Abstract
To study the effect of insulin on leucine kinetics, three groups of conscious dogs were studied after an overnight fast (16-18 h). One, saline-infused group (n = 5), served as control. The other two groups were infused with somatostatin and constant replacement amount of glucagon; one group (n = 6) received no insulin replacement, to produce acute insulin deficiency, and the other (n = 6) was constantly replaced with 600 μU/kg per min insulin, to produce twice basal hyperinsulinemia. Hepatic and extrahepatic splanchnic (gut) balance of leucine and α-ketoisocaproate (KIC) were calculated using the arteriovenous difference technique. l,4,5,[3H]Leucine was used to measure the rates (micromoles per kilogram per minute) of appearance (Ra) and disappearance (Rd), and clearance (Cl) of plasma leucine (milliliters per kilogram per minute).
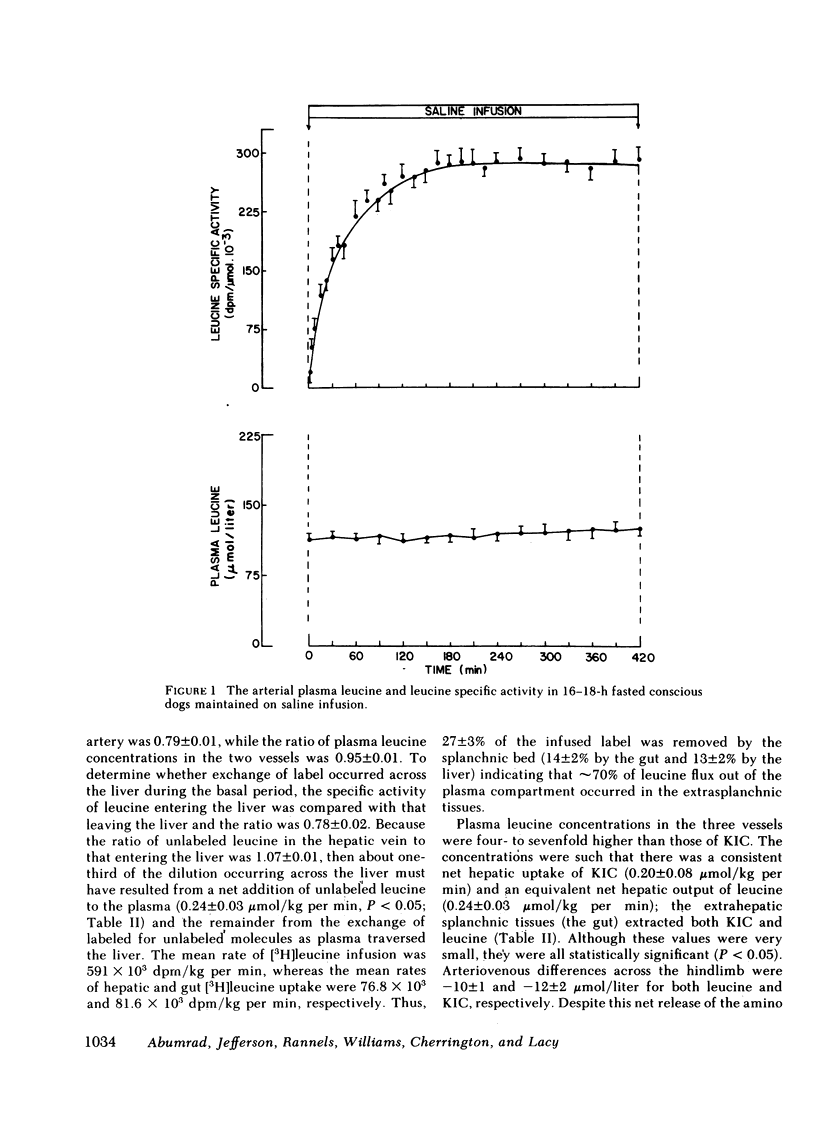
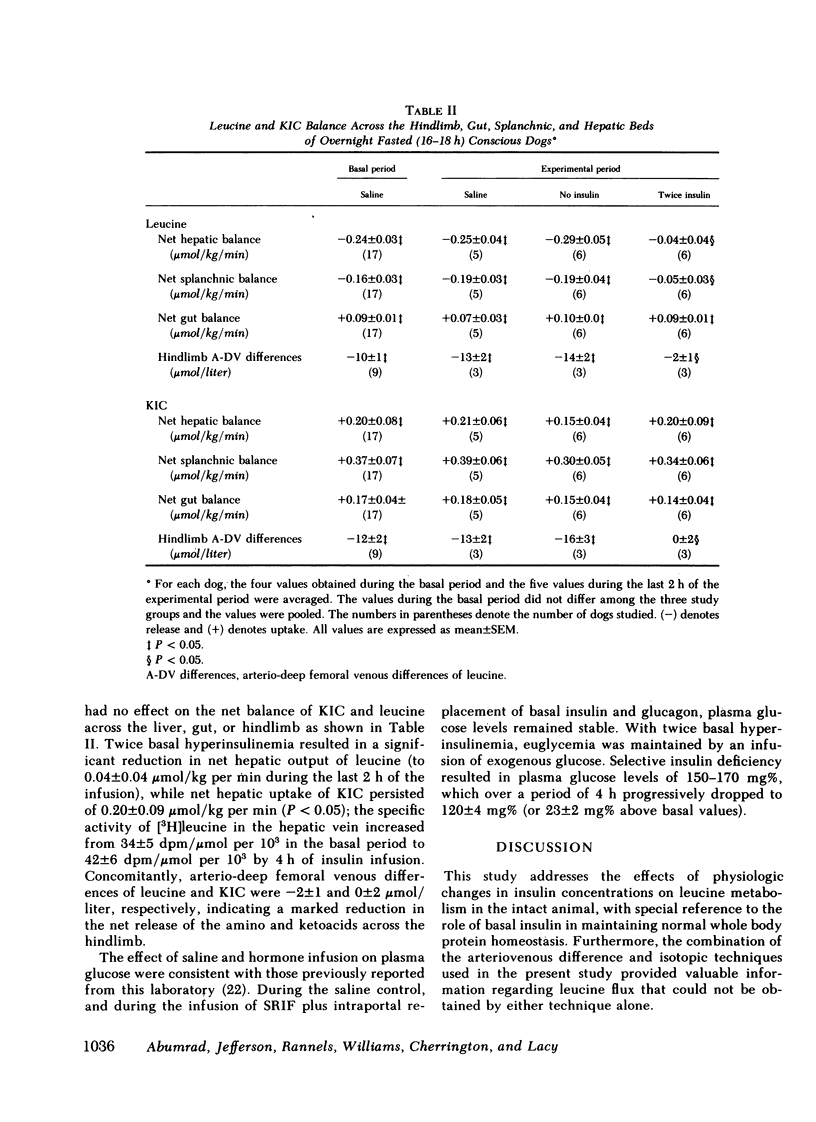
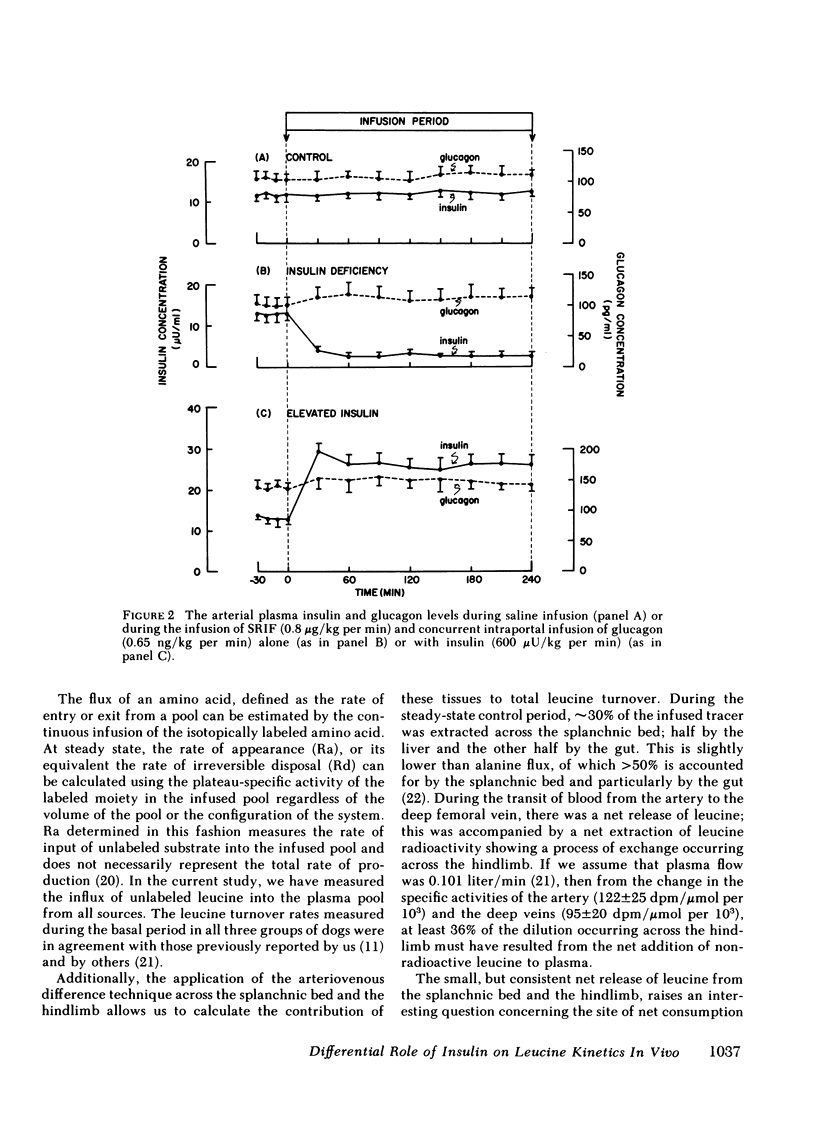
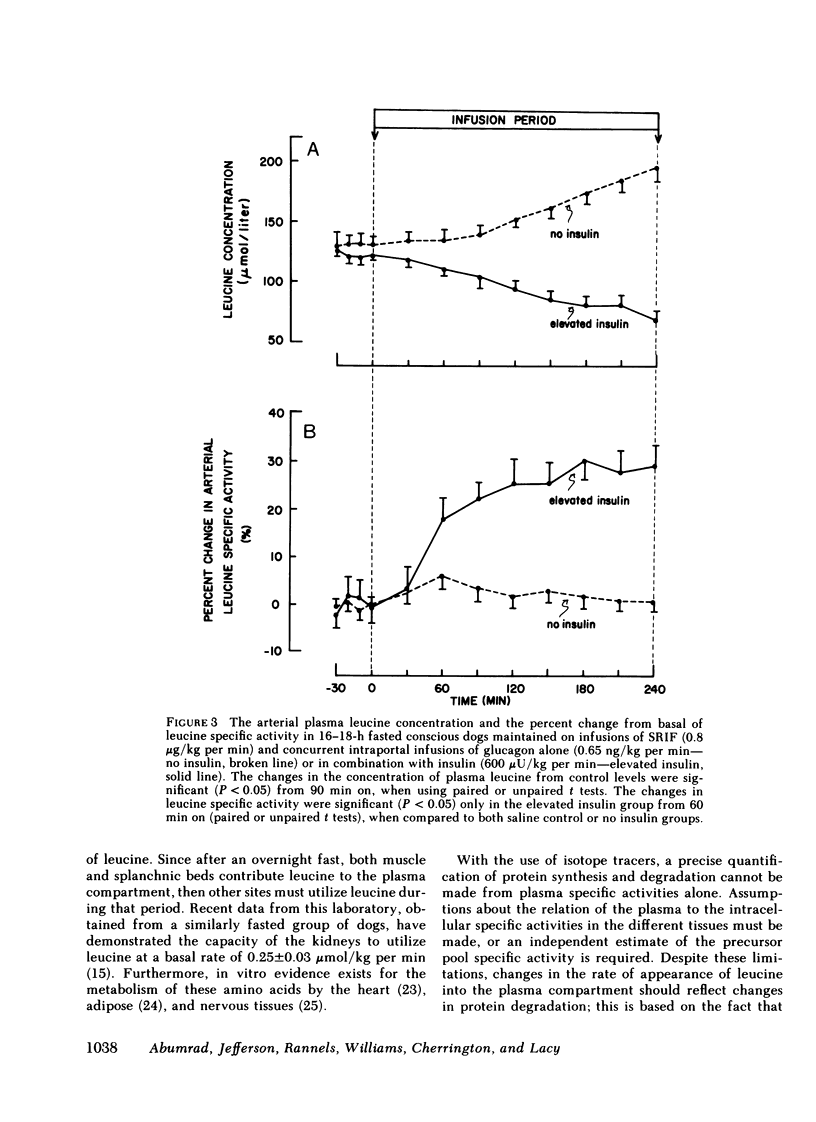
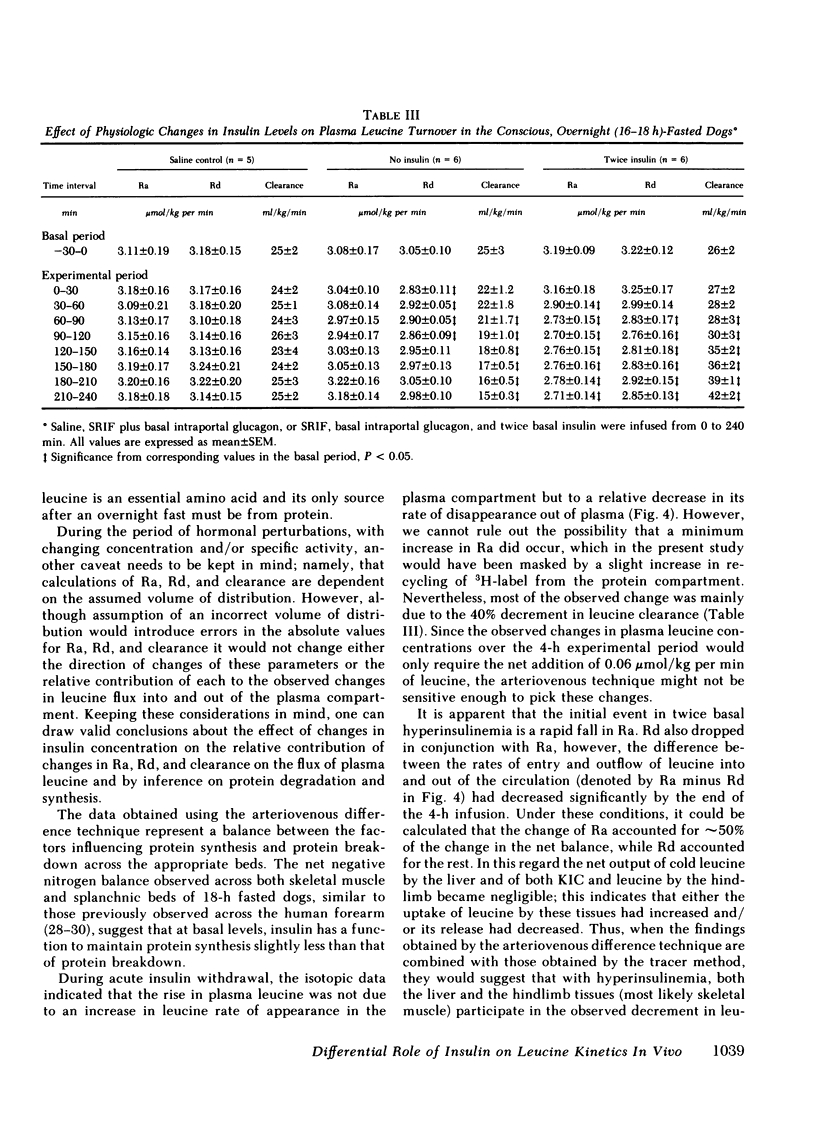
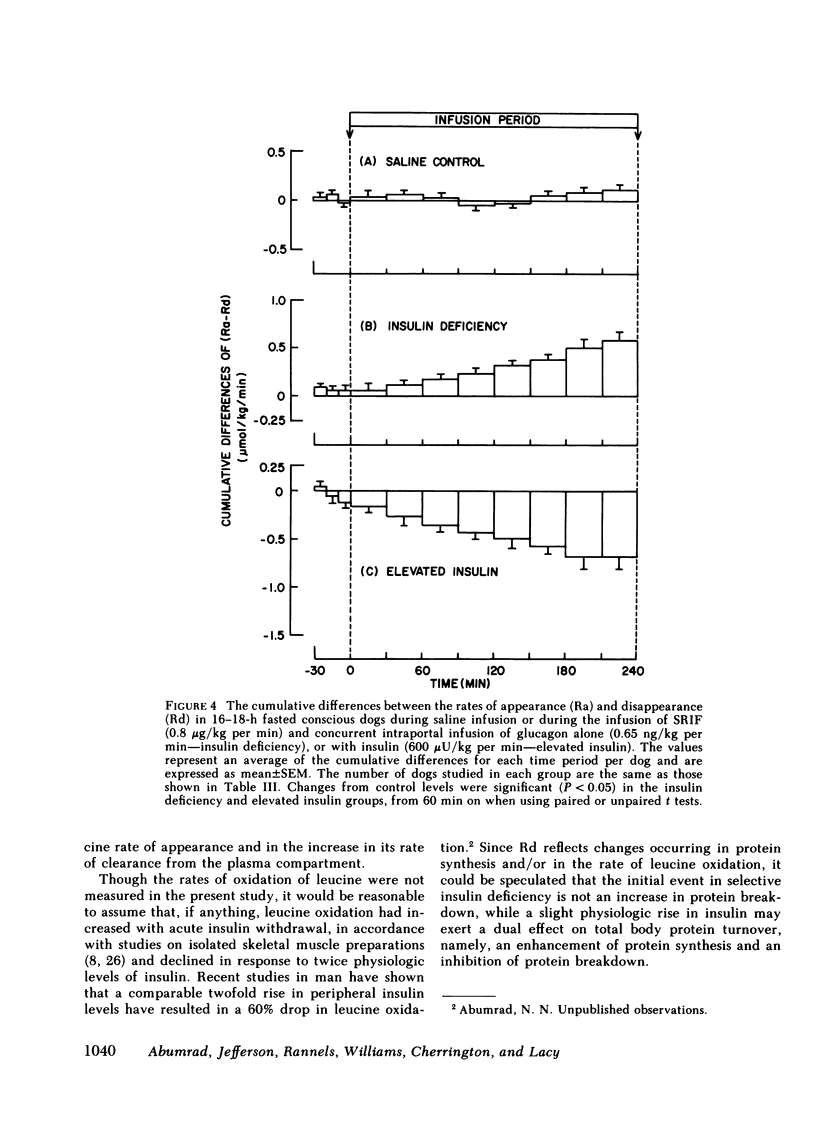
Saline infusion for 7 h resulted in isotopic steady state, where Ra and Rd were equal (3.2±0.2 μmol/kg per min). Acute insulin withdrawal of 4-h duration caused the plasma leucine to increase by 40% (P < 0.005). This change was caused by a decrease in the outflow of leucine (Cl) from the plasma, since Ra did not change. The net hepatic release of the amino acid (0.24±0.03 μmol/kg per min) did not change significantly; the arterio-deep femoral venous differences of leucine (−10±1 μmol/liter) and KIC (−12±2 μmol/liter) did not change significantly indicating net release of the amino and ketoacids across the hindlimb. Selective twice basal hyperinsulinemia resulted in a 36% drop in plasma leucine (from control levels of 128±8 to 82±7 μmol/liter, P < 0.005) within 4 h. This was accompanied by a 15% reduction in Ra and a 56% rise in clearance (P < 0.001, both). Net hepatic leucine production and net release of leucine and KIC across the hindlimb fell markedly. These studies indicate that physiologic changes in circulating insulin levels result in a differential dose-dependent effect on total body leucine metabolism in the intact animal. Acute insulin withdrawal exerts no effect on leucine rate of appearance, while at twice basal levels, insulin inhibited leucine rate of appearance and stimulated its rate of disappearance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Rabin D., Wise K. L., Lacy W. W. The disposal of an intravenously administered amino acid load across the human forearm. Metabolism. 1982 May;31(5):463–470. doi: 10.1016/0026-0495(82)90235-9. [DOI] [PubMed] [Google Scholar]
- Abumrad N. N., Wise K. L., Williams P. E., Abumrad N. A., Lacy W. W. Disposal of alpha-ketoisocaproate: roles of liver, gut, and kidneys. Am J Physiol. 1982 Aug;243(2):E123–E131. doi: 10.1152/ajpendo.1982.243.2.E123. [DOI] [PubMed] [Google Scholar]
- Adibi S. A. Metabolism of branched-chain amino acids in altered nutrition. Metabolism. 1976 Nov;25(11):1287–1302. doi: 10.1016/s0026-0495(76)80012-1. [DOI] [PubMed] [Google Scholar]
- Adibi S. A., Modesto T. A., Morse E. L., Amin P. M. Amino acid levels in plasma, liver, and skeletal muscle during protein deprivation. Am J Physiol. 1973 Aug;225(2):408–414. doi: 10.1152/ajplegacy.1973.225.2.408. [DOI] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Atchley D. W., Loeb R. F., Richards D. W., Benedict E. M., Driscoll M. E. ON DIABETIC ACIDOSIS: A Detailed Study of Electrolyte Balances Following the Withdrawal and Reestablishment of Insulin Therapy. J Clin Invest. 1933 Mar;12(2):297–326. doi: 10.1172/JCI100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Felig P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:933–955. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R., Brundin T. Splanchnic glucose and amino acid metabolism in obesity. J Clin Invest. 1974 Feb;53(2):582–590. doi: 10.1172/JCI107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galim E. B., Hruska K., Bier D. M., Matthews D. E., Haymond M. W. Branched-chain amino acid nitrogen transfer to alamine in vivo in dogs. Direct isotopic determination with [15N]leucine. J Clin Invest. 1980 Dec;66(6):1295–1304. doi: 10.1172/JCI109981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M. Site of action of insulin in promoting leucine utilization in adipose tissue. Am J Physiol. 1977 Aug;233(2):E97–103. doi: 10.1152/ajpendo.1977.233.2.E97. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Zapalowski C., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproic acid metabolism in skeletal muscle. Effects of starvation and insulin. J Biol Chem. 1980 Mar 25;255(6):2418–2426. [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Keller U., Cherrington A. D., Liljenquist J. E. Ketone body turnover and net hepatic ketone production in fasted and diabetic dogs. Am J Physiol. 1978 Aug;235(2):E238–E247. doi: 10.1152/ajpendo.1978.235.2.E238. [DOI] [PubMed] [Google Scholar]
- LEEVY C. M., MENDENHALL C. L., LESKO W., HOWARD M. M. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962 May;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H. E., Jefferson L. S., Wolpert E. B., Rannels D. E. Regulation of protein synthesis in heart muscle. II. Effect of amino acid levels and insulin on ribosomal aggregation. J Biol Chem. 1971 Apr 10;246(7):2163–2170. [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Lacy W. W., Liljenquist J. E., Keller U., Williams P. E., Cherrington A. D. Effect of glucose, independent of changes in insulin and glucagon secretion, on alanine metabolism in the conscious dog. J Clin Invest. 1980 Feb;65(2):496–505. doi: 10.1172/JCI109693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillway L. W., Weigand D. A., Buse M. G. Leucine as an in vitro precursor to lipids in rat sciatic nerve. Lipids. 1979 Feb;14(2):127–131. doi: 10.1007/BF02533861. [DOI] [PubMed] [Google Scholar]
- WALL J. S., STEELE R., DE BODO R. C., ALTSZULER N. Effect of insulin on utilization and production of circulating glucose. Am J Physiol. 1957 Apr;189(1):43–50. doi: 10.1152/ajplegacy.1957.189.1.43. [DOI] [PubMed] [Google Scholar]
- Walser M., Lund P., Ruderman N. B., Coulter A. W. Synthesis of essential amino acids from their alpha-keto analogues by perfused rat liver and muscle. J Clin Invest. 1973 Nov;52(11):2865–2877. doi: 10.1172/JCI107483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. H., Soler N. G., James H., Harvey T. C., Thomas B. J., Fremlin J. H., Fitzgerald M. G., Malins J. M. Studies in whole body potassium and whole body nitrogen in newly diagnosed diabetics. Q J Med. 1976 Apr;45(178):295–301. [PubMed] [Google Scholar]
- Ward W. F., Cox J. R., Mortimore G. E. Lysosomal sequestration of intracellular protein as a regulatory step in hepatic proteolysis. J Biol Chem. 1977 Oct 10;252(19):6955–6961. [PubMed] [Google Scholar]



