Summary
EGFR initiates a signaling cascade that leads to DNA synthesis and cell proliferation, but its role in regulating DNA replication licensing is unclear. Here, we show that activated EGFR phosphorylates the p56 isoform of Lyn, p56Lyn, at Y32, which then phosphorylates MCM7, a licensing factor critical for DNA replication, at Y600 to increase its association with other MCM complex proteins, thereby promoting DNA synthesis complex assembly and cell proliferation. Both p56Lyn Y32 and MCM7 Y600 phosphorylation are enhanced in proliferating cells and correlated with poor survival of breast cancer patients. These results establish a signaling cascade in which EGFR enhances MCM7 phosphorylation and DNA replication through Lyn phosphorylation in human cancer cells.
Introduction
MCM7 is a member of the mini-chromosome maintenance (MCM) proteins, which were originally identified through a yeast genetic screen aimed to identify mutants defective in MCM (Maine et al., 1984). The MCM proteins were subsequently found to be expressed in all eukaryotic organisms and are critical for licensing DNA replication in proliferating cells (Tye, 1999). MCM proteins 2-7 are highly conserved from yeast to humans and form a hexameric complex during the G1 phase, which is then loaded onto the replication origin to form a pre-replication complex (pre-RC) that is indispensable for initiation of DNA replication (Lei and Tye, 2001). Assembly of the MCM protein complex is essential for cell survival and proliferation, and suppressing expression of individual MCM proteins resulted in compromised DNA replication (Shi et al., 2010; Tsuji et al., 2006), possibly due to lack of stable hexameric MCM complex. In addition to its role in initiation of replication, the MCM complex has been shown to serve as a putative replication fork helicase melting DNA origins in preparation for replication (Ishimi, 1997; Labib et al., 2000; Lee and Hurwitz, 2001; Yan et al., 1993). Recent studies suggest that MCM2-7 complex interacts with CDC45 and GINS to form the CDC45-MCM-GINS complex (Moyer et al., 2006), which is required for the activation of DNA helicase (Dang and Li, 2011; Kang et al., 2012). MCM2-7 also forms a double-hexameric complex during pre-RC formation, which may be potentially required for the initiation of bidirectional replication forks in S phase (Evrin et al., 2009; Gambus et al., 2011).
Deregulation of DNA replication machinery components causes chromosome instability and tumorigenesis (Bergoglio et al., 2002; Rajagopalan et al., 2004), and the oncogenic potential of MCM7 deregulation has been well described (Luo, 2011). A transgenic mouse model demonstrates that expression of exogenous MCM7 in the epidermis accelerates squamous cell carcinoma development upon carcinogen challenge (Honeycutt et al., 2006). Additionally, increased MCM7 copy number and protein level are associated with prostate cancer relapse and metastasis, and a prostate cancer cell line ectopically expressing MCM7 exhibits increased proliferation and invasiveness (Ren et al., 2006). Similarly, MCM7 serves as a proliferation maker and is associated with tumorigenesis in various human cancers, including oral squamous cell carcinoma, colorectal cancer, ovarian cancer, glioblastoma, and esophageal carcinoma (Facoetti et al., 2006; Feng et al., 2008; Kan et al., 2009; Nishihara et al., 2008; Ota et al., 2011). MCM7 has recently been demonstrated as a prognostic marker that predicts poor cancer patient survival and a therapeutic target of non–small cell lung carcinomas (Toyokawa et al., 2011). Interestingly, MCM7 protein is a critical target of some oncogenic, e.g, androgen receptor (Shi et al., 2008), and tumor suppressor, e.g., integrin α7 and retinoblastoma (Han et al., 2010 ; Sterner et al., 1998), signaling pathways, implying that multiple layers of regulation may be involved in MCM7 biology and its oncogenic properties.
EGFR initiates a signaling cascade that leads to DNA synthesis and cell proliferation upon activation, and EGFR pathways are frequently deregulated in human cancers (Paez et al., 2004; Yarden, 2001). Previously, our mass spectrum analysis detected members of MCM hexameric ring complex in the EGFR immunoprecipitates from A431 cancer cells (Huo et al., 2010), thereby raising the possibility that EGFR signaling is functionally involved in DNA replication licensing. Since MCM7 is critical in DNA replication and involved in oncogenic signaling pathways, we set out to determine whether MCM7 is a downstream target of EGFR signaling.
Results
MCM7 is Tyr phosphorylated by Lyn kinase
We first validated the interaction between EGFR and MCM proteins by IP-western analysis and detected MCM5 and MCM7 in the EGFR immunoprecipitates (Figure S1A). Interestingly, the amount of MCM7 but not MCM5 in the EGFR immunoprecipitates was increased with EGF stimulation and decreased with the Tyr kinase inhibitor gefitinib treatment (Figure S1B), suggesting that the association between EGFR and MCM7 was dependent on EGFR kinase activity and MCM7 might be a kinase substrate of EGFR or EGFR downstream kinases.
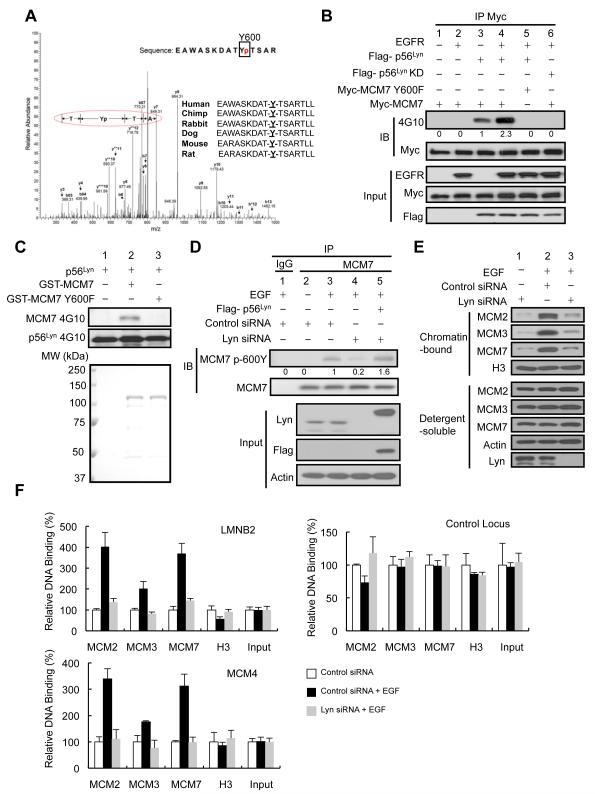
Because Tyr phosphorylation carries significant biological functions and it is unclear whether Tyr phosphorylation is involved in MCM7 function in DNA replication licensing, we investigated whether MCM7 is regulated by this post-translational modification in proliferating cells. We examined MCM7 Tyr phosphorylation in a panel of cancer cell lines stimulated with EGF using an anti-phosphoTyr antibody (4G10) and found that MCM7 was Tyr phosphorylated to various degrees in all of them except in MDA-MB-231 cells (Figure 1A, Figure S1C). The weaker MCM7 Tyr phosphorylation observed in Du145 compared with H266 cells is likely due to their lower EGFR expression level (Figure S1C). Since MCM7 is functionally involved in DNA synthesis and cell proliferation, the failure to observe EGF-induced MCM7 Tyr phosphorylation in MDA-MB-231 cells is consistent with the literature that MDA-MB-231 cells lack proliferative response to EGF (Price et al., 1999). We then asked whether MCM7’s phosphorylation status affects its ability to associate with other MCM proteins. Interestingly, the kinetics between MCM7 Tyr phosphorylation and its association with MCM2 and MCM3, but not MCM4, were positively correlated upon EGF stimulation (Figure 1B). In the absence of EGF, we did not observe MCM7 Tyr phosphorylation or increased MCM2 and MCM3 association (Figure S1D). These data suggest that EGF-mediated Tyr phosphorylation of MCM7 regulates its association with other MCM members.
Figure 1.
MCM7 is Tyr phosphorylated by Lyn kinase. (A) Immunoprecipitates of endogenous MCM7 were analyzed by Western blotting using 4G10 antibody for detecting Tyr phosphorylation in various cancer cell lines stimulated with EGF for 1 hr. (B) Western blot of endogenous MCM2, MCM3, and MCM4 in anti-MCM7 immunoprecipitates from A431 cells stimulated with EGF at various time points. The cell lysate from A431 cells treated with EGF for 2 hr was used for both the anti-MCM7 and control IgG immunoprecipitation. The right graph shows quantified densities of bands at different time points, and the band’s density in control lane (i.e. EGF 0 hr) was set as 1. (C) Cell lysates from 293T cells expressing exogenous Myc-MCM7 and Flag-p56Lyn were subjected to immunoprecipitation and immunoblotting with indicated antibodies. (D) A431 cell lysates with different treatments were subjected to immunoprecipitation and immunoblotting with indicated antibodies to detect the association of endogenous MCM7 with Lyn and MCM7 Tyr phosphorylation. (E) Western blot of Tyr phosphorylation of GST-MCM7 by recombinant p56Lyn (top) and phosphorylation of p56Lyn itself (middle) using 4G10 antibody. Bottom, Coomassie Blue staining of the GST-MCM7 input. (F) Western blot of endogenous MCM7 Tyr phosphorylation in A431 cells with or without EGF stimulation (lanes 3 and 2). Endogenous Lyn was knocked down by siRNA (targeting 3′-UTR; lane 4) and rescued with a siRNA-resistant Flag-Lyn (lane 5). See also Figure S1 and Table S1.
Although MCM7 was detected in the EGFR immunoprecipitates, MCM7 failed to be Tyr phosphorylated by recombinant EGFR in an in vitro kinase assay (data not shown). To identify the kinase that phosphorylates MCM7, we immunoprecipitated MCM7 and subjected the complex to mass spectrometry analysis. Among 94 MCM7-interacting proteins identified, Lyn was the only Tyr kinase (Table S1). The interaction between Lyn and MCM7 was validated by reciprocal immunoprecipitation and Lyn was able to Tyr phosphorylate MCM7 when both proteins are ectopically expressed (Figure 1C). EGF stimulation increased the amount of endogenous MCM7 detected in Lyn immunoprecipitates, which could be reduced by the Lyn kinase inhibitor PP2, suggesting that the kinase activity of Lyn enhances its interaction with MCM7 (Figure 1D, top). Furthermore, EGF-stimulated MCM7 Tyr phosphorylation was then abolished by PP2 (Figure 1D bottom), supporting Lyn as the Tyr kinase for MCM7. Indeed, purified recombinant Lyn kinase phosphorylated glutathione S-transferase (GST)-fused MCM7 in vitro (Figure 1E). To verify whether Lyn phosphorylates MCM7 in vivo, we knocked down Lyn expression using short interfering RNAs (siRNAs) and examined MCM7 Tyr phosphorylation in response to EGF. Upon EGF stimulation, siRNA targeting the 3′UTR of Lyn, but not other members of Src family kinases (Src, Yes, and Fyn), diminished MCM7 Tyr phosphorylation, which was rescued by reintroduction of a siRNA-resistant Flag-p56Lyn (Figure 1F). Another shRNA targeting the protein coding region of Lyn also diminished the MCM7 Tyr phosphorylation (Figure S1E), and MCM7 was detected in the Lyn but not Src, Fyn, or Yes immunoprecipitates (Figure S1F). Lyn exists as two isoforms, p53Lyn and p56Lyn (Yamanashi et al., 1991). Although both p56Lyn and p53Lyn interacted with MCM7 (Figure S1G), the kinase activity of p56Lyn but not p53Lyn toward MCM7 was potentiated upon EGF stimulation (Figure S1H), suggesting p56Lyn is the likely physiological Tyr kinase for MCM7 in response to EGF treatment in vivo. Taken together, these results demonstrate that Lyn Tyr phosphorylates MCM7 in vivo and that this event is EGF-dependent.
Lyn phosphorylates MCM7 at Y600
To further validate the MCM7 Tyr phosphorylation and identify corresponding site(s), we used mass spectrometry to analyze endogenous MCM7 immunopurified from EGF-stimulated A431 cells and identified a peptide containing phosphorylated Y600 of MCM7 (Figure 2A). The MCM7 Y600 sequence matched the consensus sequence phosphorylated by Src family kinases (Olsen et al., 2006), and Lyn was predicted as the putative kinase that phosphorylates MCM7 at Y600 (NetworKIN). Indeed, an in vivo Tyr phosphorylation assay demonstrated that it was not EGFR but Lyn that enhanced Tyr phosphorylation of MCM7 (Figure 2B, lanes 1-3). In the presence of EGFR, p56Lyn-mediated MCM7 phosphorylation was significantly augmented, suggesting that EGFR signaling potentiates p56Lyn kinase activity for MCM7 phosphorylation. Importantly, a kinase-dead (KD) p56Lyn mutant (Kasahara et al., 2004) was unable to phosphorylate MCM7 (lane 4 vs. lane 6) and p56Lyn did not phosphorylate the MCM7 Y600F mutant (lane 4 vs. lane 5). Similarly, purified recombinant p56Lyn kinase phosphorylated GST-MCM7 but not the GST-MCM7 Y600F (Figure 2C), indicating that Lyn phosphorylates MCM7 at Y600.
Figure 2.
Lyn phosphorylates MCM7 at Y600. (A) Mass spectrometry analysis of endogenous MCM7 immunopurified from A431 cells. (B) Western blot of Tyr phosphorylation of MCM7 or MCM7 Y600F from 293T cells transfected with EGFR, Flag-p56Lyn and Myc-MCM7, as indicated. The number under each band is quantified relative density with lane 3 being set as 1. (C) Western blot analysis of Tyr phosphorylation of GST-MCM7 and GST-MCM7 Y600F by recombinant p56Lyn kinase (top) and phosphorylation of p56Lyn using 4G10 antibody (middle). Bottom, Coomassie Blue staining of the GST fusion protein input. (D) Western blot of phosphorylation of the endogenous MCM7 Y600 in A431 cells with or without EGF stimulation (lanes 3 and 2). Endogenous Lyn was knocked down by siRNA (targeting 3′-UTR; lane 4) and rescued with a siRNA-resistant Flag-p56Lyn (lane 5). The number under each band is quantified relative density with lane 3 being set as 1. (E) Cell lysates from A431 cells transfected with the indicated siRNA with or without EGF stimulation were subjected to Western blot analysis of endogenous MCM2, MCM3, and MCM7 in the soluble fraction or chromatin fraction. (F) ChIP analysis of endogenous MCM2, MCM3, and MCM7 binding to the DNA replication origins in LMNB2 (Lamin B2), MCM4, and control locus (2 kb downstream of LMNB2 locus) in A431 cells transfected with the indicated siRNA and stimulated with or without EGF for 7 hr. Results are normalized to levels in cells without EGF stimulation (n = 3). Error bars, ± SD. See also Figure S2.
To facilitate the detection of MCM7 Y600 phosphorylation, we generated a polyclonal antibody that recognized Y600-phosphorylated MCM7. This antibody recognized ectopic MCM7 co-expressed with p56Lyn but not MCM7 alone or MCM7 Y600F co-expressed with p56Lyn in 293 T cells (Figure S2A) and was used to verify MCM7 Y600 phosphorylation in vivo. In A431 cells, EGF induced phosphorylation of MCM7 Y600 (Figure 2D, lane 3 vs. lane 2). Knockdown of endogenous Lyn protein by siRNA diminished MCM7 Y600 phosphorylation (lane 4), which was rescued by reintroduction of a siRNA-resistant Flag-p56Lyn (lane 5), supporting that Lyn phosphorylates MCM7 at Y600 upon EGF stimulation. We also observed EGF-induced MCM7 Y600 phosphorylation in 6 other cancer cell lines (Figure S2B).
Because MCM7 is important for licensing DNA replication and Lyn phosphorylates MCM7 upon EGF stimulation, we examined whether Lyn regulated MCM7 function in DNA replication licensing upon EGF stimulation. Indeed, knockdown of the endogenous Lyn protein by two different siRNAs compromised the EGF-induced loading of MCM 2, 3, and 7 onto the chromatin (Figures 2E and S2C) and the replication origins in the LMNB2 (Lamin B2) and MCM4 locus measured by ChIP–qPCR analysis (Figures 2F and S2D). These evidences suggest that Lyn-mediated Y600 phosphorylation may regulate MCM7 function in DNA replication licensing.
MCM7 Y600 phosphorylation enhances MCM complex assembly and cancer cell proliferation
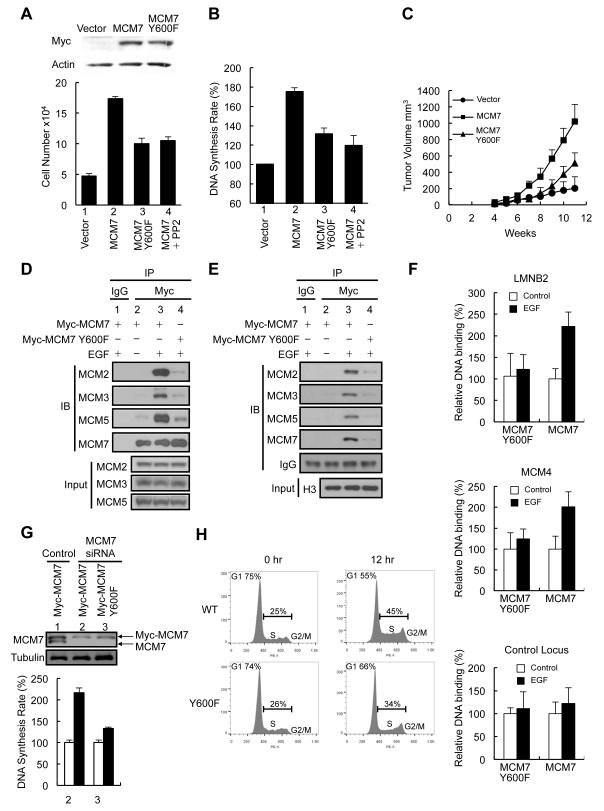
We then examined if MCM7 Y600 was also involved in cancer cell proliferation and generated MDA-MB-468 breast cancer cells expressing Myc-tagged MCM7 (468-MCM7), MCM7 Y600F (468-Y600F), or control vector (468-vector) for functional studies. MDA-MB-468 cells were chosen because Lyn was required for MCM7 phosphorylation (Figure S1E) and Lyn knockdown significantly compromised their proliferation (Figure S3A). Consistent with a previous study (Ren et al., 2006), ectopic expression of MCM7 enhanced cell proliferation and DNA synthesis rate of MDA-MB-468 cells (Figure 3A and 3B, lane 2 vs. lane 1). Compared with 468-MCM7 cells, 468-Y600F cells showed decreased cell proliferation and DNA synthesis rate (Figure 3A and 3B, lane 3 vs. lane 2). In addition, cell proliferation (Figure 3A, lane 4 vs. lane 2) and DNA synthesis rate (Figure 3B, lane 4 vs. lane 2) were decreased in 468-MCM7 cells treated with PP2. Furthermore, in an orthotopic breast cancer mouse model, 468-Y600F mammary tumor grew slower than did 468-MCM7 tumors (Figure 3C). MCM7 also enhanced the proliferation of H226 and A431 cells more significantly than did MCM7 Y600F (Figure S3B). Collectively, these results suggest that Y600 phosphorylation is involved in the MCM7-mediated DNA replication and cancer cell proliferation.
Figure 3.
MCM7 Y600 phosphorylation enhances MCM complex assembly and cancer cell proliferation. (A) MDA-MB-468 (1 × 104) cells carrying the indicated constructs were seeded in the presence or absence of 1 μM PP2, and cell number was determined at day 7 (n = 3). (B) MDA-MB-468 cells were treated as (A) and DNA synthesis rate was assayed at day 5 by detection of BrdU incorporation (n = 3). (C) In vivo tumor growth of orthotopically transplanted MDA-MB-468 cells (n = 5). (D) Western blot of endogenous MCM2, MCM3, and MCM5 in the anti-Myc-MCM7 immunoprecipitates from the soluble fraction of A431 cells with or without EGF stimulation. (E) Same as (D) except binding of MCM7 with other MCM members in chromatin fraction was assessed. (F) ChIP analysis of Myc-MCM7 or Myc-MCM7 Y600F binding to the DNA replication origins in LMNB2 (Lamin B2), MCM4, and control locus (2 kb downstream of LMNB2 locus) in A431 cells stimulated with EGF for 7 hr. Results are normalized to levels in cells without EGF stimulation (n = 5). (G) Top, HeLa cells expressing siRNA-resistant Myc-MCM7 or Myc-MCM7 Y600F were transfected with a control siRNA or a siRNA targeting 3′UTR of MCM7 as indicated. After 3 days, cells were lysed and subjected to western blot analysis using the indicated antibodies. Bottom, after siRNA transfection, 1 × 104 cells were seeded in the presence or absence of 10 ng/ml EGF and DNA synthesis rate was assayed at day 3 by detection of BrdU incorporation (n = 3). (H) HeLa cells expressing siRNA-resistant Myc-MCM7 or Myc-MCM7 Y600F were transected with siRNA targeting MCM7 3′UTR. Cells were synchronized by 48 hr of serum starvation and then stimulated with EGF for 12 hr. Cell cycle progression was analyzed by PI staining using FACS. Error bars, ± SD. See also Figure S3.
We further examined whether phosphorylation of Y600 enhances MCM7 interaction with other MCM members. Indeed, EGF stimulation enhanced association of Myc-MCM7 with MCM 2, 3, and 5, which was decreased in cells treated with PP2 (Figures 3D and S3C). In contrast, Myc-MCM7 Y600F exhibited a much weaker interaction with other MCM proteins (Figure 3D). It should be mentioned that, similar to the endogenous MCM7 (Figure 1B), Myc-MCM7 interacted with MCM2 without EGF (Figure S3D). We in addition used a phosphomimetic mutant MCM7 Y600E to determine whether the requirement of Lyn in MCM7 interaction with other MCM members could be bypassed. Indeed, in cells cultured in a low-serum medium to prevent EGFR activation, MCM7 Y600E interacted with other MCM members stronger than did MCM7 and MCM7 Y600F (Figure S3E). Furthermore, ectopic expression of MCM7 Y600E, but not MCM7, Y600F rescued the EGF-induced DNA synthesis compromised by Lyn knockdown (Figure S3F). These data suggest that MCM7 Y600 phosphorylation is critical for the MCM complex assembly and DNA synthesis initiated by the EGFR-Lyn signaling axis.
Assembled MCM complex loads onto the replication origins on the chromatin to license DNA replication. Therefore, we examined whether the association of MCM members on the chromatin was enhanced upon EGF stimulation. Indeed, EGF stimulation increased chromatin loading of MCM7 together with MCM 2, 3, and 5; however, EGF-induced chromatin loading of MCM7 Y600F was barely detectable (Figure 3E). We then performed ChIP–qPCR analysis of MCM7 or MCM7 Y600F binding to the DNA replication origins in the MCM4 and LMNB2 loci upon EGF stimulation to determine the functional important of MCM7 Y600 in DNA replication licensing. Indeed, Y600 phosphorylation enhanced MCM7-mediated DNA replication licensing, where EGF induced the loading of the Myc-MCM7 but not Myc-MCM7 Y600F onto the DNA replication origins (Figure 3F). Neither MCM7 nor MCM7 Y600F mutant exhibited increased binding to a control locus that did not contain a replication origin (2 kb downstream of LMNB2 locus) upon EGF stimulation (Figure 3F). Consistently, EGF induced the loading of the endogenous MCM2, MCM3, and MCM7 onto the DNA replication origins much more significantly in cells expressing Myc-MCM7 than those expressing MCM7 Y600F (Figure S3G), suggesting that Y600 phosphorylation aids in complex assembly at the DNA replication origins upon EGF stimulation.
To determine whether Y600 phosphorylation is critical for EGF-induced DNA synthesis, we used a siRNA to knockdown endogenous MCM7 in cells expressing the siRNA-resistant Myc-MCM7 or Myc-MCM7 Y600F and examined their responses to EGF-induced DNA synthesis and cell cycle progression. Consistently, EGF-induced DNA synthesis was higher in cells expressing Myc-MCM7 than in cells expressing Myc-MCM7 Y600F (Figure 3G). Furthermore, 12 hr of EGF treatment allowed 20% (45%-25%) of Myc-MCM7 expressing cells but only 8% (34%-26%) of Myc-MCM7 Y600F expressing cells to progress through G1 phase (Figure 3H). We also used untagged MCM7 and MCM7 Y600F to performed the DNA synthesis assay in cells with endogenous MCM7 knockdown and consistently the EGF-induced DNA synthesis was much more significant in cells expressing MCM7 than cells expressing MCM7 Y600F (Figure S3H). A BrdU pulse-chase approach was also used to evaluate the cell cycle progression of MCM7 and MCM7 Y600F cells. After BrdU labeling in S phase, the number of BrdU+ cells in G1 phase for MCM7 cells was increased more significantly than MCM7 Y600F cells at 9 hr and 12 hr chase time point, suggesting that MCM7 cells have a shorter cell cycle time than MCM7 Y600F cells (Figures S3I and S3J). Taken together, these observations suggest a pathway in which phosphorylation of MCM7 Y600 by Lyn kinase enhances MCM complex assembly and DNA replication licensing in proliferating cells.
EGFR phosphorylates p56Lyn at Y32
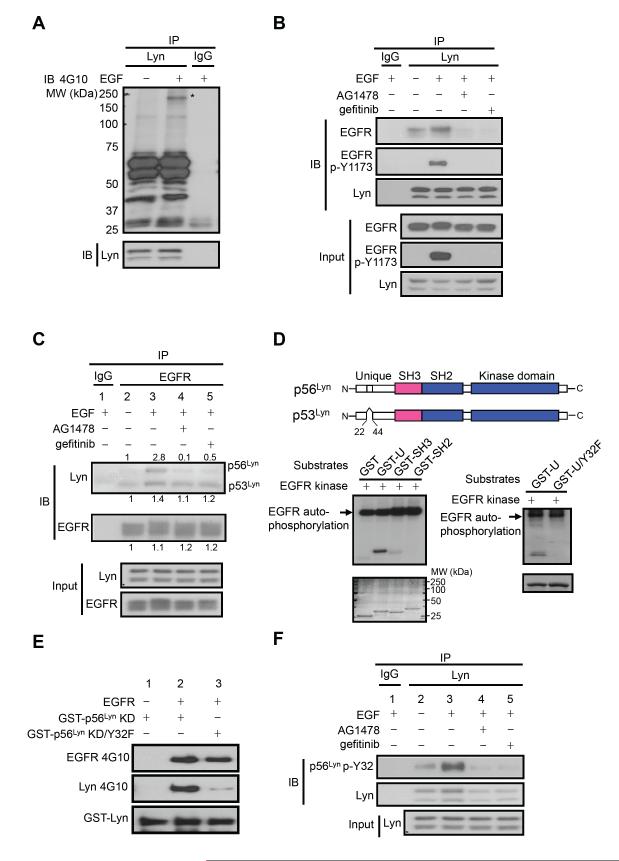
To identify potential regulators of Lyn activation after EGF stimulation, endogenous Lyn was immunoprecipitated, and the 4G10 antibody was used to detect Tyr phosphorylated Lyn-associated proteins. A single 175 kDa Lyn-interacting protein for which the Tyr phosphorylation relied on EGF stimulation was detected (starred, Figure 4A). Since EGFR itself is 175 kDa, we examined whether EGFR interacts with Lyn in response to EGF stimulation. Indeed, co-immunoprecipitation analyses in A431 cells detected EGFR in the Lyn immunoprecipitates, which increased with EGF and decreased with two different EGFR kinase inhibitors, AG1478 and gefitinib (Figure 4B), suggesting that Lyn interacted with activated EGFR.
Figure 4.
EGFR phosphorylates p56Lyn Y32. (A) Anti-Lyn immunoprecipitates from A431 cells with or without EGF stimulation were analyzed by western blotting using 4G10 antibody. The asterisk (*) indicates the protein phosphorylated by EGF stimulation in the anti-Lyn immuniprecipates. (B) Western blot of endogenous EGFR and phospho-EGFR Y1173 (EGFR p-Y1173) in the anti-Lyn immunoprecipitates from A431 cells with indicated treatments. (C) Western blot of endogenous Lyn in the anti-EGFR immunoprecipitates from A431 cells with different treatments. The number above (for p56Lyn) or under (for p53Lyn and EGFR) each band is quantified relative density with lane 2 being set as 1. (D) Western blot detection of Tyr phosphorylation of GST fused various domains of p56Lyn by recombinant EGFR in vitro. Coomassie blue staining is shown below each blot. A schematic representation of Lyn isoforms with the 21-amino acid presented in p56Lyn but not in p53Lyn indicated is shown at the top. U, the unique domain; U/Y32F, the U domain with Y32F mutation. (E) Western blot of Tyr phosphorylation of GST-p56Lyn KD and GST-p56Lyn KD/Y32F by recombinant EGFR (middle). and phosphorylation of EGFR itself (top) using 4G10 antibody. Bottom, GST-p56Lyn KD and GST-p56Lyn KD/Y32F detected by western blot using an anti-GST antibody. (F) Cell lysates of A431 cells with different treatments were subjected to anti-Lyn immunoprecipitation then immunoblotted with the phospho-p56Lyn Y32 (p56Lyn p-Y32) antibody. See also Figure S4.
To further investigate how EGFR regulates Lyn, EGFR was immunoprecipitated, and both p56Lyn and p53Lyn were detected. Moreover, EGF stimulation significantly increased the amount of p56Lyn (Figure 4C). Interestingly, EGFR kinase inhibitors diminished the amount of p56Lyn but not p53Lyn in the EGFR immunoprecipitates (Figure 4C). The interaction between EGFR and p56Lyn but not p53Lyn was dependent on EGFR kinase activity, suggesting that p56Lyn is a kinase substrate for EGFR. Indeed, purified recombinant EGFR phosphorylated a GST fusion of the domain unique to p56Lyn that contained only one Tyr residue, Y32 (Figure 4D lower left panel), and substitution of Y32 with Phe abolished the EGFR-mediated phosphorylation (Figure 4D lower right panel). Furthermore, recombinant EGFR phosphorylated kinase-dead GST-p56Lyn but not kinase-dead GST-p56Lyn Y32F (Figure 4E). Since Y32 does not exist in p53Lyn (Figure 4D, upper panel) (Yamanashi et al., 1991), this result is consistent with our observation that EGFR kinase inhibitors diminished the association of EGFR with p56Lyn but not p53Lyn (Figure 4C). To facilitate detection of p56Lyn Y32 phosphorylation, we generated a polyclonal antibody that specifically recognizes Y32-phosphorylated p56Lyn. This antibody recognized ectopic p56Lyn co-expressed with EGFR but not p56Lyn alone or p56Lyn Y32F co-expressed with EGFR in 293T cells (Figure S4). Thus, we used this antibody to detect p56Lyn Y32 phosphorylation in vivo. Phosphorylation of p56Lyn Y32 was significantly increased upon EGF stimulation but diminished by EGFR kinase inhibitors (Figure 4F). These results indicate that EGFR phosphorylates p56Lyn at Y32 in response to EGF stimulation.
Phosphorylation of Y32 enhances p56Lyn-mediated MCM7 Tyr phosphorylation and cell proliferation
Next, we investigated whether phosphorylation at p56Lyn Y32 is important for DNA synthesis and cancer cell proliferation. MDA-MB-468 cells stably expressing p56Lyn Y32F (468-Y32F) exhibited decreased cell proliferation (Figure 5A) and DNA synthesis rate (Figure 5B) compared with those expressing p56Lyn (468-Lyn), and these results are similar to MDA-MB-468 cells expressing MCM7 Y600F (Figures 3A and 3B). When treated with gefitinib or AG1478, which abolished the Lyn Y32 phosphorylation (Figure 4F), 468-Lyn cells exhibited compromised cell growth and DNA synthesis similar to 468-Y32F cells (Figures 5A and 5B). In an orthotopic breast cancer mouse model, 468-Y32F induced mammary tumor grew slower than did 468-Lyn cells (Figure 5C), which is comparable to the 468-MCM7 Y600F cells (Figure 3C). These results collectively suggest that Y32 phosphorylation is critical for the p56Lyn function. In support of this notion, EGF-induced Lyn Y397 phosphorylation, which has been shown to enhance Lyn’s kinase activity (Ingley, 2012) (Figure 5D top), and MCM7 Y600 phosphorylation (Figure 5D, middle) were clearly observed after 60 min EGF stimulation in p56Lyn cells while barely detectable in p56Lyn Y32F mutant cells. Similarly, Immunofluorescence analysis using confocal microscopy demonstrated that p56Lyn is associated with more robust Lyn Y397 phosphorylation compared with p56Lyn Y32F mutant (Figure S5A).
Figure 5.
Phosphorylation of Y32 enhances p56Lyn mediated MCM7 Tyr phosphorylation and cancer cell proliferation. (A) MDA-MB-468 cells (1 × 104) carrying indicated constructs were seeded in the presence or absence of 500 nM inhibitors. Cell number was determined at day 7 (n = 3). (B) DNA synthesis rate of MDA-MB-468 cells treated as (A) was assayed at day 5 by detection of BrdU incorporation (n = 3). (C) In vivo tumor growth of orthotopically transplanted MDA-MB-468 cells (n = 5). (D) Western blot of p56Lyn Y397 and MCM7 Y600 phosphorylation in Lyn knockdown A431 cells expressing Flag-p56Lyn Y32F or Flag-p56Lyn stimulated with EGF at indicated time point. Error bars, ± SD. See also Figure S5.
To show that Y32 phosphorylation is critical for p56Lyn function in MCM7 phosphorylation, we use a phosphomimetic mutant p56Lyn Y32E to determine whether the requirement of EGFR activation in Lyn-dependent MCM7 phosphorylation could be bypassed. Indeed, in cells cultured with a low-serum medium to prevent EGFR activation (Figure S5B right), ectopic expression of p56Lyn Y32E but not p56Lyn significantly induced MCM7 Tyr phosphorylation that was associated with increased interaction with other MCM members (Figure S5B left). Interestingly, p56Lyn Y32E interacted MCM7 much more significantly than p56Lyn (Figure S5B left). Collectively, these data suggest a cascade in which EGFR-mediated p56Lyn Y32 phosphorylation regulates the downstream MCM7 Y600 phosphorylation and DNA synthesis.
p56Lyn Y32 and MCM7 Y600 phosphorylation correlate with physiological proliferation
To investigate the biological importance of the EGFR→p56Lyn→MCM7→DNA replication licensing cascade in cells with physiological EGFR level, we first examined these events in regenerating liver. Liver regeneration is a well-established physiological model for DNA synthesis and cell proliferation and requires EGFR (Bucher, 1991; Natarajan et al., 2007). After a partial hepatectomy, robust DNA replication indicated by increased PCNA and MCM7 protein expression ensued within 24 hr (Figure 6A bottom). The increase in the MCM7 level was associated with prominent Y600 Tyr phosphorylation, suggesting that Lyn was activated in regenerating liver. Similarly, phosphorylation of p56Lyn Y32 as well as association of p56Lyn with MCM7 was increased in the regenerating liver (Figure 6A top). We in addition examined whether this cascade is associated with proliferation of normal mammary epithelial cells expressing physiological level of EGF, using MCF-10A cells as a model. It is well established that the survival and proliferation of MCF-10A cells relies on EGF and that EGF induces cell cycle progression of MCF-10A cells synchronized at G0 phase (LeVea et al., 2004) (Figure S6A). Upon EGF stimulation, phosphorylation of EGFR Y1173, p56Lyn Y32 and MCM7 Y600 were simultaneously induced, and the level of p56Lyn Y32 and MCM7 Y600 phosphorylation persisted from 8 to 24 hr (Figure 6B). Since MCM7 Y600 phosphorylation is involved in its association with other MCM members, these results are consistent with current model that MCM complex assembly begins during the G1 phase (Lei and Tye, 2001). As expected, we found that Lyn and MCM7 associate upon EGF stimulation (Figure 6C) and EGFR or Lyn kinase activity is required for the EGF-induced MCF-10A cell proliferation (Figure 6D). EGF also induced proliferation of human cancer cell line NCI-H226 and triggered p56Lyn Y32 and MCM7 Y600 phosphorylation (Figure 6E). It has been shown that when A431 cells were seeded at low density lower concentrations (3-100 pM) rather than higher concentrations of EGF induced their proliferation (Kawamoto et al., 1983). Consistently, we found 100 pM EGF enhanced A431 cell proliferation and triggered Lyn Y32 and MCM7 Y600 phosphorylation (Figure S6B). Taken together, our findings demonstrate a biological importance for phosphorylation of p56Lyn Y32 and MCM7 Y600 in cell proliferation.
Figure 6.
Phosphorylation of p56Lyn Y32 and MCM7 Y600 correlate physiological proliferation. (A) Tyr phosphorylation of p56Lyn Y32 and MCM7 Y600 in regenerating liver. N, normal liver; R, regenerating liver 24 hr after partial hepatectomy. (B) MCF-10A cells were serum starved for 48 hr and then stimulated with EGF at the indicated time points. Lysates were immunoblotted with indicated antibodies. The number under each band of anti-phospho MCM7 Y600 (MCM7 p-Y600) blot is quantified relative density with the lane 1 (0 hr) being set as 1. (C) Lysates of MCF-10A cell treated with EGF for 8 hr were subjected to Lyn immunoprecipitation and immunoblotting with indicated antibodies. (D) MCF-10A cells were serum starving for 48 hr and then stimulated with EGF in the presence of indicated inhibitors. Cell number was determined 48 hr after EGF stimulation (n = 3). (E) In the top panel, 1 × 104 NCI-H226 cells were seeded in the presence or absence of 50 ng/ml EGF in medium with 0.5% calf serum, and cell number was determined at day 5 (n = 3). In the bottom panel, NCI-H226 cells were untreated or treated with 50 ng/ml EGF for 4 hr, and the lysates were immunoblotted with the indicated antibodies. Error bars, ± SD; ** indicates p < 0.01. See also Figure S6.
p56Lyn Y32 and MCM7 Y600 phosphorylation correlate with EGFR status in human tumor samples and poor survival of breast cancer patients
To determine the pathological relevance of this signal pathway in human cancers, we analyzed the relationship between EGFR, phosphorylation of p56Lyn Y32, and MCM7 Y600 in human breast tumor samples using IHC staining. Specificity of the anti-phosphorylation antibodies for IHC staining was verified by demonstrating that the anti-phospho p56Lyn Y32 or anti-phospho MCM7 Y600 staining can be neutralized with the corresponding tyrosine-phosphorylated peptide but not with the non-phosphorylated peptide (Figure S7A). Significantly, EGFR expression level was associated with Tyr phosphorylation level of p56Lyn Y32 and MCM7 Y600 (Figures 7A and 7B). Additionally, Tyr phosphorylation level of MCM7 Y600 was correlated with the level of proliferation marker Ki-67. In the tumors with low (−/+) p-MCM7 level, only 44% (35/79) of them were identified as having the highest Ki-67 level (+++). In the tumors with higher p-MCM7 level (++ and +++), the samples identified as the highest Ki-67 level (+++) were significantly increased to 71% (10/14) and 75% (6/8) respectively (Figures 7C and 7D). These data suggested that MCM7 Y600 phosphorylation was pathologically associated with cancer cell proliferation. We also observed that Lyn and MCM7 protein levels were correlated with Ki-67 expression, suggesting their potential contributions to cell proliferation in breast tumors (Figure S7B).
Figure 7.
Phosphorylation of p56Lyn Y32 and MCM7 Y600 correlate with the EGFR protein level of human tumor samples and poor survival of breast cancer patients. (A) IHC staining of EGFR level and Tyr phosphorylation of p56Lyn Y32 and MCM7 Y600 of representative human breast tumor samples with high and low EGFR. Scale bar, 25 μm (B) Relationship between expression of EGFR, phospho-p56Lyn Y32 (p56Lyn p-Y32), phospho-MCM7 Y600 (MCM7 p-Y600), Lyn and MCM7 in human breast cancer tissues. (C) IHC staining of phospho-MCM7 Y600 and Ki-67 of representative human breast tumor samples. Scale bar, 25 μm. (D) Relationship between expression of phospho-MCM7 Y600 and Ki-67 in human breast cancer samples. (E) The Kaplan-Meier analysis of overall survival of breast cancer patients according to the phospho-Lyn Y32 level in their breast cancer. (F) The Kaplan-Meier analysis of overall survival of breast cancer patients according to the phospho-MCM7 Y600 level in their breast cancer. See also Figure S7, Table S2, S3, S4 and S5.
To determine the pathological relevance of p56Lyn activation and MCM7 Y600 phosphorylation, we analyzed the relationship between phosphorylation of p56Lyn Y32 and MCM7 Y600 in human breast tumor samples and lung cancer tissue arrays using IHC staining. Significantly, phosphorylation of p56Lyn Y32 was correlated with phosphorylation of MCM7 Y600 in human breast (p < 0.02) (Table S2) and lung cancers (p = 0.003) (Table S3). To verify Lyn is the Tyr kinase that phosphorylates MCM7 at Y600 in human tumor samples, we performed IHC staining in tissue microarrays of human lung and breast cancers. Expression of Lyn but not other Src family kinases (Src, Yes, Fyn, Lck) was significantly correlated with the phosphorylation level of MCM7 Y600 in both human lung tumor tissues (p < 0.001) (Table S4, Figure S7C) and breast tumor tissues (p = 0.002) (Table S5).
We next sought to determine whether the levels of phospho-Lyn Y32 and phospho-MCM7 Y600 expression correlated with survival of breast cancer patients. We found that the overall survival of breast cancer patients whose cancers had high level of phospho-Lyn Y32 was worse than patients whose cancers had low level of phospho-Lyn Y32 (p = 0.037; Figure 7E). Similarly, overall survival of breast cancer patients whose cancers had high level of phospho-MCM7 Y600 was worse than patients whose cancers had low level of phospho-MCM7 Y600 (p = 0.035; Figure 7F). Multivariate survival analysis using Cox’s regression model suggested that both phospho-Lyn Y32 and phospho-MCM7 Y600 were independently correlated with overall survival in the breast cancer patients (Figure S7D). Additionally, overall survival of the breast cancer patients whose cancers had high levels of both phospho-Lyn Y32 and phospho-MCM7 Y600 was much worse than patients in all other categories (p = 0.003; Figure S7E). Taken together, these findings provide a significant pathological relevance for phosphorylation of p56Lyn Y32 and MCM7 Y600 in human cancers.
Discussion
Deregulated MCM7 expression can increase cancer cell proliferation and contribute to tumorigenesis; however, how specific oncogenic pathways regulate MCM7 functions is not well understood. Here, we show that Lyn-mediated MCM7 Y600 phosphorylation increases MCM7’s association with other MCM members, consequently enhancing DNA synthesis and cell proliferation. Furthermore, the activity of p56Lyn is upregulated via Y32 phosphorylation by EGFR, resulting in potentiation of MCM7 Y600 phosphorylation. In human breast cancer, phosphorylation of both p56Lyn Y32 and MCM7 Y600 correlate with EGFR expression level in tumor samples and with poor survival of patients. Thus, we propose a Tyr phosphorylation cascade triggered by EGFR for licensing DNA replication in proliferating cells, which is augmented in cancer cells with deregulated EGFR activity. These findings suggest that Lyn may be a critical EGFR downstream mediator in licensing DNA replication and therefore may be a promising therapeutic target in treating EGFR activated cancers.
Although the therapeutic efficacy of pan-Src family kinase inhibitors is currently being evaluated in the clinic, the Lyn specific inhibitor is not yet available for treatment of human cancers. Together with the previous reports in prostate cancer (Goldenberg-Furmanov et al., 2004; Park et al., 2008), this study further identifies Lyn as a therapeutic target in breast and lung cancers. It is worthwhile to mention that EGFR Tyr kinase inhibitors (TKIs) such as erlotinib and gefitinib are well-established therapeutics for treating human cancers with deregulated EGFR, yet patients frequently develop resistance (Balak et al., 2006). Several oncogenic pathways have been shown to be involved in TKI resistance, but the detailed mechanisms are not yet completed. The current study also raises an interesting possibility that the identified Lyn→ MCM7→DNA replication licensing pathway is one of the underlying mechanisms contributing to the TKIs resistance, and therefore the Lyn specific inhibitor may be effective against the EGFR activating human cancers that have developed TKI resistance and is worthy of further development.
Several post-translational modifications have been identified in other MCM family members and shown to be functionally important in initiating DNA replication. Specifically, phosphorylation of MCM2 at Ser27, Ser41, and Ser139 by CDC7/DBF4 kinase during G1/S phase enhances the ATPase activity of MCM complex and is essential for initiating DNA replication (Tsuji et al., 2006) whereas phosphorylation of MCM2 at Ser5 by CDC7/DBF4 kinase promotes chromatin loading of MCM2 and MCM complex assembly (Chuang et al., 2009). Additionally, CDK1-dependent MCM3 phosphorylation at Ser112 regulates its incorporation into the MCM2-7 complex (Lin et al., 2008) and CDK2-dependent MCM3 phosphorylation at Thr722 regulates its loading onto chromatin in mammalian cells (Li et al., 2011). While Y600 phosphorylation that enhances MCM7 interaction with other MCM members contributes to the cancer cell proliferation triggered by the EGFR-Lyn signaling axis, the nonphosphorylatable mutant MCM7 Y600F still maintains its basal interaction with other MCM members, suggesting that MCM7 Y600F is a mutant insensitive to EGF stimulation rather than a dominant negative mutant. Thus, the EGFR/Lyn mediated-MCM7 Y600 phosphorylation plays a positive regulatory role but not an indispensable role in the MCM7 functions. Besides, MCM7 regulates other biological processes independent of its DNA replication licensing function (Hubbi et al., 2011) and other post-translational modifications of MCM7 could also regulate MCM7 functions in response to different mitogenic stimuli. Further investigation would be necessary to elucidate their biological functions.
Lyn is overexpressed in prostate cancer cells, and inhibition of Lyn expression suppresses prostate cancer cell proliferation (Goldenberg-Furmanov et al., 2004; Park et al., 2008). Intriguingly, while siRNA-mediated inhibition of Lyn suppresses cellular proliferation, siRNA inhibition of Src suppresses primarily cell migration in prostate cancer cells (Park et al., 2008). These observations indicate that Lyn and Src may not be functionally redundant and may regulate a different subset of substrates in cancer cells. In the present study, we found that Lyn is necessary for the MCM7 Y600 phosphorylation, and the expression of Lyn, but not other Src family kinases, correlated significantly with the phosphorylation level of MCM7 Y600 in both human lung tumor tissues and breast tumor tissues, suggesting that Lyn is the predominant Src family kinase to phosphorylate MCM7 Y600. The identification of Lyn specific substrate, MCM7, provides rationale for the Lyn-associated cell proliferation and implies the existence of other Lyn kinase substrates involved in cancer cell proliferation. We predict that further elucidation of the Lyn kinase substrates and their Tyr phosphorylation status would help us to understand the oncogenic functions of Lyn.
An important observation in our current study is that the activity of Lyn in MCM7 phosphorylation and cancer cell proliferation is directly regulated by EGFR-mediated Tyr phosphorylation, which suggests that Lyn may be indispensable for the cancer cells that rely on EGFR signaling to proliferate. In support of this notion, we and others have shown that Lyn knockdown significantly compromised the proliferation of Du145 and MDA-MB-468 cells (Goldenberg-Furmanov et al., 2004), both of which rely on EGFR signaling to proliferate (Matar et al., 2004). In contrast, Lyn knockdown had no growth inhibitory effect on certain cancer cell lines including HCC1954, BT-549, and MDA-MB-231 (Hochgrafe et al., 2010) that are less dependent on EGFR signaling for their proliferation (Hochgrafe et al., 2010; Krol et al., 2007; Sahin et al., 2009). Taken together, the oncogenic activity of Lyn in DNA synthesis and cell proliferation is potentiated in cancer cells that depend on EGFR signaling for their survival, and targeting Lyn may demonstrate synergistic efficacy with EGFR TKIs for treatment of this subset of cancer cells. Given that MCM7 deregulation is frequently associated with human cancers and tumorigenesis, these observations also provide clinical implications for the treatment of human cancers with deregulated MCM7 status using EGFR and Lyn kinase inhibitors.
Experimental Procedures
Cell Lysates and Immunoprecipitation
Cells were collected at the indicated time, washed with ice-cold PBS and solubilized in lysis buffer containing 1% NP-40, 25 mM NaF, 2 mM Na3VO4, 5 mM PMSF, 0.15 U/ml aprotinin followed by sonication. Cell lysates were clarified by centrifugation at 16,000 ×g for 20 min at 4 °C, and total protein concentration was determined by Bio-Rad protein assay kit using BSA as a standard. The chromatin fraction of cell lysates was isolated following a protocol described previously (Mendez and Stillman, 2000). For each immunoprecipitation assay, 2 mg cell lysates were used and 2 μg antibody was added for each reaction. The reaction was rotating at 4 °C overnight followed by addition of 50 μl of 50% protein A or G sepharose slurry and rotating for 4 hr. Beads were collected and washed with PBS buffer containing 1% NP-40 three times. Immunoprecipitates were resolved by SDS-PAGE and analyzed by immunoblotting. Free software ImageJ was used for quantification analysis of the band density of target proteins in western blot assays.
DNA Synthesis Assay
The DNA synthesis rate was measured using the BrdU (colorimetric) ELISA kit obtained from Roche following the manufacturer’s instructions.
Mouse Model for Tumorigenesis
In vivo cell growth was analyzed using an orthotopic breast cancer mouse model that has been described previously (Chang et al., 1997). Briefly, 2 × 106 MDA-MB-468 cells infected with lentivirus carrying the wild-type or mutant constructs were mixed with the Matrigel (BD Biosciences) and PBS, and injected into the mammary fat pads of female nude mice. The length (L) and width (W) of each tumor mass were measured by calipers once a week. Tumor volume (TV) was calculated by the formula (TV = 0.5 × L × W2) (Yaguchi et al., 2006). All animal procedures were conducted under regulations of Division of Laboratory Animal Medicine at The University of Texas MD Anderson Cancer Center. Animal protocols (#06-87-06139) were reviewed and approved by the Institutional Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center.
Human Tissues
The 150 cases of surgically resected human breast cancer specimens for immunohistochemistry and survival analysis were obtained retrospectively from patients undergoing surgical resection of breast cancer, as primary treatment, at The University of Texas MD Anderson Cancer Center (MDACC) between 2000 and 2006. The specimen collection and use of human tissue samples were conducted in accordance with the protocols approved by the Institutional Review Board at MDACC. Written informed consent was obtained from patients in all cases at time of enrollment. Tissue microarrays of 228 cases of breast cancer (BRC2281) and tissue microarrays of 228 cases of lung cancer (LUC2281) were obtained from Pantomics, Inc. (San Francisco, CA). Tissue microarrays of 98 cases of lung cancer (TMA IMH-305 and IMH-358) were obtained from Imgenex, Inc. (San Diego, CA).
Liver Regeneration Assay
The experiment was performed using male Fisher 344 rats according to the procedure described previously (Marti and Hug, 1995). In brief, 2/3 of rats liver were removed by hepatectomy under anesthesia and were sacrificed 24 hr after the operation. Both regenerated and normal liver tissues were washed with PBS and tissues were homogenized. The homogenized tissue was then lysed with RIPA buffer, and the protein was extracted by centrifuge and used for Western blot assay.
Statistical Analyses
Data were analyzed by the Student’s t test, Pearson Chi-Square analysis, and Cox’s Regression analysis except Kaplan-Meier analyses on survival rates. A p value of <0.05 was considered statistically significant.
The remaining experimental procedures can be found in Supplemental Information.
Supplementary Material
Highlights.
EGFR-Lyn axis regulates MCM7-mediated DNA replication licensing.
Lyn-mediated MCM7 Y600 phosphorylation enhances the formation of MCM complex.
EGFR enhances p56Lyn activity via Y32 phosphorylation, which potentiates p-MCM7 Y600.
p-p56Lyn Y32 and p-MCM7 Y600 correlate with poor survival of breast cancer patients.
Significance.
Overexpression of MCM7 is associated with various human cancers and correlated with poor prognosis; however, the mechanisms that regulate its function remain unclear. Here, we showed that EGFR enhances p56Lyn activity through Y32 phosphorylation, which potentiates the downstream MCM7 Y600 phosphorylation and positively modulates MCM complex assembly and cancer cell proliferation. We thus identified a tyrosine phosphorylation cascade namely EGFR → phospho-p56Lyn Y32→ phospho-MCM7 Y600 that links EGFR and Lyn in MCM7-mediated DNA replication licensing in human cancers. This finding links a cell surface receptor, EGFR, and a non-receptor tyrosine kinase, Lyn, to DNA replication licensing, which is an important step for initiating cell proliferation.
Acknowledgements
We thank Drs. Yekaterina Khotskaya, Jennifer L. Hsu, and Stephanie A. Miller for editing this manuscript and the Center for Biological Pathways at MD Anderson Cancer Center for support. This study was funded in part by National Institutes of Health (CA109311, CA099031, and CCSG CA16672); Susan G. Komen Foundation (SAC100016); National Breast Cancer Foundation, Patel Memorial Breast Cancer Research Fund; The University of Texas MD Anderson-China Medical University and Hospital Sister Institution Fund (to M.-C. Hung); Cancer Research Center of Excellence (DOH102-TD-C-111-005, Taiwan); Private University grant (NSC99-2632-B-039-001-MY3, Taiwan); Program for Stem Cell and Regenerative Medicine Frontier Research (NSC101-2321-B-039-001, Taiwan); International Research-Intensive Centers of Excellence in Taiwan (NSC102-2911-I-002-303, Taiwan); National Science Council Taiwan Merit Postdoctoral Scholarship (TMS-94-2B-001; to Y.-N.W.)
Footnotes
Supplemental Information Supplemental information includes supplemental experimental procedures, seven figures, and five tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- Bergoglio V, Pillaire MJ, Lacroix-Triki M, Raynaud-Messina B, Canitrot Y, Bieth A, Gares M, Wright M, Delsol G, Loeb LA, et al. Deregulated DNA polymerase beta induces chromosome instability and tumorigenesis. Cancer Res. 2002;62:3511–3514. [PubMed] [Google Scholar]
- Bucher NL. Liver regeneration: an overview. Journal of gastroenterology and hepatology. 1991;6:615–624. doi: 10.1111/j.1440-1746.1991.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Chang JY, Xia W, Shao R, Sorgi F, Hortobagyi GN, Huang L, Hung MC. The tumor suppression activity of E1A in HER-2/neu-overexpressing breast cancer. Oncogene. 1997;14:561–568. doi: 10.1038/sj.onc.1200861. [DOI] [PubMed] [Google Scholar]
- Chuang LC, Teixeira LK, Wohlschlegel JA, Henze M, Yates JR, Mendez J, Reed SI. Phosphorylation of Mcm2 by Cdc7 promotes pre-replication complex assembly during cell-cycle re-entry. Mol Cell. 2009;35:206–216. doi: 10.1016/j.molcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang HQ, Li Z. The Cdc45.Mcm2-7.GINS protein complex in trypanosomes regulates DNA replication and interacts with two Orc1-like proteins in the origin recognition complex. J Biol Chem. 2011;286:32424–32435. doi: 10.1074/jbc.M111.240143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrin C, Clarke P, Zech J, Lurz R, Sun J, Uhle S, Li H, Stillman B, Speck C. A double-hexameric MCM2-7 complex is loaded onto origin DNA during licensing of eukaryotic DNA replication. Proc Natl Acad Sci U S A. 2009;106:20240–20245. doi: 10.1073/pnas.0911500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facoetti A, Ranza E, Benericetti E, Ceroni M, Tedeschi F, Nano R. Minichromosome maintenance protein 7: a reliable tool for glioblastoma proliferation index. Anticancer research. 2006;26:1071–1075. [PubMed] [Google Scholar]
- Feng CJ, Li HJ, Li JN, Lu YJ, Liao GQ. Expression of Mcm7 and Cdc6 in oral squamous cell carcinoma and precancerous lesions. Anticancer research. 2008;28:3763–3769. [PubMed] [Google Scholar]
- Gambus A, Khoudoli GA, Jones RC, Blow JJ. MCM2-7 form double hexamers at licensed origins in Xenopus egg extract. J Biol Chem. 2011;286:11855–11864. doi: 10.1074/jbc.M110.199521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg-Furmanov M, Stein I, Pikarsky E, Rubin H, Kasem S, Wygoda M, Weinstein I, Reuveni H, Ben-Sasson SA. Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer Res. 2004;64:1058–1066. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]
- Han YC, Yu YP, Nelson J, Wu C, Wang H, Michalopoulos GK, Luo JH. Interaction of integrin-linked kinase and miniature chromosome maintenance 7-mediating integrin {alpha}7 induced cell growth suppression. Cancer Res. 2010;70:4375–4384. doi: 10.1158/0008-5472.CAN-09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochgrafe F, Zhang L, O’Toole SA, Browne BC, Pinese M, Porta Cubas A, Lehrbach GM, Croucher DR, Rickwood D, Boulghourjian A, et al. Tyrosine phosphorylation profiling reveals the signaling network characteristics of Basal breast cancer cells. Cancer Res. 2010;70:9391–9401. doi: 10.1158/0008-5472.CAN-10-0911. [DOI] [PubMed] [Google Scholar]
- Honeycutt KA, Chen Z, Koster MI, Miers M, Nuchtern J, Hicks J, Roop DR, Shohet JM. Deregulated minichromosomal maintenance protein MCM7 contributes to oncogene driven tumorigenesis. Oncogene. 2006;25:4027–4032. doi: 10.1038/sj.onc.1209435. [DOI] [PubMed] [Google Scholar]
- Hubbi ME, Luo W, Baek JH, Semenza GL. MCM proteins are negative regulators of hypoxia-inducible factor 1. Mol Cell. 2011;42:700–712. doi: 10.1016/j.molcel.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Wang YN, Xia W, Hsu SC, Lai CC, Li LY, Chang WC, Wang Y, Hsu MC, Yu YL, et al. RNA helicase A is a DNA-binding partner for EGFR-mediated transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2010;107:16125–16130. doi: 10.1073/pnas.1000743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingley E. Functions of the Lyn tyrosine kinase in health and disease. Cell communication and signaling : CCS. 2012;10:21. doi: 10.1186/1478-811X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y. A DNA helicase activity is associated with an MCM4, -6, and -7 protein complex. J Biol Chem. 1997;272:24508–24513. doi: 10.1074/jbc.272.39.24508. [DOI] [PubMed] [Google Scholar]
- Kan T, Sato F, Ito T, Matsumura N, David S, Cheng Y, Agarwal R, Paun BC, Jin Z, Olaru AV, et al. The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology. 2009;136:1689–1700. doi: 10.1053/j.gastro.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Galal WC, Farina A, Tappin I, Hurwitz J. Properties of the human Cdc45/Mcm2-7/GINS helicase complex and its action with DNA polymerase epsilon in rolling circle DNA synthesis. Proc Natl Acad Sci U S A. 2012;109:6042–6047. doi: 10.1073/pnas.1203734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K, Nakayama Y, Ikeda K, Fukushima Y, Matsuda D, Horimoto S, Yamaguchi N. Trafficking of Lyn through the Golgi caveolin involves the charged residues on alphaE and alphaI helices in the kinase domain. J Cell Biol. 2004;165:641–652. doi: 10.1083/jcb.200403011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Sato JD, Le A, Polikoff J, Sato GH, Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983;80:1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol J, Francis RE, Albergaria A, Sunters A, Polychronis A, Coombes RC, Lam EW. The transcription factor FOXO3a is a crucial cellular target of gefitinib (Iressa) in breast cancer cells. Mol Cancer Ther. 2007;6:3169–3179. doi: 10.1158/1535-7163.MCT-07-0507. [DOI] [PubMed] [Google Scholar]
- Labib K, Tercero JA, Diffley JF. Uninterrupted MCM2-7 function required for DNA replication fork progression. Science. 2000;288:1643–1647. doi: 10.1126/science.288.5471.1643. [DOI] [PubMed] [Google Scholar]
- Lee JK, Hurwitz J. Processive DNA helicase activity of the minichromosome maintenance proteins 4, 6, and 7 complex requires forked DNA structures. Proc Natl Acad Sci U S A. 2001;98:54–59. doi: 10.1073/pnas.98.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Tye BK. Initiating DNA synthesis: from recruiting to activating the MCM complex. J Cell Sci. 2001;114:1447–1454. doi: 10.1242/jcs.114.8.1447. [DOI] [PubMed] [Google Scholar]
- LeVea CM, Reeder JE, Mooney RA. EGF-dependent cell cycle progression is controlled by density-dependent regulation of Akt activation. Exp Cell Res. 2004;297:272–284. doi: 10.1016/j.yexcr.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Li J, Deng M, Wei Q, Liu T, Tong X, Ye X. Phosphorylation of MCM3 protein by cyclin E/cyclin-dependent kinase 2 (Cdk2) regulates its function in cell cycle. J Biol Chem. 2011;286:39776–39785. doi: 10.1074/jbc.M111.226464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DI, Aggarwal P, Diehl JA. Phosphorylation of MCM3 on Ser-112 regulates its incorporation into the MCM2-7 complex. Proc Natl Acad Sci U S A. 2008;105:8079–8084. doi: 10.1073/pnas.0800077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JH. Oncogenic activity of MCM7 transforming cluster. World J Clin Oncol. 2011;2:120–124. doi: 10.5306/wjco.v2.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine GT, Sinha P, Tye BK. Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics. 1984;106:365–385. doi: 10.1093/genetics/106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti U, Hug M. Acinar and cellular distribution and mRNA expression of the epidermal growth factor receptor are changed during liver regeneration. Journal of hepatology. 1995;23:318–327. [PubMed] [Google Scholar]
- Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, Tabernero J, Guzman M, Rodriguez S, Arribas J, Palacios J, Baselga J. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487–6501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci U S A. 2006;103:10236–10241. doi: 10.1073/pnas.0602400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104:17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara K, Shomori K, Fujioka S, Tokuyasu N, Inaba A, Osaki M, Ogawa T, Ito H. Minichromosome maintenance protein 7 in colorectal cancer: implication of prognostic significance. International journal of oncology. 2008;33:245–251. [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Ota T, Clayton AC, Minot DM, Shridhar V, Hartmann LC, Gilks CB, Chien JR. Minichromosome maintenance protein 7 as a potential prognostic factor for progression-free survival in high-grade serous carcinomas of the ovary. Mod Pathol. 2011 doi: 10.1038/modpathol.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim SJ, Wang Z, Gallick GE. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- Price JT, Tiganis T, Agarwal A, Djakiew D, Thompson EW. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 1999;59:5475–5478. [PubMed] [Google Scholar]
- Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- Ren B, Yu G, Tseng GC, Cieply K, Gavel T, Nelson J, Michalopoulos G, Yu YP, Luo JH. MCM7 amplification and overexpression are associated with prostate cancer progression. Oncogene. 2006;25:1090–1098. doi: 10.1038/sj.onc.1209134. [DOI] [PubMed] [Google Scholar]
- Sahin O, Frohlich H, Lobke C, Korf U, Burmester S, Majety M, Mattern J, Schupp I, Chaouiya C, Thieffry D, et al. Modeling ERBB receptor-regulated G1/S transition to find novel targets for de novo trastuzumab resistance. BMC Syst Biol. 2009;3:1. doi: 10.1186/1752-0509-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YK, Yu YP, Tseng GC, Luo JH. Inhibition of prostate cancer growth and metastasis using small interference RNA specific for minichromosome complex maintenance component 7. Cancer Gene Ther. 2010;17:694–699. doi: 10.1038/cgt.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YK, Yu YP, Zhu ZH, Han YC, Ren B, Nelson JB, Luo JH. MCM7 interacts with androgen receptor. Am J Pathol. 2008;173:1758–1767. doi: 10.2353/ajpath.2008.080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner JM, Dew-Knight S, Musahl C, Kornbluth S, Horowitz JM. Negative regulation of DNA replication by the retinoblastoma protein is mediated by its association with MCM7. Mol Cell Biol. 1998;18:2748–2757. doi: 10.1128/mcb.18.5.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokawa G, Masuda K, Daigo Y, Cho HS, Yoshimatsu M, Takawa M, Hayami S, Maejima K, Chino M, Field HI, et al. Minichromosome Maintenance Protein 7 is a potential therapeutic target in human cancer and a novel prognostic marker of non-small cell lung cancer. Mol Cancer. 2011;10:65. doi: 10.1186/1476-4598-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Ficarro SB, Jiang W. Essential role of phosphorylation of MCM2 by Cdc7/Dbf4 in the initiation of DNA replication in mammalian cells. Mol Biol Cell. 2006;17:4459–4472. doi: 10.1091/mbc.E06-03-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye BK. MCM proteins in DNA replication. Annu Rev Biochem. 1999;68:649–686. doi: 10.1146/annurev.biochem.68.1.649. [DOI] [PubMed] [Google Scholar]
- Yaguchi S, Fukui Y, Koshimizu I, Yoshimi H, Matsuno T, Gouda H, Hirono S, Yamazaki K, Yamori T. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. Journal of the National Cancer Institute. 2006;98:545–556. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y, Miyasaka M, Takeuchi M, Ilic D, Mizuguchi J, Yamamoto T. Differential responses of p56lyn and p53lyn, products of alternatively spliced lyn mRNA, on stimulation of B-cell antigen receptor. Cell Regul. 1991;2:979–987. doi: 10.1091/mbc.2.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Merchant AM, Tye BK. Cell cycle-regulated nuclear localization of MCM2 and MCM3, which are required for the initiation of DNA synthesis at chromosomal replication origins in yeast. Genes Dev. 1993;7:2149–2160. doi: 10.1101/gad.7.11.2149. [DOI] [PubMed] [Google Scholar]
- Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.