Abstract
Background:
Myocardial infarction (MI) is the acute condition of necrosis in myocardium which occurs as a result of imbalance between coronary blood supply and myocardial demand. The resultant oxidative stress excess leads to worsen the condition. The aim of this study was to investigate the effect of mebudipine, a new dihydropyridine calcium channel blocker, on lipid peroxidation and antioxidant enzymes in myocardial ischemia-reperfusion injury.
Materials and Methods:
Male Wistar rats (250-300 g) were randomly divided to Control-ischemic, mebudipine-ischemic and vehicle (ethanol-ischemic) groups. The hearts of anaesthetized rats were removed and mounted on Langendorff apparatus and perfused by Krebs-Henseleit solution under constant pressure of 75 mmHg at 37°C. Ischemic groups were received 30 min global ischemia and 120 min reperfusion and the mebudipine and vehicle groups received mebudipine (0.1 nM) or ethanol (0.01%)-enriched solution 25 min before global ischemia. Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase levels of heart tissue samples were determined by commercial specific Kits.
Results:
Mebudipine significantly reduced the MDA level (2.3 ± 0.07 nmol/mg protein) as the biochemical indicator of oxidative damage and lipid peroxidation product as compared with those of vehicle (4.6 ± 0.01 nmol/mg protein) and control groups (4.8 ± 0.09 nmol/mg protein). Furthermore, antioxidant enzymes SOD (0.1 ± 0.006 in drug vs. 0.037 ± 0.009 U/mg Protein in control), GPX (16 ± 0.009 in drug vs. 0.068 ± 0.01 U/mg Protein in control) and catalase activities (0.075 ± 0.006 in drug vs. 0.028 ± 0.002 U/mg Protein in control), activities of myocardium were significantly increased by mebudipine (P < 0.01).
Conclusion:
Our results showed that mebudipine may have antioxidant activity against myocardial ischemia-reperfusion injury since it decreased oxidative stress by enhancing the enzymatic antioxidant defense and inhibiting the lipid peroxidation. Thus, this drug can reduce the intensity of cardiac ischemic insults.
Keywords: Ischemia, lipid peroxidation, mebudipine, myocardial, oxidative stress, reperfusion
INTRODUCTION
Myocardial infarction (MI) is the acute condition of necrosis of myocardium that occurs as a result of imbalance between coronary blood supply and myocardial demand.[1,2] Reperfusion may worsen the myocardial injury beyond that generated by ischemia alone. This results in a spectrum of reperfusion-associated pathologies, collectively called ischemia- reperfusion (IR) injury.[3] Several therapeutic methods and strategies such as induction of preconditioning, post-conditioning and pharmacological agents have been examined to prevent and limit the IR injury, however, the suitable treatment modalities have not been fully proved yet. Several pathophysiological mechanisms of IR injury have been suggested including an overproduction of oxygen-derived free radicals.[4] Also intracellular Ca2+ overload or redistribution during the first minutes of reflow might be involved.[2] Hypercontracture due to calcium-overload is a candidate responsible for reperfu sion injury pathogenesis.[3] It is well recognized that ischemic tissue generates oxygen-deprived free radicals and other products which cause oxidative damage of membrane lipids, proteins, carbohydrates and DNA, leading to qualitative alteration of myocardium.[5] Large quantities of free radicals cause an overwhelming of body's endogenous antioxidant defenses. This leads to peroxidation of lipid membranes and loss of membrane integrity followed by necrosis and cell death.[6]
Ca2+ channel blockers are used for a variety of diseases including cardiac arrhythmia, hypertension and have also become established as therapeutic drugs for angina pectoris, together with β-adrenoceptor antagonists and nitrates.[7,8] Ca2+ channel blockers have several features that may relate to myocardial protection during ischemia and reperfusion. Ca2+ channel blockers decrease oxygen demand due to decrease in heart rate and myocardial contractility.[7,8] Interference of these agents with neutrophil mobilization and activation may prevent the production of free radicals and the release of proteolytic enzymes.[9] A direct protective effect may also be produced by preventing the calcium entry to cardiac cell in ischemia-induced intracellular Ca2+ overload.[10,11]
Several researchers have described the antioxidant properties of Ca2+ channel blockers as being due to either a direct scavenging effect or the preservation of the superoxide dismutase (SOD) and glutathione peroxidase (GPX) activity.[12] Also Ca2+ channel blockers may inhibit lipid peroxide formation at concentrations present in plasma.[12] Mebudipine is a new calcium channel blocker with a similar structure to dihydropyridine compounds which has comparable pharmacological effect while offering some advantages such as longer biological half time to reach peak effect and vasoselectivity.[13,14] Since the physiological functions of the heart is mainly dependent on a remarkable consumption of oxygen, so preventing the production of superoxide radicals during ischemia- reperfusion injury could be considered as a possible therapeutic interventions. As we know there is no report on the antioxidant activity of mebudipine so in the present study, we have examined the effect of mebudipine on oxidative stress and lipid peroxidation levels in myocardial ischemic-reperfusion injury in male rat.
MATERIALS AND METHODS
Animals
Thirty male Wistar rats (250-300 g) were purchased from laboratory animal house of Tabriz University of Medical Sciences. They were housed in an animal room at 22-24°C and given free access to commercial rat chow and tap water. All the experimental procedures employed, as well as rat care and handling was in accordance with guidelines provided by the Animal Care Committee of the Tabriz University of Medical Sciences. The animals were randomly divided in three groups (n = 10): Control group (ischemia without drug), drug group (ischemia with mebudipine 0.1 nM) and vehicle group (ischemia with ethanol 0.01%).
Isolated heart protocol
All animals were anesthetized with sodium pentobarbital (60 mg/kg i.p) and heparinized with sodium heparin (300 IU, i.p). After opening the chest cavity, the hearts were quickly excised and immersed in ice-cold krebs-Henseliet (K-H) solution. Then, the aorta was cannulated and the heart was retrogradely perfused via the aortic cannula in a Langendorff apparatus with K-H solution (PH = 7.4) containing: 118 mM Nacl, 4.8 mM KCl, 1.2 mM MgSO4, 1.0 mM KH2 PO4, 27.2 mM NaHCO3, 10 mM glucose and 1.25 mM CaCl2. The perfusate was bubbled with a mixture of 95% O2, and 5% CO2. Perfusate and bath temperatures were maintained at 37°C by thermostatically controlled water circulator (Satchwell Sunvic LTD). The heart was perfused at constant mean pressure of 75-80 mmHg.
Ischemia-reperfusion protocols
The isolated hearts were allowed to equilibrate for 20 min prior to each study. For ischemic-control group, the hearts were perfused with K-H solution for 20 min, and then global ischemia was conducted by interrupting the aortic flow for 30 min followed by reperfusion with K-H solution up to 120 min. In the drug and vehicle groups, before ischemia the hearts were perfused with mebudipine (0.1 nm) or ethanol (0.01%)-enriched solution for 25 min, respectively.
Preparing the drug
A dose-response study was conducted to determine the cardioprotective dose of mebudipine. For studying cardioprotective effect of an agent, a dose of agent should be administered which has no inotropic and chronotropic effects in normal hearts.[15,16,17] According to our preliminary report, mebudipine at concentration 0.1 nm was selected and examined throughout the study. Mebudipine was dissolved in 0.01% ethanol.
Tissue processing and homogenate preparation
At the end of experiments, left ventricles of hearts were dissected, weighed and quickly frozen in liquid nitrogen. For antioxidant activities measurement, cardiac homogenates were prepared at -4°C as described by Rothermel et al.[18] In brief, fifty milligrams of ventricle muscle was homogenized in 1 ml of ice-cold lysis buffer (10 mM NaCl, 1.5 mM MgCl2, 20 mM HEPES, 20% glycerol, 0.1% Triton X-100, 1 mM dithiothreitol, pH 7.4). The homogenates were centrifuged at 1000 rpm for 1 min at 4°C. The supernatant containing the cytoplasmic protein fraction was collected and a protease inhibitor cocktail (104 mM AEBSF, 0.08 mM aprotinin, 2 mM leupeptin, 4 mM bestatin A, and 1.4 mM E-64) (Sigma-Aldrich, St Louis, MO) was added to it and stored at -80°C until use. Protein concentration of supernatant was estimated using Bradford technique.[19]
Lipid peroxidation measurement
Lipid peroxides are unstable and decompose to form a series of compounds including reactive carbonyl compounds. Polyunsaturated fatty acid peroxides generate malondialdehyde (MDA) and its measurement has been used as indicator of lipid peroxidation.[12]). Lipid peroxidation is analyzed by measuring thiobarbituric acid-reactive substances (TBARS) in homogenates, as previously described by Draper and Hadley.[19] Briefly, the samples (250 μL) were mixed with 1 mL 10% trichloroacetic acid (TCA) and 1 mL of 0.67% thiobarbituric acid. Then samples were heated in a boiling water bath for 15 min and n-butyl-alcohol (2:1 v:v) were added to the solution. After centrifugation (800 g, 5 min), TBARS were determined from the absorbance at 535 nm, using a spectrophotometer (Pharmacia Biotech; England).
Enzymatic antioxidant activities
Superoxide dismutase (SOD) activity was determined using a RASOD kit (Randox Crumlin, UK) according to Delmas-Beauvieux et al.[20] SOD activity was measured at 505 nm by a spectrophotometer (Pharmacia Biotech; England). In this method, xanthine and xanthine oxidase were used to generate superoxide radicals that react with 2-(4-iodophenyl)-3 (4-nitrophenol)-5-phenyl tetrazolium chloride (ITN) to form a red formazan dye. Concentrations of substrates were 0.05 mmol/L for xanthine and 0.025 mmol/L for ITN. SOD activity was measured by the degree of inhibition of this reaction. After calculating the percent of inhibition by using related formula, SOD activity value was calculated by comparing with the standard curve and was expressed as U/mg protein.
Glutathione peroxidase (GPX) activity was determined using a RANSEL kit (Randox Crumlin, UK) according to the method of Paglia and Valentine.[21] GPX catalyses the oxidation of glutathione (at a concentration of 4 mmol/L) by cumene hydroperoxide. In the presence of glutathione reductase (at a concentration ≥ 0.5 units/L) and 0.28 mmol/L of NADPH, oxidized glutathione is immediately converted to the reduced form with concomitant oxidation of NADPH to NAD+. The decrease in absorbance at 340 nm (37°C) was measured using a spectrophotometer (Pharmacia Biotech; England), and then GPX concentration was calculated from the following formula:
GPX U/L of sample = 8412 × ΔA 340 nm/min
ΔA = difference of blank value from sample value
GPX U/mg protein = GPX U/ml/protein concentration/ml.
Catalase activity was measured using the Aebi method.[22] According to this method, measurement was performed based on dissociation rate of H2O2 in 240 nm at 20°C. Myocardial homogenate aliquots were centrifuged at 1000 g for 10 min at 4°C. The adequate amount of supernatant (60 μL equivalent to 1.5 mg tissue wet weight) was added to a reaction mixture that contained 0.002% Triton X-100, 0.1 mM EDTA, 0.5 M potassium phosphate buffer, and 15 mM H2O2 in 1 mL final volume at pH 7.0. Activity was calculated within the initial 15s decomposition rate. The initial absorbance was recorded (A240 at t = 0). Then, it was mixed well with a plastic paddle and decrease in absorbance was recorded again for about 15 sec (A240 at t = 15) and catalase activity (K) was calculated by the related formula and was expressed as U/mg protein: K =0.153 (log A240 at t = 0/A240 at t = 15)
Statistics
All numerical data are expressed as the mean ± SEM. The SOD, GPX, Catalase and MDA levels were analyzed using one-way ANOVA followed by Tukey's test. A P value less than 0.05 were considered statistically significant.
RESULTS
Effect of mebudipine on MDA level
MDA level as a marker of cardiac oxidative damage, was measured in this study. Pretreatment with 0.1 nm mebudipine before global ischemia significantly decreased the level of MDA in drug group as compared with that of control group (P < 0.01) [Figure 1]. There was no statistically significant difference in MDA level between vehicle (0.01% ethanol as a solvent for drug) and control groups.
Figure 1.
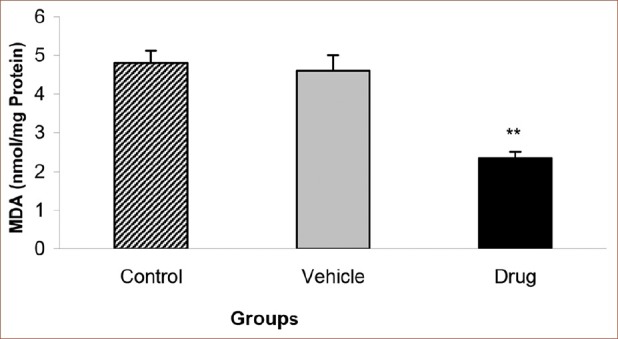
Effect of mebudipine on MDA level (nmol/mg Protein) in three groups of rats. Hearts were perfused with mebudipine (0.1 nm) or ethanol (0.01%)-enriched solution for 25 min, respectively before global ischemia. MDA= Malondialdehyde; **P<0.01 as compared with the control group
Effects of mebudipine on antioxidant enzymes activities
Administration of the mebudipine before the ischemia-reperfusion injury significantly increased the GPX activity of the myocardium (0.16 ± 0.009 U/mg Protein in drug vs. 0.068 ± 0.01 U/mg Protein in control groups; P < 0.01) [Figure 2]. Furthermore, the level of SOD activity increased significantly in drug group as compared with those of vehicle and control groups (0.1 ± 0.006 U/mg Protein in drug vs. 0.037 ± 0.009 U/mg Protein in control groups; P < 0.01) [Figure 3].
Figure 2.
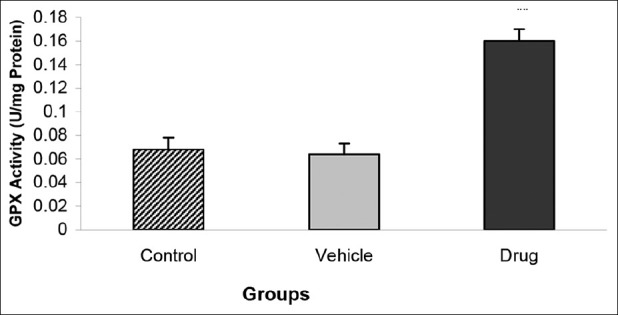
Effect of mebudipine on GPX activity (U/mg Protein) in three groups of rats. Hearts were perfused with mebudipine (0.1 nm) or ethanol (0.01%)-enriched solution for 25 min, respectively before global ischemia. GPX= Glutathione peroxidase; **P<0.01 as compared with the control group.
Figure 3.
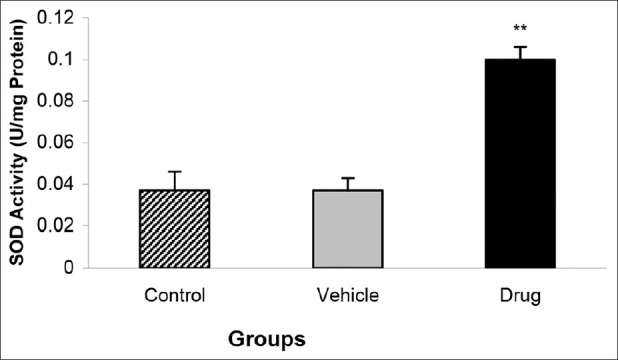
Effect of mebudipine on SOD activity (U/mg Protein) in three groups of rats. Hearts were perfused with mebudipine (0.1 nm) or ethanol (0.01%)-enriched solution for 25 min, respectively before global ischemia. SOD = superoxide dismutase; **P<0.01 as compared with the control group.
Alteration of Catalase activity of the heart was similar; so that its level was significantly higher in drug group than vehicle or control groups ((0.075 ± 0.006 U/mg Protein in drug vs. 0.028 ± 0.002 U/mg Protein in control groups; P < 0.01)) [Figure 4].
Figure 4.
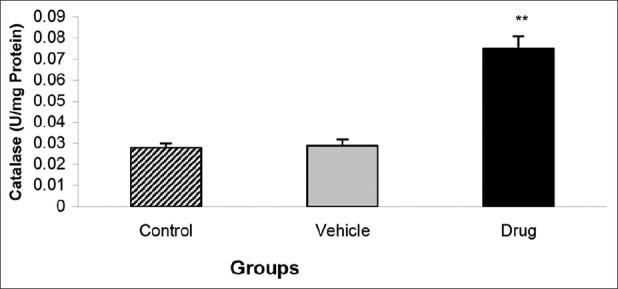
Effect of mebudipine on Catalase activity (U/mg Protein) in three groups of rats. Hearts were perfused with mebudipine (0.1nm) or ethanol (0.01%)-enriched solution for 25 min, respectively before global ischemia. **P < 0.01 as compared with the control group
DISCUSSION
In this study, we found that mebudipine significantly reduced the biochemical indicator of oxidative damage, lipid peroxidation product malondialdehyde and increased antioxidant enzymes (superoxide dismutase, glutathione peroxidase and catalase) activity in myocardium in drug group as compared with those of vehicle and control groups.
Mebudipine as a new calcium channel blocker with dihydropyridine structure possess vasoselectivity and cardioprotective effects in previous studies.[7,13,14] Also our results showed that mebudipine possess antioxidant activity in Ischemia-reperfusion heart.
Acute ischemia of the heart can result in a wide range of derangements, which range from transient reversible arrest of the myocardium to severe irreversible abnormalities. Ischemia-reperfusion (IR) injury is a complex process; the excessive production of oxygen-free radicals is the main mechanism involved in IR injury.[23,24] At the early phase of reperfusion free radicals (superoxide and hydroxyl radicals) are released. Also Ischemia-reperfusion will result in decrease in antioxidant activity which renders the myocardium extremely vulnerable.[12,16,24] The fact that free oxygen radicals play a significant role during the cardiac IR is well known, being accompanied by superoxide dismutase and glutathione peroxidase depletion and reduction of the total antioxidant capacity that act as natural oxygen radical scavengers in the organism.[24] The effects of mebudipine were assessed on ischemia-reperfusion injury in rats as a model of preconditioned-based therapy to minimize the oxidative stress. The antioxidant protection under the conditions of oxidative injury is a complex system in which the separate antioxidant elements co-operate with one another. The function of one antioxidant often potentiates the effects of another element in the system. In our study, the antioxidants, SOD, GPX and Catalase enzymes were significantly increased by mebudipine that shows enhanced detoxification of free radicals.[25] Our results showed that there was a significant decrease in the myocardial MDA values as a marker of oxidative damage in the mebudipine treated group as compared with the control group, which confirms the capability of drug to prevent lipid peroxidation and oxidative stress induced by IR injury. These findings may result from antioxidant property of mebudipine. The findings supported the beneficial changes in the treated hearts than controls.
It can be deduced from the results of our experiment that the administration of mebudipine before ischemia has a cardioprotective potential against ischemia-reperfusion induced injury in rat hearts. This was confirmed by the increase in the antioxidant enzyme values. This effect is also demonstrated in the functional indicators of hearts, LVDP, LVEDP and + dP/dtmax that we have obtained in our previous work (unpublished data). Similar to our study, other studies have showed the antioxidant properties of Ca2+ channel blockers as being due to either a direct scavenging effect or the preservation of the SOD and GPX activities and they inhibit lipid peroxide formation at concentrations present in plasma.[12,26,27] Other studies have found that two dihydropyridine Ca2+ channel blockers, lacidipine and nifedipine, decrease the formation of free radicals and have antioxidant capacity.[27] Since in this study, pre ischemia-treatment with mebudipine at the dose 0.1 nm significantly decreased the myocardial lipid peroxide production of MDA, we can conclude its beneficial effects on heart function may be mediated through the inhibition of lipid peroxidation and oxidative stress. Other studies showed that mebudipine is a potent vasodilator with greater tissue selectivity, with significant negative chronotropic effect and no noticeable negative effect on the contractility of the atrium,[13,14] so in regard to our results, it may be more effective than other dihydropyridine Ca2+ channel blockers in diminishing myocardial IR injury.
In summary, the results of this study showed that mebudipine significantly reduced the biochemical indicator of oxidative damage, lipid peroxidation product malondialdehyde as compared with those of vehicle and control groups. Furthermore, antioxidant enzymes (SOD, GPX and catalase) activities of myocardium were significantly increased by mebudipine. Our results indicate that mebudipine may have antioxidant activity against myocardial ischemia-reperfusion injury, thus, this drug can reduce the intensity of cardiac ischemic insults.
Limitation: since the control group should be behaved as the test group/s except for treatment, so in our protocol it would be preferable to have stabilization for all groups, i.e., it was better if the control group was stabilized with perfusion with K-H buffer for 45 min before ischemia, and for mebudipine and ethanol for 20 min (perfusion with K-H buffer) followed by 25 min perfusion with either mebudipine or ethanol, respectively.
ACKNOWLEDGMENTS
The work was supported by a grant (project No: K/87/80) from the Applied Drug Research Center, Tabriz University of Medical Sciences, Tabriz, Iran and Physiology Research Center, Kerman University of Medical Sciences, Kerman, Iran.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Baroldi G. Letter: Myocardial necrosis: the need for definition. J Mol Cell Cardiol. 1974;6:401–2. doi: 10.1016/0022-2828(74)90081-9. [DOI] [PubMed] [Google Scholar]
- 2.Boudina S, Laclau MN, Tariosse L, Daret D, Gouverneur G, Bonoron-Adele S, et al. Alteration of mitochondrial function in a model of chronic ischemia in vivo in rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H821–31. doi: 10.1152/ajpheart.00471.2001. [DOI] [PubMed] [Google Scholar]
- 3.Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, et al. Nitric oxide and cardiac function. Life Sci. 2007;81:779–93. doi: 10.1016/j.lfs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Gross GJ, Auchampach JA. Reperfusion injury: Does it exist? J Mol Cell Cardiol. 2007;42:12–8. doi: 10.1016/j.yjmcc.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda VS, Naik SR. Cardioprotective activity of Ginkgo biloba Phytosomes in isoproterenol-induced myocardial necrosis in rats: A biochemical and histoarchitectural evaluation. Exp Toxicol Pathol. 2008;60:397–404. doi: 10.1016/j.etp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–90. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Ghyasi R, Mohammadi M, Badalzadeh R, Rashidi B, Sepehri G. The effect of mebudipine on cardiac function and activity of the myocardial nitric oxide system in ischaemia-reperfusion injury in rats. Cardiovasc J Afr. 2011;22:319–23. doi: 10.5830/CVJA-2010-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsumata N, Ma X, Higuchi H. Protective effect of diltiazem against ischemia-induced decreases in regional myocardial flow in rat heart. Eur J Pharmacol. 2000;398:83–91. doi: 10.1016/s0014-2999(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau G, Provost P, Tran D, Caille G, Latour JG. Clentiazem given at reperfusion improves subendocardial reflow and reduces myocardial infarct size in the dog. J Pharmacol Exp Ther. 1994;268:1252–60. [PubMed] [Google Scholar]
- 10.Tamura K, Suzuki Y, Koga T, Akima M, Kato T, Nabata H. Actions of CP-060S on veratridine-induced Ca2+ overload in cardiomyocytes and mechanical activities in vascular strips. Eur J Pharmacol. 1996;312:195–202. doi: 10.1016/0014-2999(96)00460-8. [DOI] [PubMed] [Google Scholar]
- 11.Ver Donck L, Van Reempts J, Vandeplassche G, Borgers M. A new method to study activated oxygen species induced damage in cardiomyocytes and protection by Ca2+-antagonists. J Mol Cell Cardiol. 1988;20:811–23. doi: 10.1016/s0022-2828(88)80006-3. [DOI] [PubMed] [Google Scholar]
- 12.Godfraind T. Antioxidant effects and the therapeutic mode of action of calcium channel blockers in hypertension and atherosclerosis. Philos Trans R Soc Lond B Biol Sci. 2005;360:2259–72. doi: 10.1098/rstb.2005.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirkhani H, Dirin M, Youssef-Zayeh I. Mechanism of vasoselective action of mebudipine, a new calcium channel blocker. Vascul Pharmacol. 2004;42:23–9. doi: 10.1016/j.vph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Rouzrokh A, Ebrahimi SA, Rahbr-Roshandel N, Mahmoudian M. Effects of mebudipine and dibudipine, two new calcium channel blockers on voltage-activated calcium currents of PC12 cells. Acta Physiol Hung. 2007;94:199–207. doi: 10.1556/APhysiol.94.2007.3.5. [DOI] [PubMed] [Google Scholar]
- 15.Becker BF, Mobert J. Low-dose calcium antagonists reduce energy demand and cellular damage of isolated hearts during both ischemia and reperfusion. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:287–94. doi: 10.1007/s002109900053. [DOI] [PubMed] [Google Scholar]
- 16.Massoudy P, Becker BF, Seligmann C, Gerlach E. Preischaemic as well as postischaemic application of a calcium antagonist affords cardioprotection in the isolated guinea pig heart. Cardiovasc Res. 1995;29:577–82. [PubMed] [Google Scholar]
- 17.Vander Heide RS, Schwartz LM, Reimer KA. The novel calcium antagonist Ro 40-5967 limits myocardial infarct size in the dog. Cardiovasc Res. 1994;28:1526–32. doi: 10.1093/cvr/28.10.1526. [DOI] [PubMed] [Google Scholar]
- 18.Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–25. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 19.Siu PM, Wang Y, Alway SE. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009;84:468–81. doi: 10.1016/j.lfs.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delmas-Beauvieux MC, Peuchant E, Dumon MF, Receveur MC, Le Bras M, Clerc M. Relationship between red blood cell antioxidant enzymatic system status and lipoperoxidation during the acute phase of malaria. Clin Biochem. 1995;28:163–9. doi: 10.1016/0009-9120(94)00071-3. [DOI] [PubMed] [Google Scholar]
- 21.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 22.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 23.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton KL. Antioxidants and cardioprotection. Med Sci Sports Exerc. 2007;39:1544–53. doi: 10.1249/mss.0b013e3180d099e8. [DOI] [PubMed] [Google Scholar]
- 25.Necas J, Bartosikova L, Florian T, Klusakova J, Suchy V, Naggar EM, et al. Protective effects of the flavonoids osajin and pomiferin on heart ischemia-reperfusion. Ceska Slov Farm. 2006;55:168–74. [PubMed] [Google Scholar]
- 26.Allanore Y, Borderie D, Perianin A, Lemarechal H, Ekindjian OG, Kahan A. Nifedipine protects against overproduction of superoxide anion by monocytes from patients with systemic sclerosis. Arthritis Res Ther. 2005;7:R93–100. doi: 10.1186/ar1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noronha-Dutra AA, Steen-Dutra EM, Woolf N. An antioxidant role for calcium antagonists in the prevention of adrenaline mediated myocardial and endothelial damage. Br Heart J. 1991;65:322–5. doi: 10.1136/hrt.65.6.322. [DOI] [PMC free article] [PubMed] [Google Scholar]


