Abstract
Background:
Ketogenic enteral nutrition (KEN) is a modification of the protein sparing modified fast in which a protein solution is introduced with a continuous infusion through a nasogastric tube over 10-days cycles. The aim of the study was to perform a retrospective analysis of the safety, compliance, weight loss and body composition changes after 3 sequential 10-days cycles of KEN therapy.
Materials and Methods:
From a large number of patients who underwent KEN therapy in our department over a 5-year period, we selected 188 patients who participated in 3 KEN cycles with 10-13 days of break between them. Before and after the treatment cycles, body composition was analyzed by bioelectric impedance; a final assessment was made 10 days after the end of last cycle. During each rest period all the patients were on a low-carbohydrate, normal caloric diet.
Results:
Most patients (97%) successfully tolerated the nasogastric treatment and lost an average of 14.4 kg of body weight, 10.6 kg of fat mass and 3.4 kg of body cell mass. Adverse effects were recorded as mild gastric hypersecretion (2%) and constipation (5%). Patients continued to lose fat during the 10-day follow up period after the end of each KEN Cycle. This effect may be explained by abnormality of water distribution during the rapid weight loss inducing the observed change in fat mass.
Conclusion:
Ten-days KEN treatment cycles can induce rapid weight loss and reduction of fat mass in obese patients. Furthermore, preservation of lean mass can be achieved by infusing 1.9 g of protein/kg of BCM.
Keywords: Body composition, enteral nutrition, obesity, protein
INTRODUCTION
Obesity is a public health problem with worldwide effects. The presence of obesity is associated with increased risk of death, especially from cardiovascular causes and cancer.[1] Body mass index (BMI; weight in kg/height in m2) is a strong predictor of obesity-associated mortality. Life expectancy in obese patients is reduced such that patients with a BMI of 30-35 kg/m2 have a median survival reduced by 2-4 years; those with a BMI of 40-45 kg/m2 have a median survival reduced by 8-10 years.[2] Obesity is also associated with chronic illnesses, including diabetes, hypertension, osteoarthritis, gallstones, gout to name a few,[3] as well as reduced quality of life.[4] Adult obesity and overweight are responsible for up to 6% of health care expenditures and 10-13% of deaths in the European Region; in addition, they impose indirect costs (due to the loss of lives, productivity and related income) that are at least two times higher.[5]
In several randomized controlled trials, a low-carbohydrate, ketogenic diet (LCKD) has resulted in significant weight loss over a 6- to 12-month period.[6,7,8] Moreover, recent studies suggest that an LCKD results in less hunger,[9,10,11] seems to be safe even in the long term[12,13] and preserves lean mass even during hypo energetic conditions of weight loss,[14,15] although there is still not enough evidence for a full consensus on the use of this kind of diet.[16]
The aim of this study was to evaluate retrospectively body composition changes and the efficacy and safety of an LCKD supplied via nasogastric tube, called ketogenic enteral nutrition (KEN). KEN is a short-term treatment for obesity developed in our Department of Surgery Paride Stefanini of the University La Sapienza in Rome. The KEN diet is a modified LCKD similar to the protein sparing modified fast (PSMF). In essence, the PSMF diet is a low-calorie diet that is high in protein (1.2 g of protein per kg of ideal body weight) in the form of lean meat, fish, or fowl,[17] i.e., foods that are naturally rich in high quality proteins but low in fats and very low in carbohydrates. Like the PSMF diet, the KEN diet can be performed at home and both diets promote moderate ketosis in all patients. However, the KEN diet differs from the PSMF diet in several key points;[17]
Protein intake is supplemented continuously both day and night via infusion through a small nasogastric tube. This ensures a steady protein intake at all times, avoids the unpleasant taste of less-palatable proteins and allows a more effective control of protein intake.
Daily protein intake is reduced to 50 g for women and 65 g for men (an average of 0.86 g/kg of ideal body weight) considering that we are using only high biological value protein. Furthermore, with a slow-flow tube infusion (2.1-2.7 g of protein/h) we can suppose 100% intestinal absorption.
Carbohydrate intake is completely eliminated; during each treatment cycle patients are permitted to drink only water or tea without sugar.
Length of treatment is reduced to a 10-day cycle. Patients can repeat multiple cycles after a rest period of at least 10 days.
The aim of this study was to evaluate the safety of KEN treatment, patient compliance, and efficacy with regard to weight loss and body composition changes after three subsequent 10-day cycles of KEN treatment.
Methods and procedures
The study was performed within the Clinical Nutrition Service of the Department of Surgery Paride Stefanini at the University La Sapienza in Rome and included obese and overweight patients who did not have success with previous dietetic treatments for obesity.
Exclusion criteria included pregnancy or lactation, type 1 diabetes, renal failure, hepatic failure, heart failure, history of cardiac arrhythmias and/or long QT syndrome, eating disorders and/or severe psychiatric disturbances, and age < 16 or > 75 years. The patients were self-referred and each patient had to sign an informed consent release before the beginning of the treatment. The procedures were in accordance with the Helsinki Declaration of 1975 (as revised in 1983).
The cases analyzed for this study were selected from a very large population of more than 19,000 patients who received KEN treatment during a 5-year period (from 2006 to 2011). From this pool we selected only patients who underwent the first KEN cycle and then came back for 2 more subsequent cycles with rest periods of 10-13 days between each treatment.
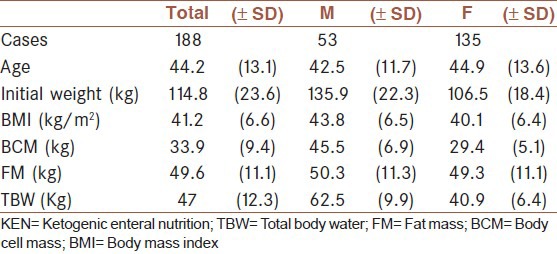
The patients’ ages varied from 16 to 74 years (with a mean age of 44.2 years) [Table 1]. The male:female ratio was 2:5. The average weight at the start of the treatment for all patients was 114.8 kg and was higher for men than women (135.9 kg versus 106.5 kg, respectively). The mean BMI was 41.2 kg/m2 (obesity class 3) and was also higher in the men than in the women (43.8 kg versus 40.1 kg). The men had a very high body cell mass (BCM) compared with the women (45.5 kg versus 29.4 kg), however, the average fat mass (FM) was nearly the same for men and women (50.3 kg versus 49.3 kg).
Table 1.
Baseline anthropometric profiles of the patients by gender. The results are presented as mean (SD)
Each patient had been received a medical visit before the beginning treatment and during the treatment cycles that were domiciliary, a medical doctor (MD) was available by telephone 24 h/day. At the beginning of each cycle the patients received a 6-fr polyurethane tube inserted through the nose. The tube was held in place on the patient's cheek and ear by transparent tape.
The patients were given a short course to educate them about using the infusion pump. The infusion rate was controlled by a small peristaltic pump providing a daily dose of 50 g of protein for women and 65 g for men (resulting in an average of 0.85 g/kg of ideal body weight in women and 0.89 g/kg in men) and 13-17 mEq of potassium [Table 2]. In addition, each patient received a supplement providing the RDA for all vitamins and essential minerals except calcium and phosphorus. They were also given a 50-g dose of Macrogol 4000 to be taken on the first, fourth and seventh days of treatment in order to make up for the absence of fibers in the protein solution.
Table 2.
Composition of the KEN diet. The whey proteins and hydrolyzed collagen in the solution are of bovine origin and potassium chloride was used

We decided to administer the feeding formula via a nasogastric tube for two reasons, namely because the formula is not highly palatable and also providing food continuously throughout the day helped reduce hunger. The treatments were administered over 10 days for every patient as this time period is free from the hazards associated with long-term liquid protein diets.[18,19] During treatment cycles the patients were not allowed to eat anything by mouth, and they were asked to drink only water or other unsweetened beverages ad libitum.
Every day during the cycle the patients were asked to monitor their level of Ketonuria by using the Ketur test. Patients affected by hypertension were suggested to reduce or to stop their usual medication to avoid symptomatic hypotension, to control daily their blood pressure and to contact the MD in case of increa sed blood pressure. Even diabetic patients were suggested to reduce or stop their usual medications to avoid hypoglycemia. They were also asked to monitor glycemia 3 times a day and to contact the MD in case of blood glucose > 160 mg%. Patients affected by gout and/or hyperuricemia were supplied with daily doses of allopurinol.
Measurements of body weight and bioimpedance (using a Handy 3000 DS Medica, single frequency, 50Khz) as well as clinical and laboratory data were taken at the beginning and at the end of the KEN cycles. The body composition was calculated by a computerized program which was provided by the manufacturer, according to the three-compartment model.[20]
After each 10-day KEN treatment, patients returned to the unit for tube removal, to report all treatment data (daily evaluation of hunger level, body weight and ketonuria) and to undergo another body composition evaluation. Between cycles, each patient was given a rest period during which he/she would be advised to follow a low–carbohydrate, normal caloric diet. After this 10-day rest period they returned for a new assessment of body composition before beginning the next treatment cycle.
RESULTS
Ketonuria was observed in most patients by the second day of KEN treatment and increased within 2-3 days to 100-120 mg%. Commonly the Ketonuria was accompanied by moderate halitosis; however, this did not prevent the patients from carrying out normal daily activities.
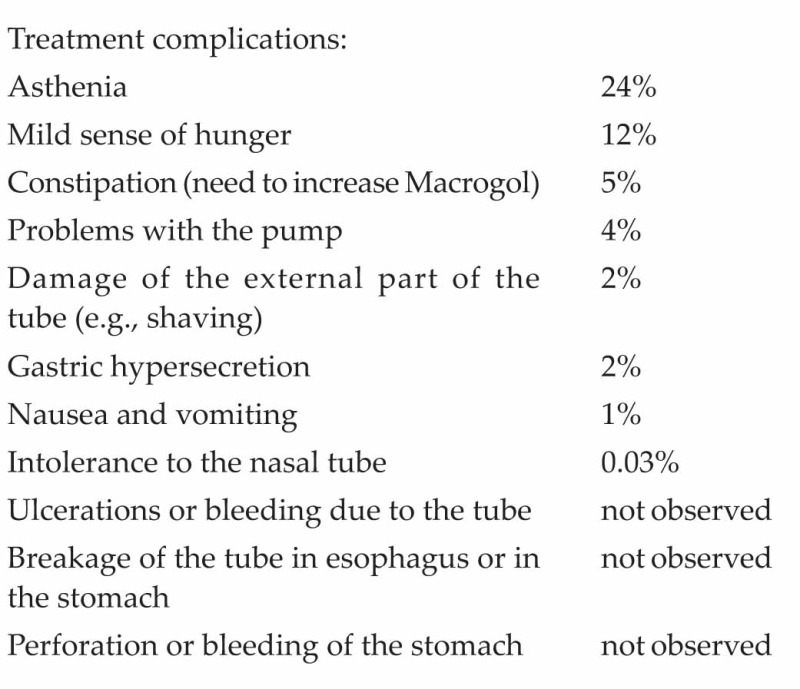
By the fifth day of treatment 24% of patients reported a strong sense of asthenia, even if they had normal blood pressure levels. By the end of the treatment, 12% of patients reported a mild sense of hunger.
Laboratory examinations revealed moderate hyper-cholesterolemia in 35% of the patients at baseline; the values did not change after KEN therapy. Nearly one quarter (22%) of the patients were diabetic and receiving treatment for their condition at the start of treatment. As reported by other authors,[21] 92% of the diabetics in our study had to suspend their medications during the treatments period because the lack of carbohydrate in the nutritional solution was sufficient to lower their glycemia on its own. Of these patients, who suspended anti-diabetic therapy, 22% reported glucose values as low as 50-60 mg/dL, but no cases of hypoglycemia were reported. Similarly, 80% of hypertensive patients also suspended their medication because the KEN treatment had a very powerful anti-hypotensive effect. Reduced hypertension during and after PSMF has been reported by Dornfeld.[22]
After three 10-day KEN cycles, patients lost an average of 14.4 kg of body weight [Figure 1], 3.5 kg of BCM, 10.6 kg of FM, and 2.6 kg of TBW [Figure 2]. The weight loss was higher in male patients (18.1 kg in males versus 12.9 kg in females) but female patients lost a higher percentage of FM (76% in females versus 62% in males). Fat mass decreased not only during KEN cycles but also during the rest periods.
Figure 1.
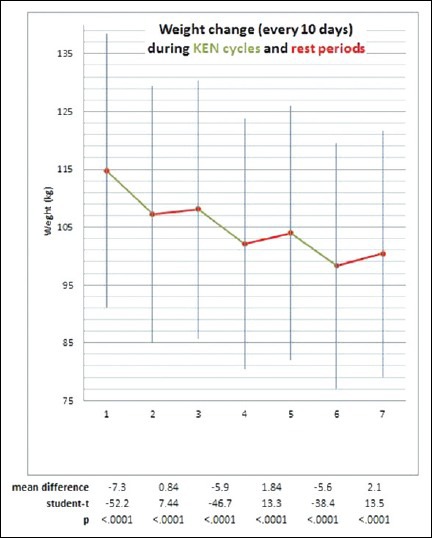
Weight change during 3 cycles of KEN (green) and 3 rest periods (red) of 10-13 days. Total weight loss is 14.4 kg, 12.5% of the initial body weight. Student's paired t-test was used for the statistical analysis
Figure 2.
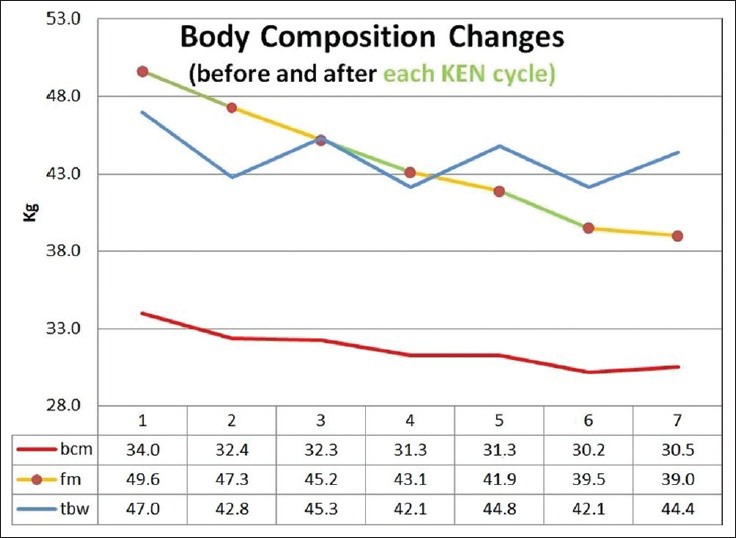
Body composition changes (in kg) after three cycles of KEN therapy and 10 days of follow-up. The patients lost 3.5 ± 3.3 kg of BCM and 10.6 ± 3.7 kg of fat. (KEN-Ketogenic Enteral Nutrition; TBW-Total body water; FM-Fat mass; BCM-Body cell mass)
Treatment complications:
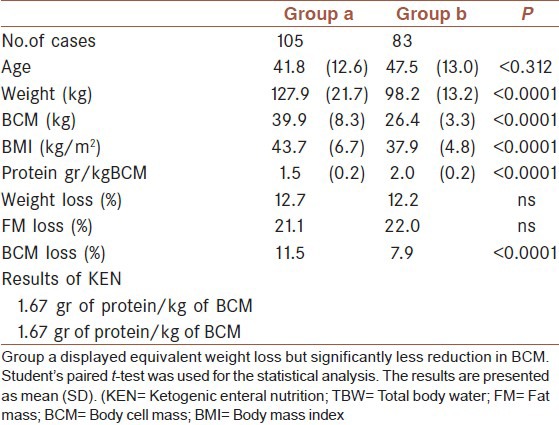
All patients received the same amount of protein during the treatments: 50 g/d for women and 65 g/d for men, corresponding to an average of 1.67 ± 0.3 g of protein/kg of BCM/day. To compare the positive predictive value of loss of fat mass and sparing of BCM, we split the patients into two groups [Table 3]. One group consisting of 105 patients who received less than 1.67 g protein/kg of BCM/day (group A, receiving an average of 1.45 g protein/kg of BCM/day) and a second group of 83 patients who received more than 1.67 g protein/kg of BCM/day (group B, receiving an average of 1.95 g protein/kg of BCM/day). Table 3 shows the results from these two groups. Percent weight loss was similar between the groups, but group B showed significantly less reduction to BCM.
Table 3.
Comparison between patients who were infused with 1.45 g of protein/kg of BCM (Group a) and patients who were infused with 1.9 g of protein (Group b)
DISCUSSION
KEN is a well-tolerated treatment that produces significant, rapid weight loss. This gives the patient a psychological boost because he/she sees immediate results. Few patients were not able to tolerate the nasal tube (0.03%) or had problems with the nutritional pump (4%). This is important because the nasal intubation and pump system are essential to control the intake of proteins during both day and night to avoid the catabolism of lean mass during the treatment period.
After three 10-day KEN cycles, patients lost an average of 12.5% of their initial body weight. Judging from the changes in body composition, loss of FM accounted for 73.9% of the weight loss while BCM loss accounted for 24.1%. Interestingly, while the loss of BCM is only during KEN cycles, the loss of fat continues after the end of KEN therapy. It is unclear why FM continued to decline despite overall body weight increased during the normal caloric feeding period of the 10-day follow-up. One possible explanation for the effect on FM may be that to assess accurately water content after the rapid weight loss we should wait a few days for the stabilization of body compartments. We know that bioimpedance does not measure fat directly; rather, it measures BCM and extracellular water and by subtracting these two values from total body weight the remainder is assumed to be FM. Because of this assumption, any underestimation of body water will be reported as an increase of FM, and the actual reduction of fat will not be accounted for at that time. After the stabilization of water compartments an apparent additional loss of fat will be registered. This mechanism may justify the reported data and account for bioimpedance inaccuracies in patients rapidly gaining or losing FM.
The significant difference in BCM loss in the patients given 1.45 g of protein/kg of BCM compared with those given 1.95 g of protein indicates optimal results can be obtained by infusing each patient with the higher amount of protein proportional to initial BCM.
If we compare the results of this study with other PSMF studies we find that weight loss after 17 weeks of PSMF reported by Palgi[23] was 21 kg versus 14.4 kg in 3 weeks of the present study. Van Gaal[24] results are closer to our results: 15 patients with 6 weeks of PSMF with a lactalbumin derived containing 60 g/day protein lost 14.4 kg. Wadden[25] patients lost around 8 kg in 4 weeks either using a lean meat, fish, and fowl 450 kcal diet or an isocaloric protein-formula-liquid diet. All these authors do not report of any significant clinical complications. Wadden found that a liquid formula diet given by mouth was less tolerated than a food diet. Comparing these PSMF studies with our results we find out that our patients lost more weight in less time and probably this is due to the fact that using a 24 h infusion we could reduce protein intake always sparing BCM mass. This is the main rationale behind the tube infusion.
CONCLUSION
This study demonstrates that KEN therapy administered over 10-day cycles can induce rapid weight loss; in this sample of 188 obese patients we obtained a 12.5% total weight loss, 73.9% of which was FM. Comparing with other PSMF studies weight loss is increased. KEN therapy can also be used to treat diabetic and/or hypertensive patients, but their therapies have to be reformulated during the treatment period. The nasal tube is well-tolerated and complications during the nasal insertion and treatment are very low and generally tolerable.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Aleksandrova K, Boeing H, Jenab M, et al. Metabolic syndrome and risks of colon and rectal cancer: The European prospective investigation into cancer and nutrition study. Cancer Prev Res (Phila) 2011;4:1873–83. doi: 10.1158/1940-6207.CAPR-11-0218. [DOI] [PubMed] [Google Scholar]
- 2.Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. [Google Scholar]
- 3.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 4.Vasiljevic N, Ralevic S, Kolotkin RL, Marinkovic J, Jorga J. The Relationship Between Weight Loss and Health-related Quality of Life in a Serbian Population. Eur Eat Disord Rev. 2012;20:162–8. doi: 10.1002/erv.1114. [DOI] [PubMed] [Google Scholar]
- 5.Akdag R, Danzon M. Istanbul, Turkey: World Health Organization Europe; 2006. [Last accessed on 2013 Jan 24]. European charter on counteracting obesity; pp. 1–5. Available from: http://www.euro.who.int/__data/assets/pdf_file/0009/87462/E89567.pdf . [Google Scholar]
- 6.Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 7.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–81. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 8.Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 9.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403–11. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 10.McClernon FJ, Yancy WS, Jr, Eberstein JA, Atkins RC, Westman EC. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity. 2007;15:182–7. doi: 10.1038/oby.2007.516. [DOI] [PubMed] [Google Scholar]
- 11.Nickols-Richardson SM, Coleman MD, Volpe JJ, Hosig KW. Perceived hunger is lower and weight loss is greater in overweight premenopausal women consuming a low-carbohydrate/high-protein vs high-carbohydrate/low-fat diet. J Am Diet Assoc. 2005;105:1433–7. doi: 10.1016/j.jada.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Hussain TA, Mathew TC, Dashti AA, Asfar S, Al-Zaid N, Dashti HM. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition. 2012;28:1016–21. doi: 10.1016/j.nut.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Dashti HM, Al-Zaid NS, Mathew TC, Al-Mousawi M, Talib H, Asfar SK, et al. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol Cell Biochem. 2006;286:1–9. doi: 10.1007/s11010-005-9001-x. [DOI] [PubMed] [Google Scholar]
- 14.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta regression. Am J Clin Nutr. 2006;83:260–74. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- 15.Volek JS, Sharman MJ, Love DM, Avery NG, Gómez AL, Scheett TP, et al. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism. 2002;51:864–70. doi: 10.1053/meta.2002.32037. [DOI] [PubMed] [Google Scholar]
- 16.Bravata DM, Sanders L, Huang J, Krumholz HM, Olkin I, Gardner CD, et al. Efficacy and safety of low-carbohydrate diets: A systematic review. JAMA. 2003;289:1837–50. doi: 10.1001/jama.289.14.1837. [DOI] [PubMed] [Google Scholar]
- 17.Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: Results in 668 outpatients. Am J Public Health. 1985;75:1190–4. doi: 10.2105/ajph.75.10.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation. 1979;60:1401–12. doi: 10.1161/01.cir.60.6.1401. [DOI] [PubMed] [Google Scholar]
- 19.Lantigua RA, Amatruda JM, Biddle TL, Forbes GB, Lockwood DH. Cardiac arrhythmias associated with a liquid protein diet for the treatment of obesity. N Engl J Med. 1980;303:735–8. doi: 10.1056/NEJM198009253031305. [DOI] [PubMed] [Google Scholar]
- 20.Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77:331–40. doi: 10.1093/ajcn/77.2.331. [DOI] [PubMed] [Google Scholar]
- 21.Bistrian BR, Blackburn GL, Flatt JP, Sizer J, Scrimshaw NS, Sherman M. Nitrogen metabolism and insulin requirements in obese diabetic adults on a protein-sparing modified fast. Diabetes. 1976;25:494–504. [Google Scholar]
- 22.Dornfeld LP, Maxwell MH, Waks AU, Schroth P, Tuck ML. Obesity and hypertension: Long-term effects of weight reduction on blood pressure. Int J Obes. 1985;9:381–9. [PubMed] [Google Scholar]
- 23.Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: Results in 668 outpatients. Am J Public Health. 1985;75:1190–4. doi: 10.2105/ajph.75.10.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr. 1985;41:540–4. doi: 10.1093/ajcn/41.3.540. [DOI] [PubMed] [Google Scholar]
- 25.Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: Protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr. 1985;41:533–9. doi: 10.1093/ajcn/41.3.533. [DOI] [PubMed] [Google Scholar]


