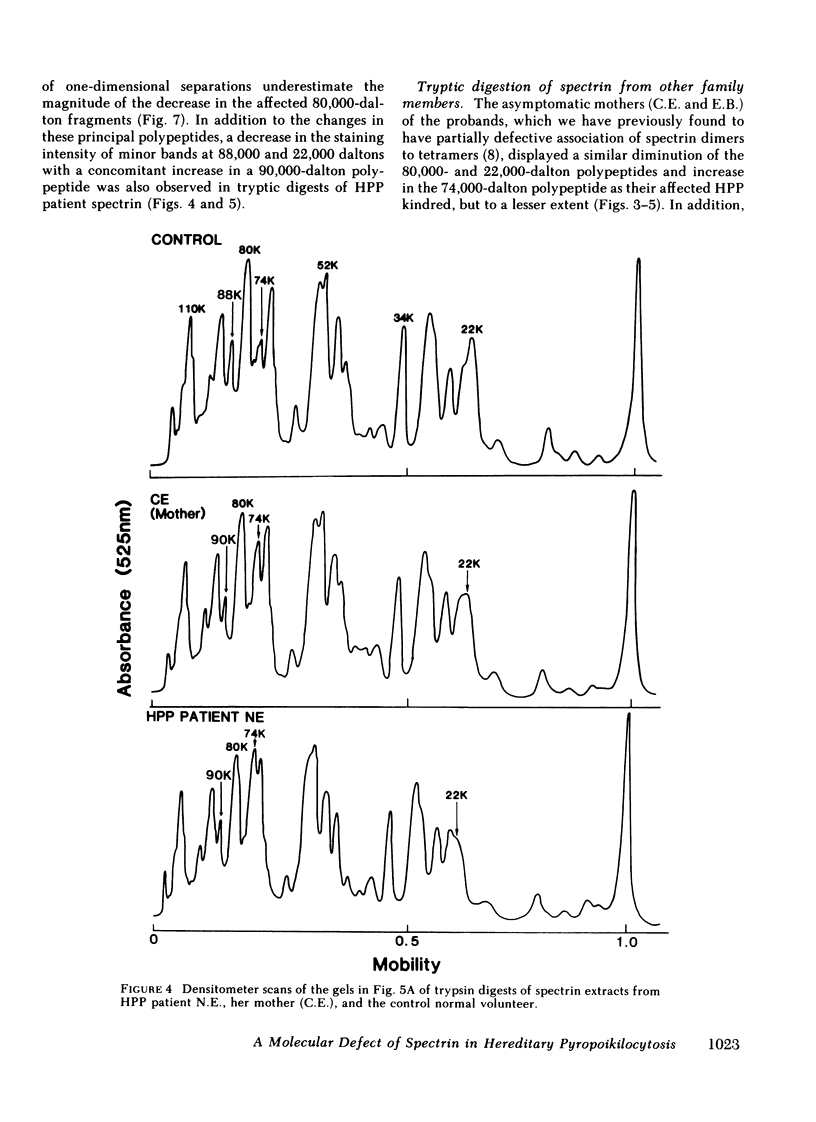
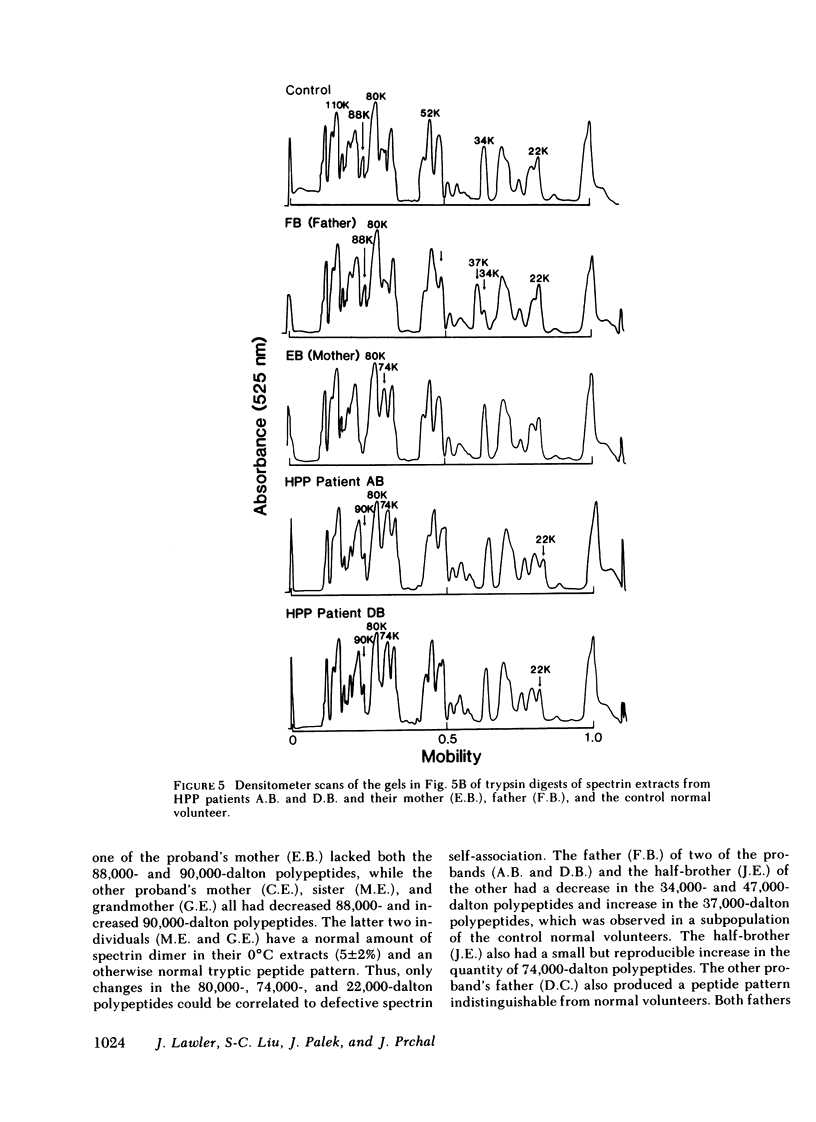
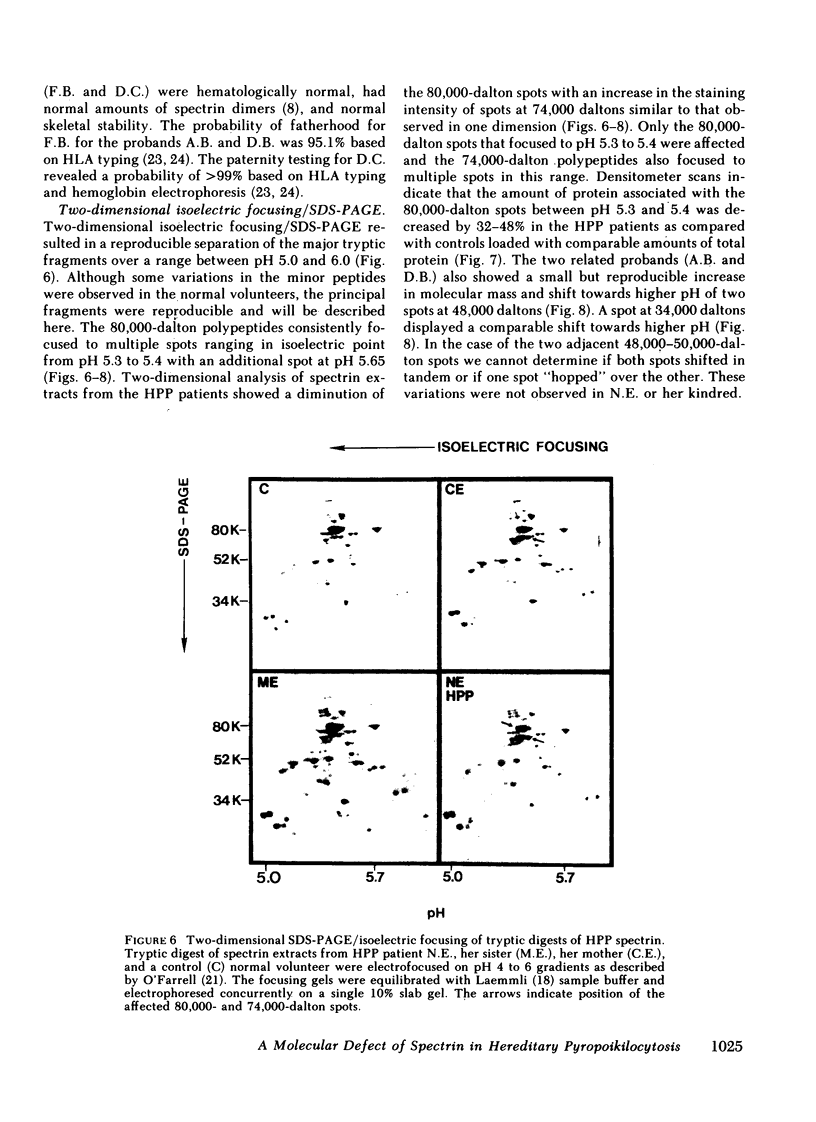
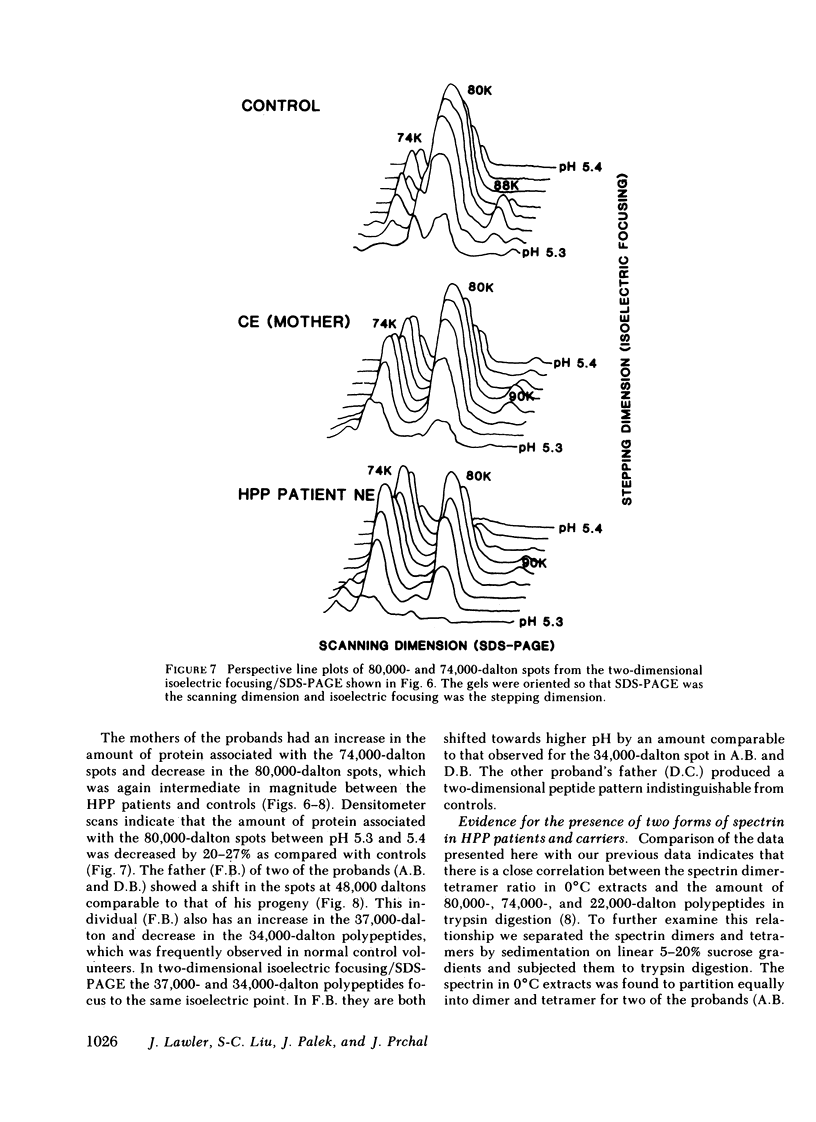
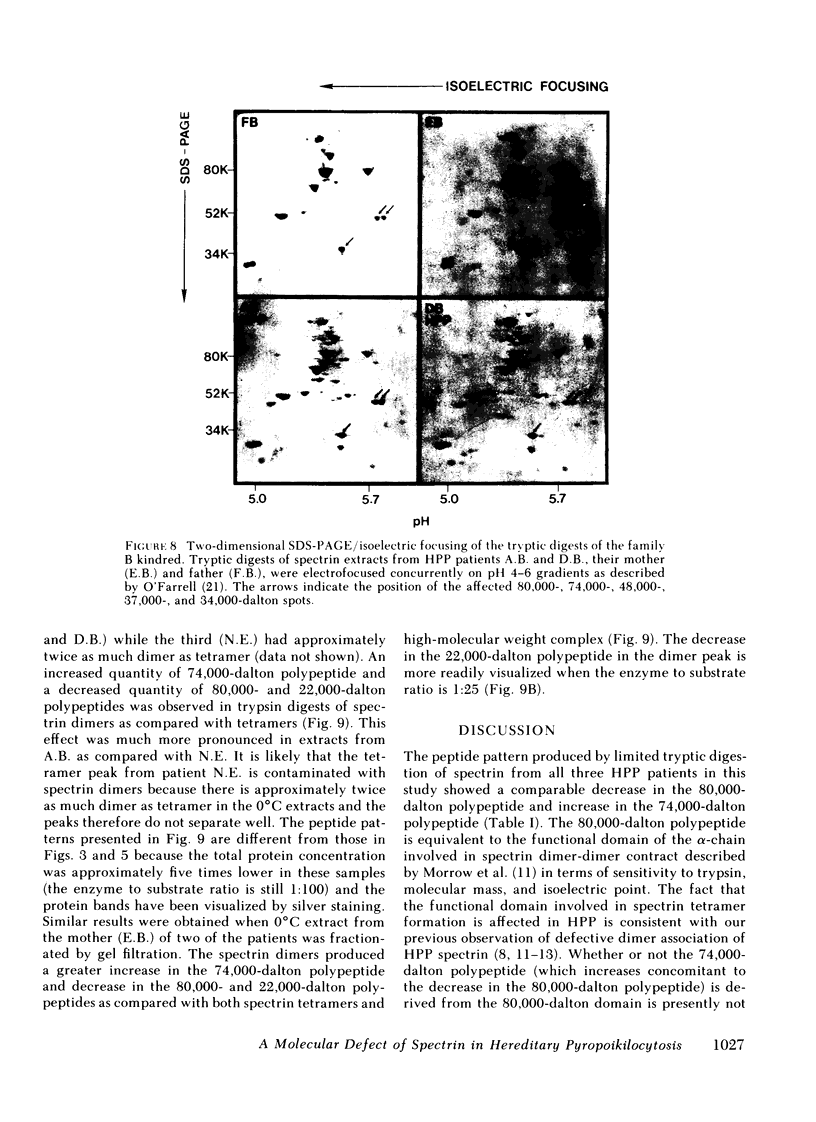
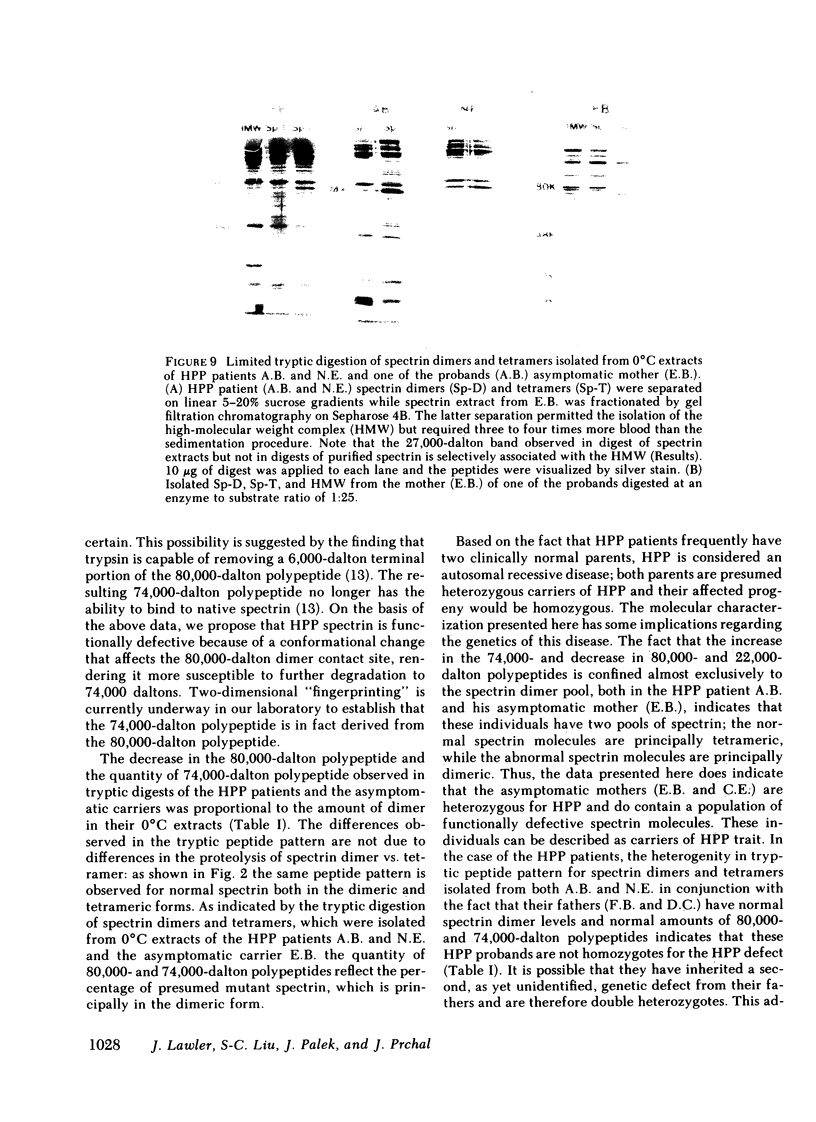
In hereditary pyropoikilocytosis (HPP) the erythrocyte membrane skeleton exhibits mechanical instability that can be correlated to defective self-association of spectrin heterodimers. To detect structural changes in the functional domains of HPP spectrin we have examined the peptide pattern produced by limited tryptic digestion of spectrin extracts from two families that contain three HPP patients. Limited tryptic digestion of all three HPP patients revealed a similar and reproducible decrease in the staining intensity of an 80,000-, and 22,000-, and an 88,000-dalton polypeptide with a concomitant increase in a 74,000- and a 90,000-dalton polypeptide as compared with controls. Only changes in the 80,000-, and 74,000-, and 22,000-dalton polypeptides could be correlated to defective spectrin self-association and the amount of spectrin dimers in 0°C extracts of the HPP patients and their affected kindred. Similar results were obtained when the tryptic digests were analyzed by two-dimensional isoelectric focusing/sodium dodecyl sulfate-polyacrylamide gel electrophoresis with the affected 74,000- and 80,000-dalton polypeptides focusing into multiple spots ranging in isoelectric point from 5.3-5.4. When HPP spectrin dimers and tetramers were separated and subjected to trypsin digestion, changes in the 80,000-, 74,000-, and 22,000-dalton polypeptides were found predominantly in the spectrin dimer pool. Similar results were obtained for spectrin from two of the probands' mother, whom we have identified as an HPP carrier. We conclude that these HPP patients contain a population of normal, (principally tetrameric) and mutant (principally dimeric) spectrin. The latter is characterized by a defective spectrin dimer self-association due to conformational changes that affect the 80,000-dalton domain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Orringer E. P., Chui D. H., Bennett V. A molecular defect in two families with hemolytic poikilocytic anemia: reduction of high affinity membrane binding sites for ankyrin. J Clin Invest. 1981 Dec;68(6):1566–1576. doi: 10.1172/JCI110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Coetzer T., Zail S. S. Tryptic digestion of spectrin in variants of hereditary elliptocytosis. J Clin Invest. 1981 May;67(5):1241–1248. doi: 10.1172/JCI110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Ji T. H., Kiehm D. J., Middaugh C. R. Presence of spectrin tetramer on the erythrocyte membrane. J Biol Chem. 1980 Apr 10;255(7):2990–2993. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Fairbanks G., Palek J. Spontaneous, reversible protein cross-linking in the human erythrocyte membrane. Temperature and pH dependence. Biochemistry. 1977 Sep 6;16(18):4066–4074. doi: 10.1021/bi00637a020. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J., Castleberry R. P. Altered spectrin dimer-dimer association and instability of erythrocyte membrane skeletons in hereditary pyropoikilocytosis. J Clin Invest. 1981 Sep;68(3):597–605. doi: 10.1172/JCI110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Marchesi V. T. Functional proteins of the human red blood cell membrane. Semin Hematol. 1979 Jan;16(1):3–20. [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Speicher D. W., Knowles W. J., Hsu C. J., Marchesi V. T. Identification of functional domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6592–6596. doi: 10.1073/pnas.77.11.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Palek J., Liu S. C., Liu P. Y., Prchal J., Castleberry R. P. Altered assembly of spectrin in red cell membranes in hereditary pyropoikilocytosis. Blood. 1981 Jan;57(1):130–139. [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. Identification of proteolytically resistant domains of human erythrocyte spectrin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5673–5677. doi: 10.1073/pnas.77.10.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S., Maddy A. H. The molecular basis of the defect in phosphorylation of spectrin in human hereditary spherocytosis. Biochim Biophys Acta. 1981 Nov 20;649(1):38–44. doi: 10.1016/0005-2736(81)90006-7. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Zarkowsky H. S. Heat-induced erythrocyte fragmentation in neonatal elliptocytosis. Br J Haematol. 1979 Apr;41(4):515–518. doi: 10.1111/j.1365-2141.1979.tb05889.x. [DOI] [PubMed] [Google Scholar]
- Zarkowsky H. S., Mohandas N., Speaker C. B., Shohet S. B. A congenital haemolytic anaemia with thermal sensitivity of the erythrocyte membrane. Br J Haematol. 1975 Apr;29(4):537–543. doi: 10.1111/j.1365-2141.1975.tb02740.x. [DOI] [PubMed] [Google Scholar]