Abstract
Background:
Recently, omega-3 fatty acids are in the center of attention for their potent anti-inflammatory effects. Osteoporosis as a chronic senile disease is associated with inflammation, and the role of inflammatory mediators has been demonstrated in the recent years. The beneficial effects of n-3 fatty acids on bone were proven in many animal studies, while to date, no conclusive data is available in human. The aim of this study was to evaluate the impact of n-3 fatty acids on bone biomarkers in osteoporotic women.
Material and Methods:
Forty osteoporotic post-menopausal women were recruited in the study and randomized in receiving either 40 g canola oil or the same amount sunflower oil per day as their dietary oil for 3 months. Serum levels of osteocalcin, bone alkaline phosphatase (BALP), N telo peptide collagen (NTX) and 25- hydroxy vitamin D3 were measured at baseline and at the end of the third month in both groups.
Results:
In the canola oil group, BALP and NTX were increased after 3 months while Osteocalcin decreased in both groups slightly; however,none of these changes were significant. In both groups, serum vitamin D3 was increased significantly; however, this change between groups was not significant.
Conclusion:
Canola oil did not affect bone formation and resorption significantly after 3 months consumption. Further investigations with longer follow up are recommended.
Keywords: Bone alkaline phosphatase, canola oil, N telo peptide collagen, osteocalcin, osteoporosis, sunflower oil
INTRODUCTION
Osteoporosis is a common skeletal disorder among the elderly. Symptomatic osteoporosis is due to a reduction in bone mineral density (BMD).[1] The World Health Organization defined osteoporosis as a bone density score at or below 2.5 standard deviations (T-score) below normal peak bone values for young adults.[2] Low BMD values are strongly associated with osteoporotic fracture. Approximately 50% of all women suffer from osteoporosis; in these women, BMD values fall progressively with age. Several risk factors contribute to osteoporotic fracture, such as advanced age, low body mass index, previous fracture, muscle weakness and a family history of fracture.[1]
Bone density, which defines bone strength, is a contraction between bone formation and bone resorption. In the bone renewal process, special cells such as osteoclasts and osteoblasts contribute, resulting in production of specific bone resorption and formation markers. From two decades ago, the role of receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin (OPG) pathway have been demonstrated in bone remodeling. RANKL is expressed on osteoclasts and stimulates bone resorption, while osteoblasts produce OPG as a receptor for RANKL, then osteoclast formation is inhibited. Therefore, the balance of RANKL/OPG is considered as a controlling module for bone metabolism.[3,4] Given this fact, every factor affecting this pathway will influence bone-remodeling cycle. Regarding the pathogenesis of osteoporosis, various investigations have demonstrated the association between inflammation, hormones, growth factors, paracrine and autocrine extractions, oxidative stress, and bone fragility.[5,6,7]
Several cytokines influence the balance between the bone-resorbing activity of osteoclasts and the bone-forming activity of osteoblasts. In vitro studies have shown that interleukin (IL)-1 stimulates the differentiation and bone-resorbing activity of osteoclasts.[8,9] IL-1 also affects osteoblasts by inhibiting their bone matrix synthetic activity[10] and stimulating their secretion of IL-6.[11] IL-6, in turn, promotes development of osteoclasts and stimulates bone resorption.[12] Tumor necrosis factor (TNF)-α promotes the fusion of monocytes into osteoclasts while inhibiting differentiation of osteoblasts from progenitor cells.[13]
Over the past two decades, strong evidence supported the benefits of n-3 fatty acids in bone health. Several mechanisms have been proposed; however, neither the exact benefit nor the exact mechanism of action of essential fatty acids was determined. It has been postulated that polyunsaturated fatty acids can alter the production of IL-1, IL-6, and TNF-α by modulating prostaglandin production. Therefore, this modifying effect plays a crucial role in the pathogenesis of osteoporosis. The limited numbers of clinical trials for evaluating the relationship between bone health and n-3 fatty acids forwarded us into designing a randomized clinical trial comparing the effect of canola oil, as a natural source of n-3 fatty acids,[14,15] and sunflower oil, as a natural source of n-6 fatty acids, on bone health in patients with osteoporosis.
MATERIALS AND METHODS
The study was authorized by the ethics committee of the National Nutrition and Food Technology Research Institute, and all of the patients filled up the informed consent. Forty osteoporotic women were entered into the study. All patients’ osteoporosis was diagnosed by dual energy X-ray absorptiometry at femur neck and lumbar vertebrae. Their profile was assessed to obtain information about age, smoking, alcohol consumption, and past medical history. Patients with history of malignancies, heart disease, liver disease, renal failure, hyperlipidemia, obesity, diabetes, acute infection, endocrinologic disorders, drug history of corticosteroids, hormones, GnRH analogs, anti-convulsive drugs, heparin, aluminum-containing antacids, thyroid hormones, and smoking or alcohol consumption were excluded.
After a 2-wk run-in period, the subjects were randomly assigned to groups receiving either 40 g canola oil or 40 gr sunflower oil as their dietary oil. Fatty acid concentrations in the oils were determined by a specialized laboratory accredited by the Deputy of Food and Drug of the Iranian Ministry of Health. All patients were advised a normal diet with seafood less than 3 servings per week, not to consume much dairy products, and seriously not to change the food regimen until the end of the study. Both oils were similar in taste, texture, and appearance. The patients were instructed to consume oils for 3 months. Also, they were recommended to inform us upon any changes in their medication used or any lifestyle changes.
At first visit, the objectives and the protocol of the study were fully described for the subjects before they signed an informed written consent form. Then, a general questionnaire on demographic data, medical and drug history was completed. All of the subjects were visited at approximately biweekly intervals to assess their compliance and to deliver the oils for the next 2 weeks.
All of the participants were also given a consumption instruction manual including “oils consumption table” that consisted of empty boxes for each week. They were also asked to keep the empty bottles and to bring them back on their next visit. Compliance was assessed by checking the consumption tables, counting the empty bottles, and making weekly phone calls. Dietary (24-h recall), anthropometric, and laboratory assessments were done for all of the participants before (baseline) and after the intervention period.
Diet
The participants were instructed to follow a weight-maintenance normal diet for 2 wk (run-in period), then their dietary oil were replaced by either canola oil or sunflower oil by a dietitian. Dietary intake was assessed at the beginning and at the end of the intervention period by using a validated 24-h recall questionnaire[16] for 2 days (including a weekend).
Anthropometric measures and blood pressure were measured by using a digital scale to the nearest of 0.1 kg (Seca 808; Seca, Hamburg, Germany) while the participants were wearing light clothing and no shoes. Height was determined with a stadiometer to the nearest of 0.1 cm (Seca). BMI was calculated as weight (kg)/height2 (m). WC was measured at the midpoint between the lower rib and iliac crest at the end of expiration by using a measuring tape to the nearest of 0.1 cm. Blood pressure was measured with a digital system (BC 08; Beurer, Ulm, Germany), whereas the subject was seated for 10 min. The average of duplicate measurements was considered for blood pressure whereas the subjects were fasting and not allowed to smoke.
Laboratory investigations
Blood samples were collected at baseline, and post-treatment. At these two time occasions, in the early morning and fasting state, blood samples were collected and kept at room temperature for 30-60 min. The samples were then centrifuged at 2000 g at room temperature. Sera recovered and transferred to fresh micro tubes in aliquots and kept at -80°C until the day of analysis. Serum levels of bone biomarkers such as osteocalcin (OC), bone alkaline phosphatase (BALP), N telo peptide collagen (NTX), as well as serum 25-hydroxy vitamin D3 were measured. Serum OC, BALP and NTX were measured by enzyme-linked-immunosorbent serologic assay (ELISA) (Cusabio, Japon), serum vitamin D3 was measured by Immunodiagnostic Systems Ltd (IDS Ltd), (Boldon, UK), In this study, vitamin D3 status was defined on the basis of serum concentrations of 25 (OH) D3 as sufficient (>50 nmol/L), insufficient (27.5 to, 50 nmol/L), and deficient (<27.5 nmol/L).[17]
Statistical analyses
Data are expressed as means ± SDs. The normality of data distribution was assessed by using the Kolmogorov-Smirnov goodness-of-fit test. Two-factor repeated-measures analysis of variance (ANOVA) was used to test time group interactions, with time and treatment as factors. In case of a significant time group interaction, a between group comparison of changes at 12mo was done by using ANOVA followed by Tukey's post hoc analysis. When the time effect was significant, the within-group comparison of values was performed by paired-samples t test with Bonferroni correction. Differences in proportions were evaluated by using a Man Whitney test. Correlations between variables were evaluated by using either Pearson (r) (for data with normal distribution) or Spearman (rs) (for data with non-normal distribution) correlations. All statistical analyses were done by using the Statistical Package for Social Sciences (SPSS version 16; SPSS Inc, Chicago, IL). P, 0.05 was considered significant.
RESULTS
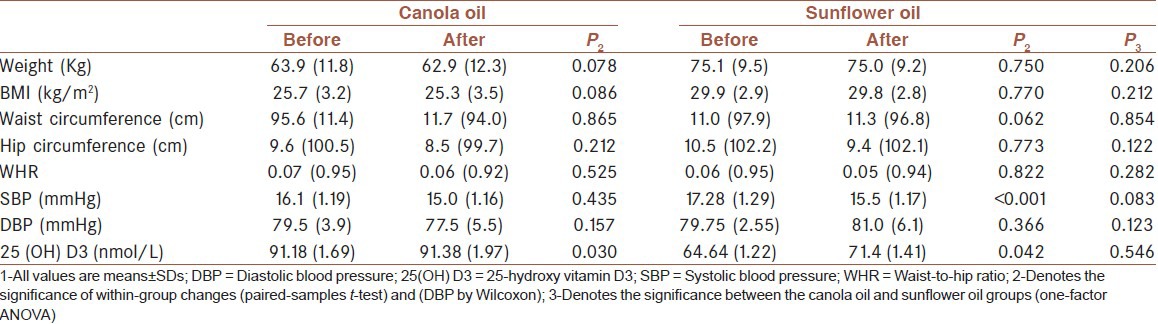
We enrolled a total of 40 osteoporotic women in the study aged 50.7 ± 6.1 y. All participants completed the study, and overall compliance by the subjects was estimated as being 100%. None of these women smoked. The demographic characteristics of two groups’ patients who completed the study are shown in Table 1.
Table 1.
The demographic characteristics of two group patients
Deficiency is defined as, 27.5 nmol/L, insufficiency as 27.5 to, 50 nmol/L, and sufficiency as 50 nmol/L. 2 Denotes the significance of differences in the distribution of vitamin D categories between the 2groups (Mann-Whitney test).
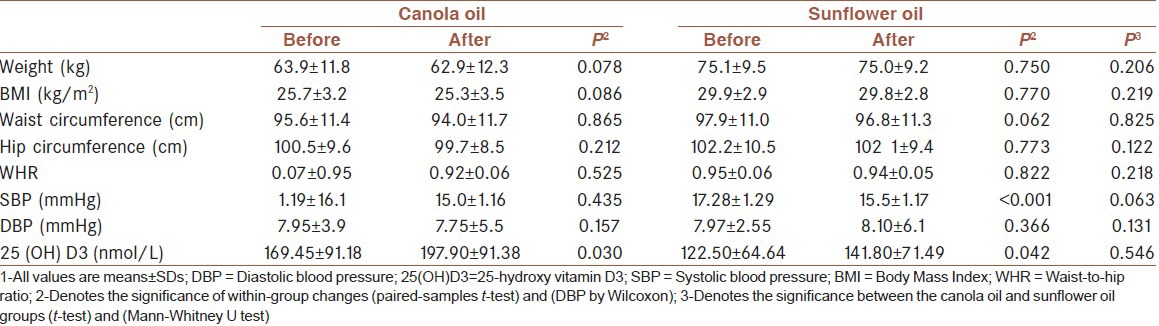
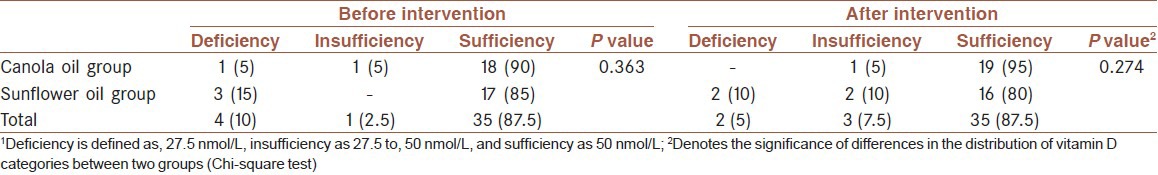
Suboptimal vitamin D status was observed in 10% of the subjects at baseline in canola group and 15% in sunflower group at the end of the intervention. In the canola group, however, the percentage of the suboptimal vitamin was 5% and in the sunflower group 20%. In the two groups, however, the vitamin D status did not change significantly from the beginning to the end of the study in both groups [Table 2].
Table 2.
Characteristics of patients at baseline and after the intervention[1]
Deficiency is defined as, 27.5 nmol/L, insufficiency as 27.5 to, 50 nmol/L, and sufficiency as 50 nmol/L. 2 Denotes the significance of differences in the distribution of vitamin D categories between the 2 groups (Chi-square test) [Table 3].
Table 3.
Comparison of vitamin D status based on serum concentrations of 25.hydroxy vitamin D3 between two groups after the intervention[1]
Changes of bone formation and resorption markers in 3 months:
No significant changes were observed in serum level of bone formation markers. BALP increased in canola oil group non-significantly. the changes of OC and N telo peptide collagen (NTX) level were not significant across the two groups (P > 0.05, Tables 4 and 5).
Table 4.
Baseline and final bone formation markers and comparison of changes within and between groups after the intervention[1]
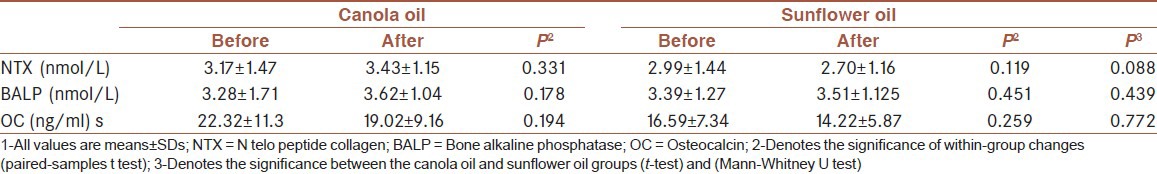
Table 5.
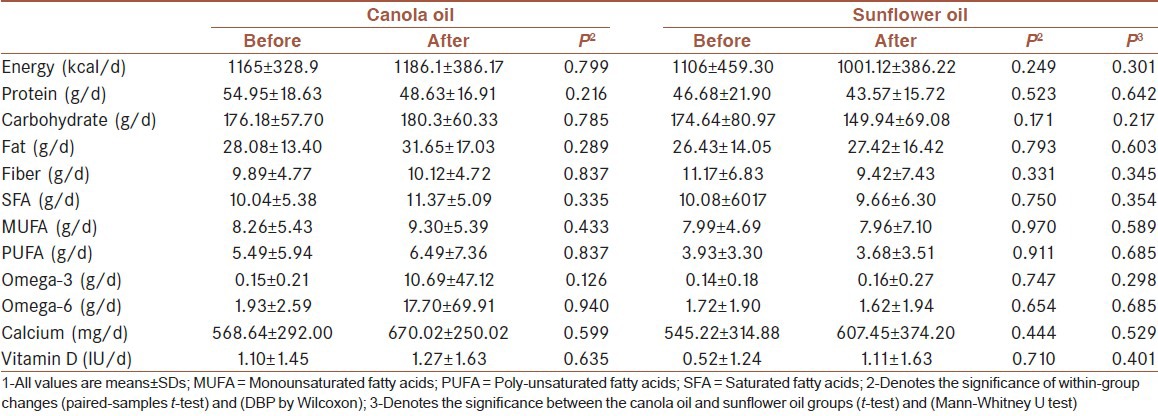
Comparison of changes within and between groups macronutrient and micronutrient intake after the intervention[1]
DISCUSSION
While the importance of the essential fatty acids in bone health and calcium homeostasis was confirmed in several animal studies,[7] to date, no conclusive data is available in human. Although, our study demonstrated that canola oil can increase BALP and NTX while decreasing Osteocalcin, these alterations were not significant. Then, adjustment for serum 25(OH)D3), these alterations were not significant. Recent studies evaluating the effect of n-3 fatty acids on bone formation have shown controversial results[18,19] indicating the type and amount of poly-unsaturated fatty acids (PUFAs) influence bone formation in animal models and osteoblastic cell functions in culture. In growing rats, supplementing the diet with omega-3 PUFA resulted in greater bone formation rates and moderates ex vivo prostaglandin E(2) production in bone organ cultures, and increased alkaline phosphatase activity. Experiments with mouse calvarial origin (MC3T3-E1) osteoblast-like cells support findings in vivo where omega-3 PUFA modulated cycloxygenase-2 (COX-2) protein expression, reduced prostaglandin E(2) production, and increased alkaline phosphatase activity.[20] Tumor necrosis factor-α, a cytokine that may promote bone resorption, is stimulated by PGE2.[21] Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) supplementation in the form of fish oil reduce tumor necrosis factor-α by 70-77% and PGE2 by 28-55%, depending on the linoleic acid (LA) and alpha-linolenic acid (ALA) content of the diet.[22] On the other hand, PGE2 decreases osteoprotegerin production and increases receptor activator of nuclear transcription factor κB ligand (RANKL) expression. This action lowers the osteoprotegerin: Receptor activator of nuclear transcription factor-κB ligand ratio, which is critical in the pathogenesis of resorptive bone disease.[23]
It is hypothesized that after 20 weeks, an increase in omega-3 intake can increase bone mineral content and cortical + subcortical BMD, bone-specific alkaline phosphatase activity and increasing, 1,25-(OH) 2 vitamin D3.[24] Our studies had a short intervention period (12 weeks) that was limitation. Another study showed, n-3 fatty acids supplement can decrease bone resorption urine concentration of pyridinoline (Pyd)); however, could not affect bone formation (serum bone-specific alkaline phosphatase and osteocalcin) significantly after 6 months in osteoporotic post-menopausal women.[25]
Recent evidence has shown that consumption of whole flaxseeds (predominantly ALA) did not lead to a marked improvement of osteoporotic bones in humans and animals. However, when combined with estrogen therapy, flaxseed supplementation offered an extra benefit to bone in animal models. Similar results were found in studies conducted with flaxseed oil.[26]
It has been shown that the relative amounts of dietary poly-unsaturated fatty acids may play a crucial role in maintaining skeletal integrity in the aged patients.[27] Another study showed significant increase in serum OC level in the fish oil patients receiving 4 g/day of fish oil after 16 weeks in comparison to the evening primrose oil group, while BALP decreased significantly in the fish oil group.[28] It was observed that maintaining lumbar bone mass and increasing femoral bone mass in post-menopausal women is available by consuming poly-unsaturated fatty acids for 18 months that accompanied with decreasing OC and deoxypyridinoline levels in both treatment and control groups.[29] A significant decrease in the total body BMD in both treatment and control groups after consumption of 440 mg fish oil or calcium for 1 year in the healthy post-menopausal women has been reported while they showed significant decreases in the serum markers of bone formation (OC and BALP) without any significant change in urine N-telopeptide (NTx) concentration in both groups.[30]
Greater BMD loss in the femoral neck was associated with increased intake of poly-unsaturated fatty acids in another study.[31] In renal transplant patients, a link was found between plasma phospholipid n-3 poly-unsaturated fatty acids content and BMD.[32] Although, no change in levels of BALP in n-3 fatty acid consuming group was found, a significant decrease in NTx levels was evident.[33] Some recent studies indicate a positive relationship between n-3 fatty acids especially DHA and peak BMD in young men.[34] Recently, the influence of moderate energy restriction and seafood on bone turnover was evaluated, and a significant decrease in serum OC during 8 weeks in young adults was reported while serum level of BALP did not change significantly. In addition, a significant increases in the bone resorption markers (N-telopeptides of type 1 collagen in urine and C-terminal telopeptide of type 1 collagen) was found.[35] Some recent studies indicate the addition of DHA to oral calcium, vitamin D (3) for 12 months by osteopenic individuals no effect demonstrated on serum c-terminal telopeptides.[36]
Some other investigators reported negative impact of poly-unsaturated fatty acids on bone as increased risk of fractures in elderly population.[37] A large observational study reported in post-menopausal women with a low intake of marine n-3 FAs, a higher intake of n-6 FAs may modestly decrease total fracture risk.[38] because the mean EPA + DHA consumption was 0.13 g/d, considerably less than the ≈ 0.5 g/d minimally recommended by many global organizations.[39] There was little variation in the range of EPA + DHA consumed by this studies, which made it difficult to examine associations with higher marine n – 3 FA intakes and fracture. Interestingly, women who consumed the most EPA + DHA consumed the least calcium and vitamin D.
Comparing our results with other studies, our results are similar to some of the studies, while those are not conclusive and mostly confirm the beneficial effects of n-3 fatty acids on bone by decreasing bone resorption. It has been presumed that n-3 fatty acids can affect bone via different mechanisms (by affecting bone formation, bone resorption, serum calcium and vitamin D, and inflammatory mediators), but the exact mechanism of action has not been determined yet. This is because of lack of enough clinical trial studies that mostly have been performed by food questionnaire, and no data is present about the dose of n-3 fatty acids. In our study, forty gram canola oil per day could not affect bone formation and resorption markers significantly, which might be related to low dose and short duration of exposure.
Study limitations
Time shortage was one of our study limitations. Our study was done in 3 months, and it seems more reasonable to design a study with a longer duration of at least 1 to 2 years to evaluate the changes of BMD in parallel with bone markers, which can give us more reliable results. In addition, it was shown that about 6-7 weeks after resorption, osteoblast enrollment occurs.[40] Also, our study lacks data on BMD and physical activity; however, the sample size seems to be insufficient. Insufficient resources forced us into a limited sample size with limited numbers of bone markers.
Therefore, it seems reasonable to perform a multicenter clinical trial in a vast majority of subjects in a longer period of time with performing the survey on inflammatory mediators and their relationship with bone markers.
ACKNOWLEDGMENT
This study was supported by a grant from National Nutrition and Food Technology Research Institute. OILA Company provided the canola and sunflower oils. Authors wish to thank the physicians and nurses who helped in sampling and technicians who assisted in measurements.
Footnotes
Source of Support: Supported by the National Nutrition and Food Technology Research Institute. The oils were produced and donated by the Oila Company of Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Stewart TL, Ralston SH. Role of genetic factors in the pathogenesis of osteoporosis. J Endocrinol. 2000;166:235–45. doi: 10.1677/joe.0.1660235. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy. JAMA. 2001;285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvestrini G, Ballanti P, Patacchioli F, Leopizzi M, Gualtieri N, Monnazzi P, et al. Detection of osteoprotegerin (OPG) and its ligand (RANKL) mRNA and protein in femur and tibia of the rat. J Mol Histol. 2005;36:59–67. doi: 10.1007/s10735-004-3839-1. [DOI] [PubMed] [Google Scholar]
- 5.Abdollahi M, Larijani B, Rahimi R, Salari P. Role of oxidative stress in osteoporosis. Ther. 2005;2:787–96. [Google Scholar]
- 6.Yousefzadeh G, Larijani B, Mohammadirad A, Heshmat R, Dehghan G, Rahimi R, et al. Determination of oxidative stress status and concentration of TGF-beta 1 in the blood and saliva of osteoporotic subjects. Ann N Y Acad Sci. 2006;1091:142–50. doi: 10.1196/annals.1378.062. [DOI] [PubMed] [Google Scholar]
- 7.Salari P, Rezaie A, Larijani B, Abdollahi M. A systematic review of the impact of n-3 fatty acids in bone health and osteoporosis. Med Sci Monit. 2008;14:37–44. [PubMed] [Google Scholar]
- 8.Ralston SH, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20:2492–506. doi: 10.1101/gad.1449506. [DOI] [PubMed] [Google Scholar]
- 9.Jimi E, Nakamura I, Duong LT, Ikebe T, Takahashi N, Rodan GA, Suda T. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp Cell Res. 1999;247:84–93. doi: 10.1006/excr.1998.4320. [DOI] [PubMed] [Google Scholar]
- 10.Evans DB, Bunning RA, Russell RG. The effects of recombinant human interleukin-1 beta on cellular proliferation and the production of prostaglandin E2, plasminogen activator, osteocalcin and alkaline phosphatase by osteoblast-like cells derived from human bone. Biochem Biophys Res Commun. 1990;166:208–16. doi: 10.1016/0006-291x(90)91932-i. [DOI] [PubMed] [Google Scholar]
- 11.Lacey DL, Grosso LE, Moser SA, Erdmann J, Tan HL, Pacifici R, et al. IL-1-induced murine osteoblast IL-6 production is mediated by the type 1 IL-1 receptor and is increased by 1,25 dihydroxyvitamin D3. J Clin Invest. 1993;91:1731–42. doi: 10.1172/JCI116383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–11. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 13.Nanes MS. Tumor necrosis factor-α: Molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 14.Morris DH. Methodologic challenges in designing clinical studies to measure differences in the bioequivalence of n-3 fatty acids. Mol Cell Biochem. 2003;246:83–90. [PubMed] [Google Scholar]
- 15.Bourre JM. Dietary omega-3 fatty acids for women. Biomed Pharmacother. 2007;61:105–12. doi: 10.1016/j.biopha.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Kalantari N, Ghafari M. Iran, 2001-2003 (na-tional report) Tehran, Iran: Nutrition and Food Science Institute; 2005. National comprehensive study on household food consumption pattern and nutritional status. [Google Scholar]
- 17.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial US adolescent population: The National Health and Nutrition Examination Survey III. Pediatrics. 2009;123:797–803. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]
- 18.Shen CL, Yeh JK, Rasty J, Li Y, Watkins BA. Protective effect of dietary long-chain n-3 polyunsaturated fatty acids on bone loss in gonad-intact middle-aged male rats. Br J Nutr. 2006;95:462–8. doi: 10.1079/bjn20051664. [DOI] [PubMed] [Google Scholar]
- 19.Watkins BA, Li Y, Seifert MF. Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: Actions on bone mineral and serum biomarkers in ovariectomized rats. J Nutr Biochem. 2006;17:282–9. doi: 10.1016/j.jnutbio.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Watkins BA, Li Y, Lippman HE, Feng S. Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68:387–98. doi: 10.1016/s0952-3278(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 21.Griel AE, Kris-Etherton PM, Hilpert KF, Zhao G, West SG, Corwin RL. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr J. 2007;6:2. doi: 10.1186/1475-2891-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish. Am J Clin Nutr. 1996;63:116–22. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 23.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/rankl/rank system for bone and vascular diseases. JAMA. 2004;292:490–5. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 24.Shen CL, Yeh JK, Rasty J, Li Y, Watkins BA. Protective effect of dietary long-chain n-3 polyunsaturated fatty acids on bone loss in gonad-intact middle-aged male rats. Br J Nutr. 2006;95:462–8. doi: 10.1079/bjn20051664. [DOI] [PubMed] [Google Scholar]
- 25.Salari Sharif P, Asalforoush M, Ameri F, Larijani B, Abdollahi M. The effect of n-3 fatty acids on bone biomarkers in Iranian postmenopausal osteoporotic women: A randomized clinical trial. Age (Dordr) 2010;32:179–86. doi: 10.1007/s11357-009-9122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, Ilich JZ. Implications of dietary α-linolenic acid in bone health. Nutrition. 2011;27:1101–7. doi: 10.1016/j.nut.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Weiss LA, Barrett-Connor E, von Muhlen D. Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: The Rancho Bernardo Study. Am J Clin Nutr. 2005;81:934. doi: 10.1093/ajcn/81.4.934. [DOI] [PubMed] [Google Scholar]
- 28.Papendrop DH, Coetzer H, Kruger MC. Biochemical profile of osteoporotic patients on essential faty acid supplementation. Nutr Res. 1995;15:325. [Google Scholar]
- 29.Kruger MC, Coetzer H, de Winter R, Gericke G, van Papendorp DH. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging (Milano) 1998;10:385–94. doi: 10.1007/BF03339885. [DOI] [PubMed] [Google Scholar]
- 30.Bassey EJ, Littlewood JJ, Rothwell MC, Pye DW. Lack of effect of supplementation with essential fatty acids on bone mineral density in healthy pre- and postmenopausal women: Two randomized controlled trials of Efacal v. Calcium alone. Br J Nutr. 2000;83:629–35. doi: 10.1017/s0007114500000805. [DOI] [PubMed] [Google Scholar]
- 31.Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: Evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. 2004;79:155–65. doi: 10.1093/ajcn/79.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Baggio B, Budakovic A, Ferraro A, Checchetto S, Priante G, Musacchio E, et al. Relationship between plasma phospholipid polyunsaturated fatty acid composition and bone disease in renal transplantation. Transplantation. 2005;80:1349–52. doi: 10.1097/01.tp.0000179152.57167.c1. [DOI] [PubMed] [Google Scholar]
- 33.Griel AE, Kris-Etherton PM, Hilpert KF, Zhao G, West SG, Corwin RL. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr J. 2007;6:2. doi: 10.1186/1475-2891-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogstrom M, Nordstrom P, Nordstrom A. n-3 fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: The NO2 study. Am J Clin Nutr. 2007;85:803–7. doi: 10.1093/ajcn/85.3.803. [DOI] [PubMed] [Google Scholar]
- 35.Lucey AJ, Paschos GK, Cashman KD, Martinez JA, Thorsdottir I, Kiely M. Influence of moderate energy restriction and seafood consumption on bone turnover in overweight young adults. Am J Clin Nutr. 2008;87:1045–52. doi: 10.1093/ajcn/87.4.1045. [DOI] [PubMed] [Google Scholar]
- 36.Vanlint SJ, Ried K. Efficacy and tolerability of calcium, vitamin D and a plant-based omega-3 oil for osteopenia: A pilot RCT. Maturitas. 2012;71:44–8. doi: 10.1016/j.maturitas.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Ramirez MJ, Palma S, Martinez-Gonzalez MA, Delgado-Martinez AD, de la Fuente C, Delgado-Rodriguez M. Dietary fat intake and the risk of osteoporotic fractures in the elderly. Eur J Clin Nutr. 2007;61:1114–20. doi: 10.1038/sj.ejcn.1602624. [DOI] [PubMed] [Google Scholar]
- 38.Orchard TS, Cauley JA, Frank GC, Neuhouser ML, Robinson JG, Snetselaar L, et al. Fatty acid consumption and risk of fracture in the Women's Health Initiative. Am J Clin Nutr. 2010;92:1452–60. doi: 10.3945/ajcn.2010.29955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gebauer SK, Psota TL, Harris WS, Kris-Etherton PM. n – 3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006;83:1526 S–35 S. doi: 10.1093/ajcn/83.6.1526S. [DOI] [PubMed] [Google Scholar]
- 40.Eriksen EF, Gundersen HJ, Melsen F, Mosekilde L. Reconstruction of the formative site in iliac trabecular bone in 20 normal individuals employing a kinetic model for matrix and mineral apposition. Metab Bone Dis Relat Res. 1984;5:243–52. doi: 10.1016/0221-8747(84)90066-3. [DOI] [PubMed] [Google Scholar]


