Abstract
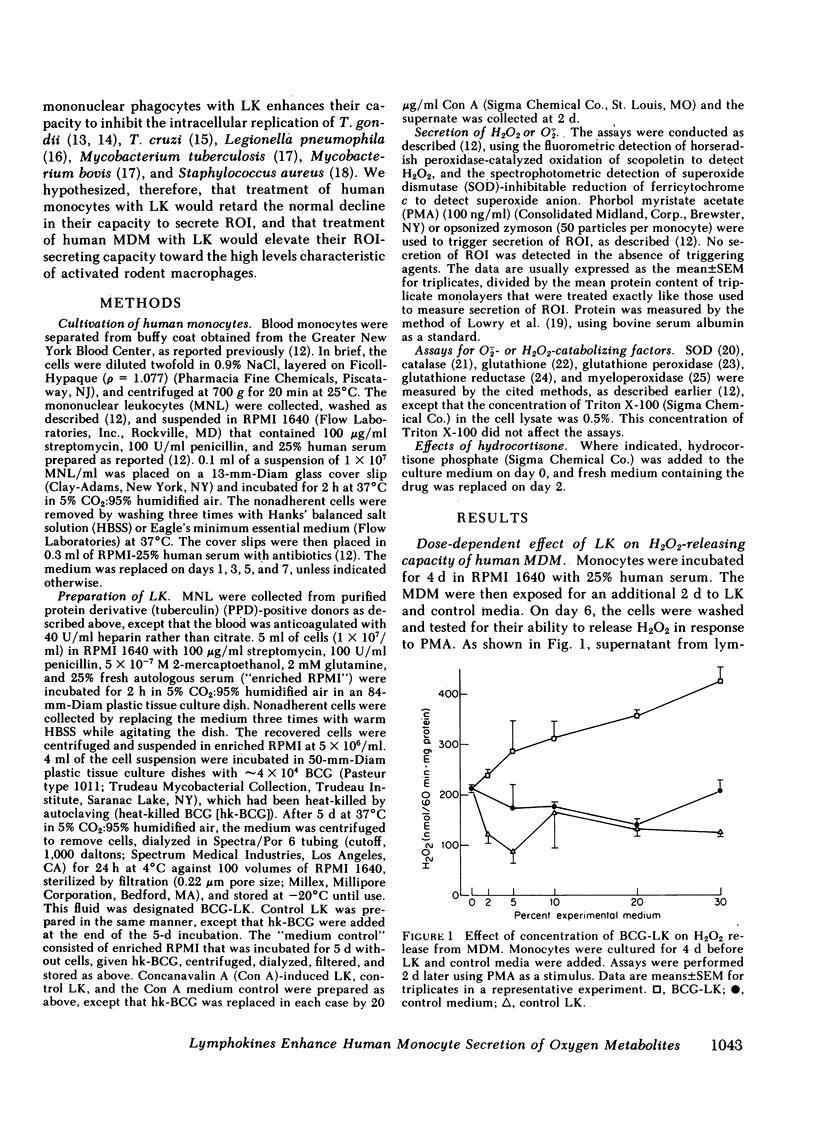
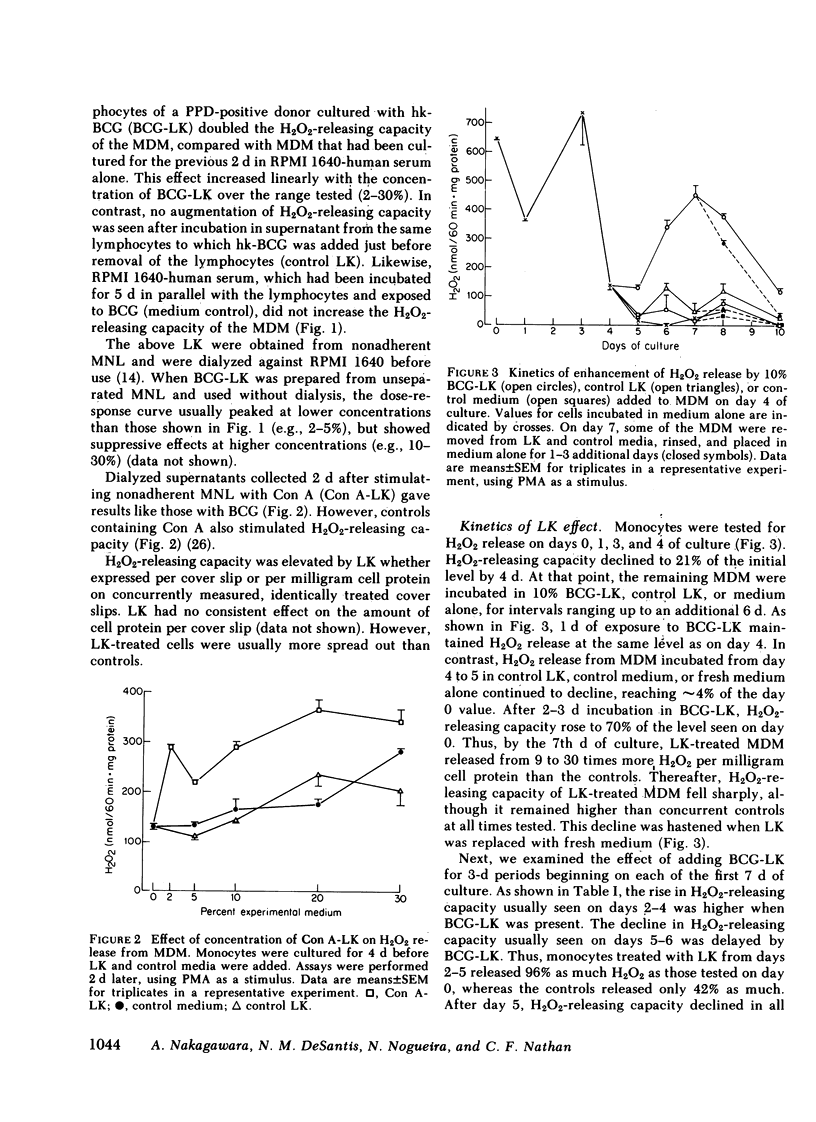
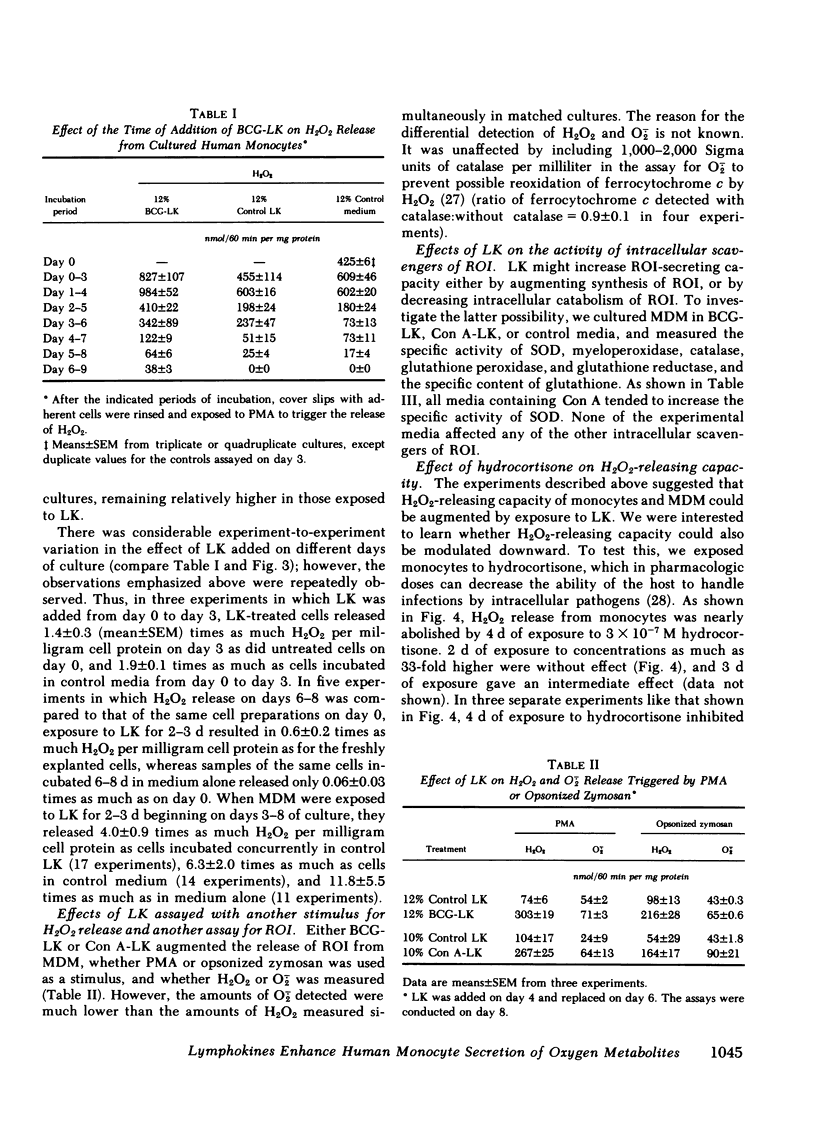
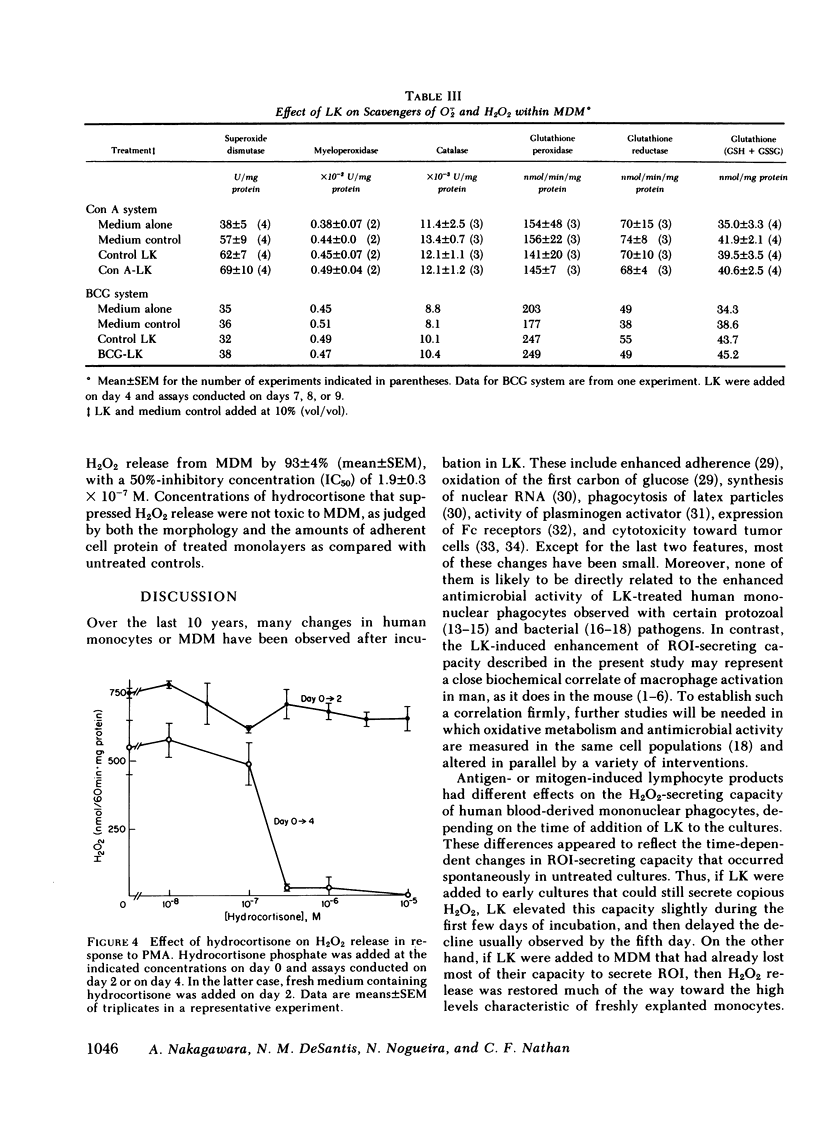
Supernatants from mitogen- or antigen-stimulated human blood mononuclear cells enhanced the capacity of human monocytes or monocyte-derived macrophages (MDM) to release H2O2 or O2̇̄ in response to phorbol myristate acetate or zymosan. The stimulatory effect of lymphokines (LK) lasted ∼5 d, regardless of the time of their addition. However, the magnitude of stimulation depended on whether LK were added to freshly explanted monocytes or to MDM. When LK were added on day 0 of culture, they enhanced MDM H2O2-releasing capacity ∼40% measured on day 3, when H2O2-releasing capacity in the controls was maximal. Addition of LK on day 2 retarded the decline in H2O2-releasing capacity normally seen by day 5, so that LK-treated cells released about twice as much H2O2 as the controls. Addition of LK to MDM that had already lost most of their H2O2-releasing capacity (e.g., on day 4-6) restored it to an average of 60% of the values seen with freshly explanted monocytes. In this case, LK-treated cells were about 12 times more active than cells incubated in medium alone. The effects of LK were dose- and time-dependent, with maximal effects requiring 3 d of exposure. The specific activities of superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and myeloperoxidase, and the specific content of glutathione were not diminished in LK-treated MDM, suggesting that increased synthesis of H2O2 rather than decreased catabolism probably explained the greater release of H2O2 from LK-treated cells. In contrast, release of H2O2 was suppressed 93±4% by exposing monocytes for 4 d to hydrocortisone (50%-inhibitory concentration, 1.9±0.3 × 10−7 M). Thus, the oxidative metabolism of human mononuclear phagocytes can be markedly modulated in vitro: augmented by mediators released from lymphocytes during an immune response, and suppressed by antiinflammatory corticosteroids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Bautista S., Remington J. S. Induction of resistance to Toxoplasma gondii in human macrophages by soluble lymphocyte products. J Immunol. 1976 Aug;117(2):381–387. [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges J. S., Johnson W. D., Jr Inhibition of multiplication of Toxoplasma gondii by human monocytes exposed to T-lymphocyte products. J Exp Med. 1975 Feb 1;141(2):483–496. doi: 10.1084/jem.141.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation: possible involvement of oxygen metabolites in killing of Leishmania enrietti by activated mouse macrophages. J Reticuloendothel Soc. 1981 Mar;29(3):181–192. [PubMed] [Google Scholar]
- Cameron D. J., Churchill W. H. Cytotoxicity of human macrophages for tumor cells. Enhancement by human lymphocyte mediators. J Clin Invest. 1979 May;63(5):977–984. doi: 10.1172/JCI109398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., May M. Preliminary demonstration of human tuberculoimmunity in vitro. Infect Immun. 1981 Jan;31(1):453–464. doi: 10.1128/iai.31.1.453-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale D. C., Petersdorf R. G. Corticosteroids and infectious diseases. Med Clin North Am. 1973 Sep;57(5):1277–1287. doi: 10.1016/s0025-7125(16)32228-3. [DOI] [PubMed] [Google Scholar]
- Greineder D. K., Connorton K. J., David J. R. Plasminogen activator production by human monocytes. I. Enhancement by activated lymphocytes and lymphocyte products. J Immunol. 1979 Dec;123(6):2808–2813. [PubMed] [Google Scholar]
- Guyre P. M., Crabtree G. R., Bodwell J. E., Munck A. MLC-conditioned media stimulate an increase in Fc receptors on human macrophages. J Immunol. 1981 Feb;126(2):666–668. [PubMed] [Google Scholar]
- Hammerström J., Unsgaard G., Lamvik J. Activation of human monocytes by mediators from lymphocytes stimulated with Corynebacterium parvum. Acta Pathol Microbiol Scand C. 1979 Jun;87C(3):167–175. [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. A comparison of the superoxide-releasing response in human polymorphonuclear leukocytes and monocytes. J Immunol. 1980 Jul;125(1):359–364. [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmeyer J. E., Johnston R. B., Jr Effect of anti-inflammatory drugs and agents that elevate intracellular cyclic AMP on the release of toxic oxygen metabolites by phagocytes: studies in a model of tissue-bound IgG. Clin Immunol Immunopathol. 1978 Apr;9(4):482–490. doi: 10.1016/0090-1229(78)90144-7. [DOI] [PubMed] [Google Scholar]
- Masur H., Murray H. W., Jones T. C. Effect of hydrocortisone on macrophage response to lymphokine. Infect Immun. 1982 Feb;35(2):709–714. doi: 10.1128/iai.35.2.709-714.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Chaplan S., Reesink M., Tydings J., Cohn Z. A. Trypanosoma cruzi: induction of microbicidal activity in human mononuclear phagocytes. J Immunol. 1982 May;128(5):2142–2146. [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W. Activation of mouse macrophages for tumor cell killing. I. Quantitative analysis of interactions between lymphokine and lipopolysaccharide. J Immunol. 1981 May;126(5):1863–1867. [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Reiss M., Roos D. Differences in oxygen metabolism of phagocytosing monocytes and neutrophils. J Clin Invest. 1978 Feb;61(2):480–488. doi: 10.1172/JCI108959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold H. G., McCarthy P. L., Jr, Mednis A. D. Purification of guinea pig pH 3 migration inhibitory factor. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4088–4091. doi: 10.1073/pnas.78.7.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E., Winston C. T., David J. R. Activation of human blood monocytes by products of sensitized lymphocytes. J Clin Invest. 1974 Feb;53(2):559–564. doi: 10.1172/JCI107590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Rossi F. Reversible metabolic stimulation of polymorphonuclear leukocytes and macrophages by concanavalin A. Nat New Biol. 1973 May 23;243(125):111–112. [PubMed] [Google Scholar]
- Roos D., Weening R. S., Voetman A. A., van Schaik M. L., Bot A. A., Meerhof L. J., Loos J. A. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979 May;53(5):851–866. [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. E., Douglas S. D., Rubin A. D. Human monocyte activation by supernatants from concanavalin A (con A) stimulated lymphocytes. Cell Immunol. 1973 Oct;9(1):45–59. doi: 10.1016/0008-8749(73)90166-4. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffet S. M., Russell S. W. Macrophage-mediated tumor cell killing: regulation of expression of cytolytic activity by prostaglandin E. J Immunol. 1981 Feb;126(2):424–427. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Superoxide generation by human monocytes and macrophages. Am J Hematol. 1978;4(1):1–8. doi: 10.1002/ajh.2830040102. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]


