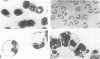
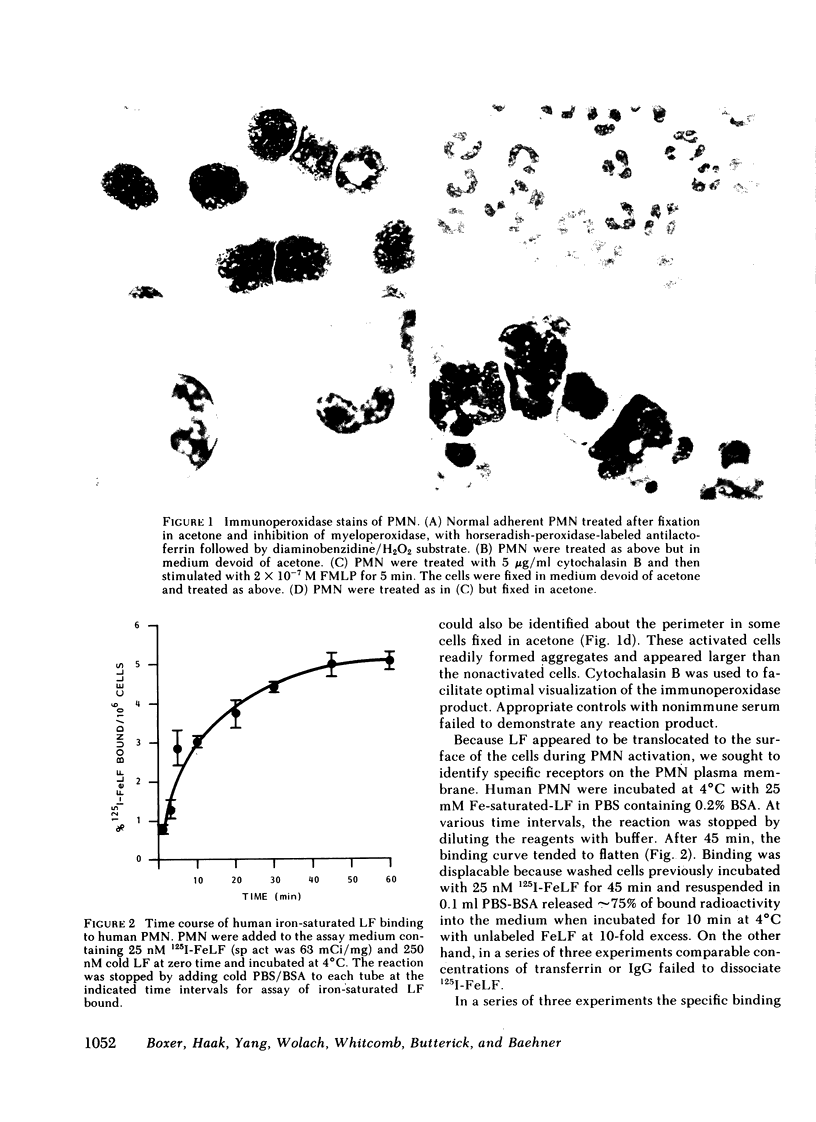
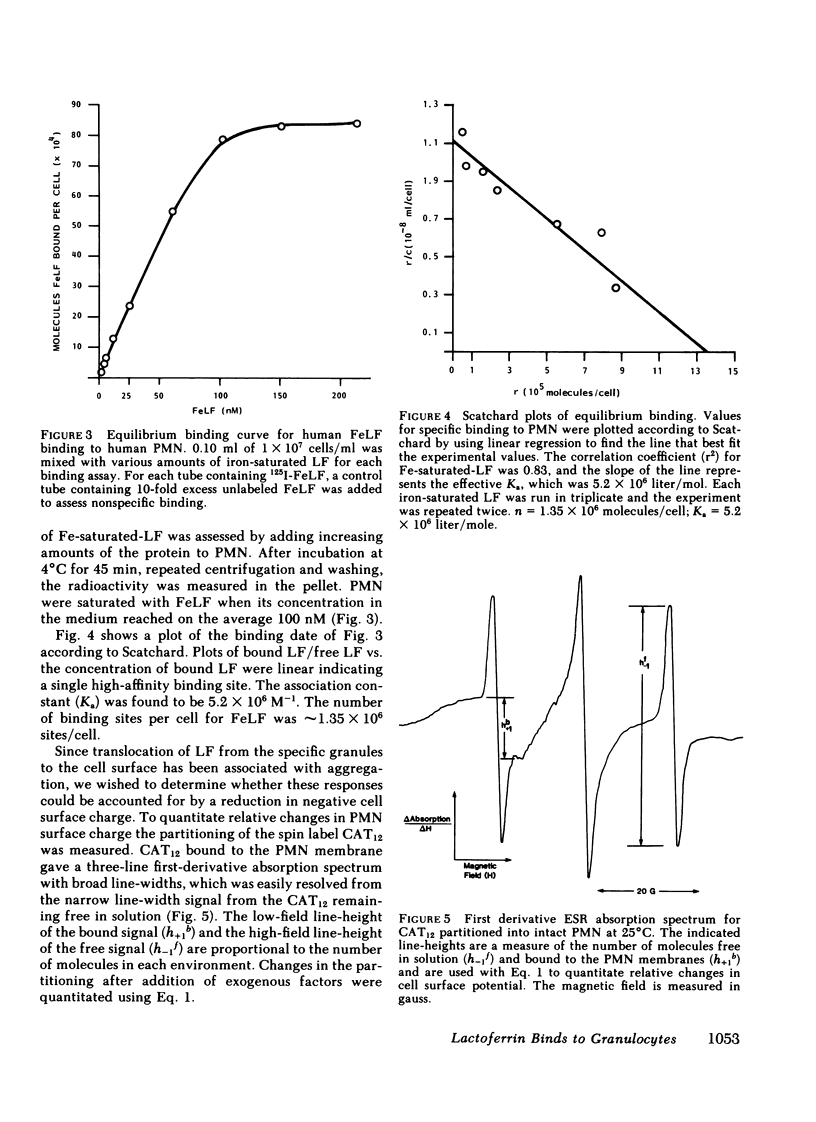
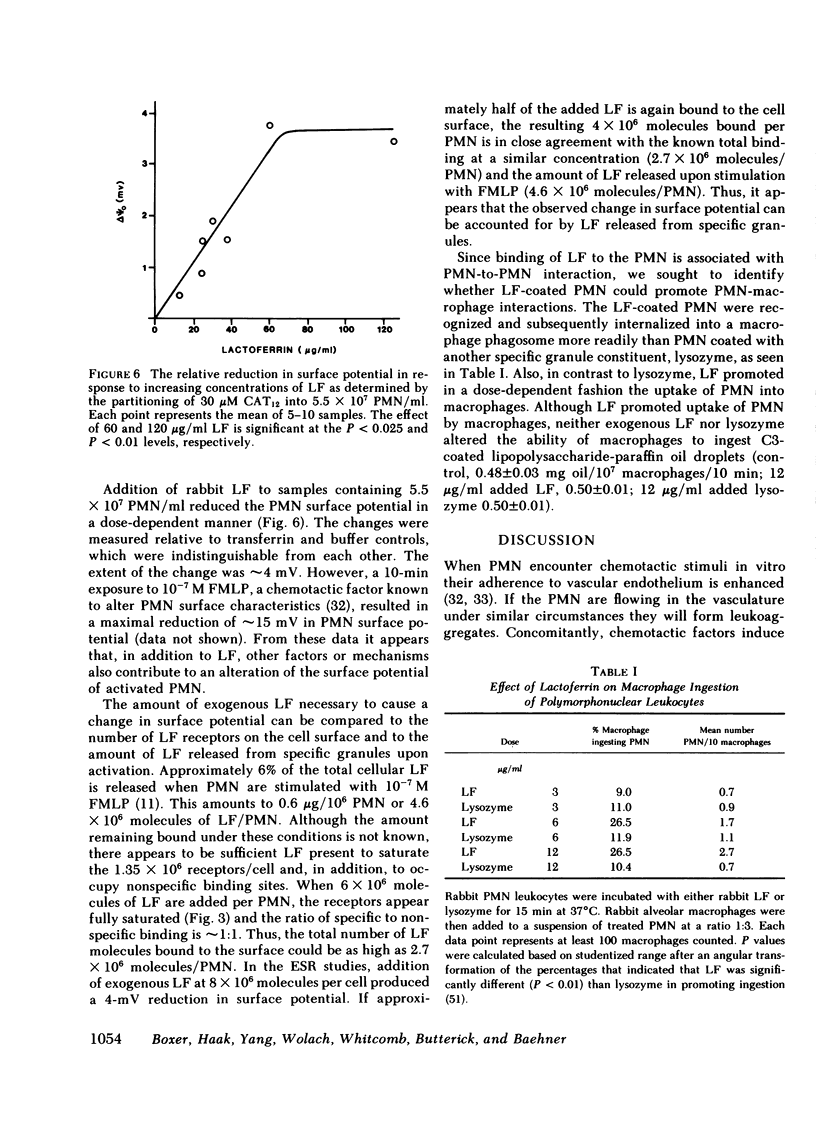
Abstract
Polymorphonuclear leukocytes (PMN) aggregate and avidly attach to endothelium in response to chemotactic agents. This response may be related in part to the release of the specific granule constituent lactoferrin (LF). We found by using immunohistology and biochemical and biophysical techniques that LF binds to the membrane and alters the surface properties of the PMN. Upon exposure of PMN treated with 5 micrograms/ml cytochalasin B to 2 x 10(-7) M formyl-methionine-leucine-phenylalanine for 5 min, the PMN mobilized LF to their surface as observed by immunoperoxidase staining for LF. At added LF levels ranging from 4 to 15 micrograms/10(7) PMN there was a dose-dependent reduction in PMN surface charge reaching 4 mV, when the partitioning into the membrane of a charged amphipathic nitroxide spin label was measured by electron spin resonance spectroscopy, whereas transferrin was without effect. When 125I-FeLF was added to human PMN in increasing amounts and the results corrected for the residual amount of free LF contaminating the cells, the PMN were saturated with LF at concentrations between 100 and 200 nM in the medium. Human PMN bound 1.35 x 10(6) molecules per cell and the calculated value for the association constant for these receptors was 5.2 x 10(6) M-1. Additionally, 6 micrograms/ml LF served as an opsonin for rabbit MN to promote PMN uptake by rabbit macrophages, when assessed by electron microscopy, but lysozyme did not. These studies indicate that LF can bind to the surface of the PMN and reduce its surface charge. This correlates with enhanced "stickiness" leading to a variety of cell-cell interactions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Mannik M. The macrophage receptor for IgG: number and affinity of binding sites. J Immunol. 1973 Jun;110(6):1455–1463. [PubMed] [Google Scholar]
- BENNETT I. L., Jr, BEESON P. B. The properties and biologic effects of bacterial pyrogens. Medicine (Baltimore) 1950 Dec;29(4):365–400. doi: 10.1097/00005792-195012000-00003. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Gonwa T. A., Szejda P., Cousart M. S., DeChatelet L. R., McCall C. E. Eosinopenia of acute infection: Production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest. 1980 Jun;65(6):1265–1271. doi: 10.1172/JCI109789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. M., Bagby G. C., Davis J. Calcium-dependent polymerization of lactoferrin. Biochem Biophys Res Commun. 1981 Jul 16;101(1):88–95. doi: 10.1016/s0006-291x(81)80014-9. [DOI] [PubMed] [Google Scholar]
- Bennett R. M., Davis J. Lactoferrin binding to human peripheral blood cells: an interaction with a B-enriched population of lymphocytes and a subpopulation of adherent mononuclear cells. J Immunol. 1981 Sep;127(3):1211–1216. [PubMed] [Google Scholar]
- Bentwood B. J., Henson P. M. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [PubMed] [Google Scholar]
- Boxer L. A., Baehner R. L., Davis J. The effect of 2-deoxyglucose on guinea pig polymorphonuclear leukocyte phagocytosis. J Cell Physiol. 1977 Apr;91(1):89–102. doi: 10.1002/jcp.1040910110. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Björkstén B., Björk J., Yang H. H., Allen J. M., Baehner R. L. Neutropenia induced by systemic infusion of lactoferrin. J Lab Clin Med. 1982 Jun;99(6):866–872. [PubMed] [Google Scholar]
- Boxer L. A., Coates T. D., Haak R. A., Wolach J. B., Hoffstein S., Baehner R. L. Lactoferrin deficiency associated with altered granulocyte function. N Engl J Med. 1982 Aug 12;307(7):404–410. doi: 10.1056/NEJM198208123070704. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Stossel T. P. Effects of anti-human neutrophil antibodies in vitro. Quantitative studies. J Clin Invest. 1974 Jun;53(6):1534–1545. doi: 10.1172/JCI107704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carmeli C., Quintanilha A. T., Packer L. Surface charge changes in purple membranes and the photoreaction cycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4707–4711. doi: 10.1073/pnas.77.8.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle J. D., Hubbell W. L. Estimation of membrane surface potential and charge density from the phase equilibrium of a paramagnetic amphiphile. Biochemistry. 1976 Nov 2;15(22):4818–4831. doi: 10.1021/bi00667a011. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D. E., Moldow C. F., Yamada O., Jacob H. S. Granulocyte aggregation as a manifestation of membrane interactions with complement: possible role in leukocyte margination, microvascular occlusion, and endothelial damage. Semin Hematol. 1979 Apr;16(2):140–147. [PubMed] [Google Scholar]
- Gaffney B. J., Mich R. J. Letter: A new measurement of surface charge in model and biological lipid membranes. J Am Chem Soc. 1976 May 12;98(10):3044–3045. doi: 10.1021/ja00426a076. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Degranulating stimuli decrease the neagative surface charge and increase the adhesiveness of human neutrophils. J Clin Invest. 1980 Feb;65(2):298–306. doi: 10.1172/JCI109672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Fletcher M. P., Seligmann B. E., Hoffstein S., Cehrs K., Mounessa N. Human neutrophil-specific granule deficiency: a model to assess the role of neutrophil-specific granules in the evolution of the inflammatory response. Blood. 1982 Jun;59(6):1317–1329. [PubMed] [Google Scholar]
- Haak R. A., Ingraham L. M., Baehner R. L., Boxer L. A. Membrane fluidity in human and mouse Chediak-Higashi leukocytes. J Clin Invest. 1979 Jul;64(1):138–144. doi: 10.1172/JCI109432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L., Imai K., Goren M. B. Expression of peroxidase-dependent iodination by macrophages ingesting neutrophil debris. J Reticuloendothel Soc. 1980 Oct;28(4):391–404. [PubMed] [Google Scholar]
- Hunter R. Standardization of the chloramine-T method of protein iodination. Proc Soc Exp Biol Med. 1970 Mar;133(3):989–992. doi: 10.3181/00379727-133-34611. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Transferrin receptors on human B and T lymphoblastoid cell lines. Biochim Biophys Acta. 1979 Apr 3;583(4):483–490. doi: 10.1016/0304-4165(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Farrell C., Taylor C. R. The detection of intracellular antigens in human leucocytes by immunoperoxidase staining. Br J Haematol. 1975 Nov;31(3):361–370. doi: 10.1111/j.1365-2141.1975.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Metal-combining properties of human lactoferrin (red milk protein). 1. The involvement of bicarbonate in the reaction. Eur J Biochem. 1968 Dec 5;6(4):579–584. doi: 10.1111/j.1432-1033.1968.tb00484.x. [DOI] [PubMed] [Google Scholar]
- McCall C. E., De Chatelet L. R., Brown D., Lachmann P. New biological activity following intravascular activation of the complement cascade. Nature. 1974 Jun 28;249(460):841–843. doi: 10.1038/249841a0. [DOI] [PubMed] [Google Scholar]
- Mehlhorn R. J., Packer L. Membrane surface potential measurements with amphiphilic spin labels. Methods Enzymol. 1979;56:515–526. doi: 10.1016/0076-6879(79)56049-2. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Ward P. A. Neutropenia induced by systemic infusion of chemotactic factors. J Immunol. 1977 May;118(5):1586–1589. [PubMed] [Google Scholar]
- O'Flaherty J. T., Ward P. A. Leukocyte aggregation induced by chemotactic factors: a review. Inflammation. 1978 Jun;3(2):177–194. doi: 10.1007/BF00910738. [DOI] [PubMed] [Google Scholar]
- Oseas R. S., Boxer L. A., Butterick C., Baehner R. L. Differences in polymorphonuclear leukocyte aggregating responses among several species in response to chemotactic stimulation. J Lab Clin Med. 1980 Aug;96(2):213–221. [PubMed] [Google Scholar]
- Oseas R., Yang H. H., Baehner R. L., Boxer L. A. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981 May;57(5):939–945. [PubMed] [Google Scholar]
- Pryzwansky K. B., MacRae E. K., Spitznagel J. K., Cooney M. H. Early degranulation of human neutrophils: immunocytochemical studies of surface and intracellular phagocytic events. Cell. 1979 Dec;18(4):1025–1033. doi: 10.1016/0092-8674(79)90215-0. [DOI] [PubMed] [Google Scholar]
- Quintanilha A. T., Packer L. Surface potential changes on energization of the mitochondrial inner membrane. FEBS Lett. 1977 Jun 15;78(2):161–165. doi: 10.1016/0014-5793(77)80296-2. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Majno G. Acute inflammation. A review. Am J Pathol. 1977 Jan;86(1):183–276. [PMC free article] [PubMed] [Google Scholar]
- Seligmann B., Chused T. M., Gallin J. I. Human neutrophil heterogeneity identified using flow microfluorometry to monitor membrane potential. J Clin Invest. 1981 Nov;68(5):1125–1131. doi: 10.1172/JCI110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Goetzl E. J. Molecular and cellular mechanisms of leukocyte chemotaxis. Science. 1981 Aug 21;213(4510):830–837. doi: 10.1126/science.6266014. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sullivan S. J., Zigmond S. H. Chemotactic peptide receptor modulation in polymorphonuclear leukocytes. J Cell Biol. 1980 Jun;85(3):703–711. doi: 10.1083/jcb.85.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L. The binding of human lactoferrin to mouse peritoneal cells. J Exp Med. 1976 Dec 1;144(6):1568–1580. doi: 10.1084/jem.144.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]