Abstract
AIM: To investigate mucin expression profiles in colorectal carcinoma (CRC) histological subtypes with regard to clinicopathologic variables and prognosis.
METHODS: Mucin (MUC)2 and MUC5AC expressions were assessed by immunohistochemistry for a total of 250 CRC cases that underwent surgical resection. CRCs included 63 well-to-moderately differentiated adenocarcinomas (WMDAs), 91 poorly differentiated adenocarcinomas (PDAs), 81 mucinous adenocarcinoma (MUAs), and 15 signet-ring cell carcinomas (SRCCs). MUC2 and MUC5AC were scored as positive when ≥ 25% and ≥ 1% of cancer cells were stained positive, respectively. The human mutL homolog 1 and human mutS homolog 2 expressions were assessed by immunohistochemistry in PDAs to investigate mismatch-repair (MMR) status. Tumors that did not express either of these two were considered MMR-deficient. Results were analyzed for associations with clinicopathologic variables and the prognosis in individual histological CRC subtypes.
RESULTS: MUC2-positive and MUC5AC-positive WMDA percentages were 49.2% and 30.2%, respectively. In contrast, MUC2-positive and MUC5AC-positive PDA percentages were 9.5% and 51.6%, respectively. MUC2 levels tended to decrease and MUC5AC levels tended to increase from WMDA to PDA. In 21 tumors comprising both adenoma and adenocarcinoma components in a single tumor (4 WMDAs, 7 PDAs, and 10 MUAs), MUC2 was significantly downregulated in PDA and MUC5AC was downregulated in PDA and MUA in the adenoma-carcinoma sequence. These results suggested that MUC2 levels might be associated with malignant potential and that MUC5AC expression was an early event in tumorigenesis. Despite worse prognoses than WMDA, high MUC2 expression levels were maintained in MUA (95.1%) and SRCC (71.5%), which suggested a pathogenesis for these subtypes distinct from that of WMDA. No significant associations were found between MUC2 expression and any clinicopathologic variables in any histological subtype. MUC5AC expression in PDA was closely associated with right-sided location (P = 0.017), absence of nodal metastasis (P = 0.010), low tumor node metastasis stage (P = 0.010), and MMR deficiency (P = 0.003). MUC2 expression in WMDA was a marginal prognostic factor for recurrence/metastasis-free survival (RFS) by univariate Cox analysis (P = 0.077) but not by multivariate Cox analysis (P = 0.161). MUC5AC expression in PDA was a significant prognostic factor for RFS by univariate Cox analysis (P = 0.007) but not by multivariate Cox analysis (P = 0.104). Kaplan-Meier curves and log-rank tests revealed that MUC2 expression was marginally associated with a better WMDA prognosis [P = 0.064 for RFS and P = 0.172 for overall survival (OS)] but not for PDA. In contrast, MUC5AC expression was significantly and marginally associated with a better PDA prognosis in terms of RFS and OS, respectively (P = 0.004 for RFS and P = 0.100 for OS), but not for WMDA and MUA.
CONCLUSION: Mucin core protein expression profiles and clinical significance differ according to histological CRC subtypes. This may reflect different pathogeneses for these tumors.
Keywords: Mucin 2, Mucin 5AC, Microsatellite instability, Mismatch repair, Colorectal carcinoma, Poorly differentiated adenocarcinoma, Pathogenesis, Adenoma-carcinoma sequence, Prognosis
Core tip: Altered mucin expression may be correlated with biological behavior and possibly with the prognosis of colorectal carcinoma (CRC). However, many contradictory results make it difficult to interpret its clinical significance, possibly because of CRC variations. Therefore, we examined mucin (MUC)2 and MUC5AC expressions in different pathological CRC subtypes by immunohistochemistry to determine their true clinical significance. Our results suggest that the expression profiles and the clinical significance of these mucin core proteins are different according to histological subtypes. This may reflect different pathogeneses for these tumors.
INTRODUCTION
Mucins are a diverse family of high-molecular-weight glycoproteins that are widely expressed in epithelial tissues and are characterized by the presence of tandem repeat sequences that are rich in highly O-glycosylated serine and threonine residues[1]. Mucins can be classified as either membrane-associated or secretory glycoproteins. To date, a total of 20 human mucins have been identified. Secreted mucins can be gel-forming or non-gel-forming and include mucin (MUC)2, MUC5AC, MUC5B, MUC6, MUC7, MUC8, MUC9, and MUC19. Transmembrane mucins include MUC1, MUC3A, MUC3B, MUC4, MUC11, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, and MUC21; these are anchored to the plasma membranes of various cells through a transmembrane domain. These mucin proteins are encoded by various MUC genes[2]. The genes for gel-forming mucins MUC2 and MUC5AC are found in a cluster on chromosome 11p15.5[3]. The MUC2 gene codes for a typical secretory mucin, which is predominantly found in colorectal goblet cells, and the MUC5AC gene is mainly expressed in gastric and tracheal-bronchial mucosa.
Altered expressions of MUC2 and MUC5AC may be significantly correlated with the biological behavior of and, possibly, the prognosis for colorectal carcinoma (CRC). However, many contradictory results make it difficult to interpret their clinical significance. For example, MUC2 expression is significantly decreased according to CRC disease progression[4-6]. MUC2-positive CRC shows a relatively good prognosis or a low incidence of liver and nodal metastasis[7,8]. Suppressing the MUC2 gene expression in colon carcinoma cell lines in vitro was associated with methylation of its promoter region[7]. In contrast, other studies reported that MUC2 expression was not a significant marker of tumor invasion depth, liver metastasis, or overall survival[9,10]. However, the absence of MUC5AC expression can be a prognostic indicator of a more aggressive colorectal tumor. Highly villous adenoma with severe dysplasia expressed a less MUC5AC than larger adenomas of moderate villous histology and dysplasia[11]. Carcinomas with low grade atypia exhibited a higher incidence of MUC5AC expression as compared with carcinomas showing high grade atypia[6]. Consistently, MUC5AC expression analysis combined with survival analysis has demonstrated that those patients with MUC5AC-negative CRC had lower rates for disease-free status and of overall survival[12].
Most studies analyzed CRC without detailed classifications. However, in the World Health Organization (WHO) classification, CRC consists of various histological subtypes, such as conventional adenocarcinoma, mucinous adenocarcinoma (MUA), signet-ring cell carcinoma (SRCC), squamous cell carcinoma, adenosquamous carcinoma, medullary carcinoma, undifferentiated carcinoma, and other very rare variants[13]. Conventional adenocarcinoma is further sub-classified into well-to-moderately differentiated adenocarcinoma (WMDA) and poorly differentiated adenocarcinoma (PDA) based on the percentage of the area showing a gland-like structure[13]. Most CRCs encountered in the clinic are WMDA and poorly differentiated/undifferentiated carcinomas are rare, accounting for up to 16% of all CRCs in the United States[14,15]. The purpose of this study was to assess MUC2 and MUC5AC expressions in different pathological CRC subtypes by immunohistochemistry and to determine their true clinical significance.
MATERIALS AND METHODS
Patients and tumor samples
For this study, WMDA included all consecutive cases that were surgically resected in Tokyo Kosei Nenkin Hospital from April 1998 to March 2000, but excluded 10 other histological CRC subtypes. These cases included 63 tumors from 63 patients. In addition, a total of 187 histological CRC subtypes other than WMDA were collected from all CRC cases resected in Dokkyo Medical University Koshigaya Hospital between 1990 and 2011 and Tokyo Kosei Nenkin Hospital between 1991 and 2010. Formalin-fixed, paraffin-embedded tissue blocks were obtained from the archival material stored in the pathology departments of the both hospitals. A sufficient number of samples to provide for complete investigations were available for all these cases. Patients whose medical records were sufficiently complete were included in survival analysis. Patients with invasive cancers originating from other sites were excluded from the analysis. Clinicopathologic classifications and stage groupings were based on the WHO classification of colorectal tumors and the tumor node metastasis (TNM) staging by the American Joint Committee on Cancer[13,16]. Our study protocol was approved by the ethical review boards of the participating hospitals.
Immunohistochemistry
Tumor specimens were fixed in 10% neutral-buffered formalin for 48 h, embedded in paraffin, and cut into 4-μm-thick sections, and then mounted on silane-coated glass slides. Antigen-retrieval was done by autoclaving (121 °C) for 5 min in pH 9 Antigen Retrieval Liquid (Nichirei, Tokyo, Japan) for MUC2, MUC5AC, and hMSH2, and by microwave irradiation for 10 min in pH 9 Antigen Retrieval Liquid for human mutL homolog 1 (hMLH1). Primary antibodies used were the mouse monoclonal antibody for MUC2 (1:100 dilution; clone Ccp58, Novocastra, Newcastle Upon Tyne, United Kingdom), the mouse monoclonal antibody for MUC5AC (1:100 dilution; CLH2, Novocastra), the rabbit anti-MLH1 monoclonal antibody (1:400 dilution; EPR3894, GeneTex, San Antonio, TX, United States), and the rabbit anti-MSH2 polyclonal antibody (1:200 dilution; 15520-1-AP, Proteintec, Chicago, IL, United States). Samples were treated overnight with each primary antibody at 4 °C. Immunostaining was performed blindly by an investigator (Fukuda K) who was unaware of the clinical information using an N-Histofine Simple Stain MAX-PO kit (Nichirei).
The immunostaining results for mucin core proteins were assessed semi-quantitatively: 0, no staining; 1, < 5% of cells; 2, 5% to < 25% of cells; 3, 25% to < 50% of cells; 4, ≥ 50% of cells (Figure 1). The immunostaining results for mismatch repair (MMR) proteins were either completely negative (negative) or nearly 100% positive (positive). Immunoreactivity was independently evaluated by two investigators (Fukuda K and Imai Y), and discrepancies were resolved by discussion.
Figure 1.
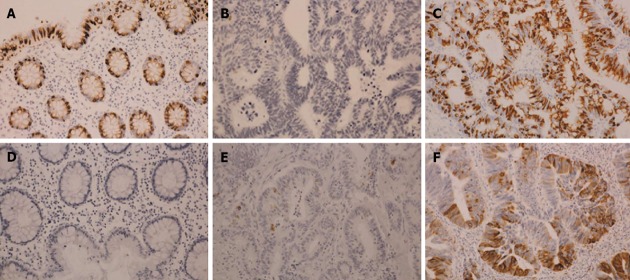
Expression of mucin 2 and mucin 5AC in colorectal carcinomas. A: Mucin (MUC)2 expression in normal colonic mucosa; B: MUC2 expression in cancer: level 0; C: MUC2 expression in cancer: level 4; D: MUC5AC expression in normal colonic mucosa; E: MUC5AC expression in cancer: level 1; F: MUC5AC expression in cancer: level 4 (immunohistochemical staining, × 10).
In light of their expression levels in normal colonic mucosa, levels 3-4 for MUC2 and levels 1-4 for MUC5AC were evaluated as positive.
Statistical analysis
Comparisons of two cohorts with or without a specific clinicopathologic variable were made by a χ2 test with/without a Yates’ correction or Fisher’s exact probability test based on the expected values in a contingency table. Age was compared with Mann-Whitney U test. Comparisons of the mucin expression levels between adenoma and adenocarcinoma components in a single tumor were made by Wilcoxon signed-rank test for sample numbers of ≥ 6. Univariate analysis by Cox regression analysis was used to identify possible prognostic predictors. Variables for which P values were < 0.10 were entered into multivariate regression analysis (forced entry method). Survival curves were generated using the Kaplan-Meier method, and curves were compared by log-rank test. P value < 0.05 was considered significant. Statistical analysis was performed using IBM SPSS Statistics 20 (IBM, Armonk, NY, United States).
RESULTS
Clinicopathologic characteristics
WMDA cases included 63 tumors from 63 patients. Five of these patients (5 tumors) had a family history of CRC. Additional chemotherapy and irradiation were administered for 17 tumors in 17 patients and 4 tumors in 4 patients, respectively. CRCs other than WMDA included a total of 187 tumors: 91 PDAs (90 patients); 81 MUAs (81 patients); and 15 SRCCs (15 patients). One patient had triple cancers (No. 148: two PDAs and one MUA) and one patient had double cancers (No. 170: one PDA and one MUA). Ten of these patients (12 tumors) had a family history of CRC, one of which was proven to be a hereditary non-polyposis colorectal cancer pedigree (No. 148). Predominant occurrence in females, right-sided location, depth of tumors beyond muscularis propria, lymphatic invasion, nodal involvement (except for MUA), TNM stage III/IV (except for MUA) were more frequent in CRCs other than WMDA as compared with WMDA (Table 1). In addition to surgery, chemotherapy and irradiation were administered for 80 tumors in 80 patients and 6 tumors in 6 patients, respectively.
Table 1.
Clinicopathologic characteristics in colorectal carcinoma n (%)
| Variables | WMDA | PDA | MUA | SRCC | |
| (n = 63) | (n = 91) | (n = 81) | (n = 15) | ||
| Gender | Male | 39 (61.9) | 45 (49.5) | 46 (56.8) | 6 (40.0) |
| Female | 24 (38.1) | 46 (50.5) | 35 (43.2) | 9 (60.0) | |
| Age (yr) | Range | 32-87 | 35-92 | 26-90 | 30-82 |
| Median | 65 | 64 | 71 | 70 | |
| Family history of CRC | Yes | 5 (7.9) | 5 (6.1) | 5 (6.3) | 2 (13.3) |
| No | 58 (92.1) | 77 (93.9) | 75 (93.7) | 13 (86.7) | |
| Unknown | 9 | 1 | |||
| Location | Left-sided | 43 (68.3) | 38 (41.8) | 40 (49.4) | 6 (40.0) |
| Right-sided | 20 (31.7) | 53 (58.2) | 41 (50.6) | 9 (60.0) | |
| Depth | Up tp MP | 10 (15.9) | 4 (4.4) | 5 (6.2) | 1 (6.7) |
| Beyond MP | 53 (84.1) | 87 (95.6) | 76 (93.8) | 14 (93.3) | |
| Venous invasion | Yes | 48 (76.2) | 77 (85.6) | 41 (50.6) | 14 (93.3) |
| No | 15 (23.8) | 13 (14.4) | 40 (49.4) | 1 (6.7) | |
| Unknown | 1 | ||||
| Lymphatic invasion | Yes | 35 (55.6) | 82 (91.1) | 54 (66.7) | 12 (80.0) |
| No | 28 (44.4) | 8 (8.9) | 27 (33.3) | 3 (20.0) | |
| Unknown | 1 | ||||
| Nodal metastasis | Yes | 34 (54.0) | 69 (78.4) | 37 (46.8) | 9 (64.3) |
| No | 29 (46.0) | 19 (21.6) | 42 (53.2) | 5 (35.7) | |
| Unknown | 3 | 2 | 1 | ||
| Chemotherapy | Yes | 17 (27.0) | 42 (53.8) | 31 (41.9) | 7 (46.7) |
| No | 46 (73.0) | 36 (46.2) | 43 (58.1) | 8 (53.3) | |
| Unknown | 13 | 7 | |||
| Irradiation | Yes | 4 (6.3) | 2 (2.6) | 3 (4.1) | 1 (7.1) |
| No | 59 (93.7) | 76 (97.4) | 71 (95.9) | 14 (92.9) | |
| Unknown | 13 | 7 | |||
| TNM stage | I/II | 29 (46.0) | 17 (18.9) | 40 (50.0) | 4 (26.7) |
| III/IV | 34 (54.0) | 73 (81.1) | 40 (50.0) | 11 (73.3) | |
| Unknown | 1 | 1 | 0 |
CRC: Colorectal carcinoma; WMDA: Well-to-moderately differentiated adenocarcinoma; PDA: Poorly differentiated adenocarcinoma; MUA: Mucinous adenocarcinoma; SRCC: Signet-ring cell carcinoma; MP: Muscularis propria; TNM: Tumor node metastasis.
The clinicopathologic characteristics of each CRC subtype are summarized in Table 1. CRC patient prognosis was significantly associated with these histological subtypes (Figure 2).
Figure 2.
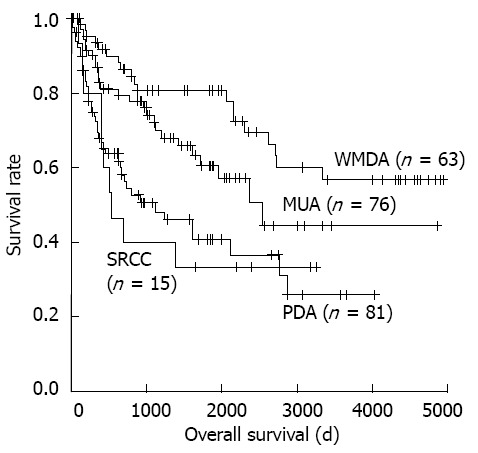
Colorectal carcinoma overall survival curves generated by the Kaplan-Meier method. WMDA: Well-to-moderately differentiated adeno-carcinoma; PDA: Poorly differentiated adenocarcinoma; MUA: Mucinous adenocarcinoma; SRCC: Signet-ring cell carcinoma.
Expression of MUC2 and MUC5AC in CRCs
Expression of the mucin core proteins was assessed individually in different pathological subtypes. MUC2 was expressed in the perinuclear cytoplasm of goblet cells in normal colonic mucosa and diffusely in the cytoplasm of cancer cells. MUC5AC was not expressed in normal colonic mucosa but was occasionally expressed in pericancerous normally appearing colonic mucosa. About half of WMDA cases (49.2%) were positive for MUC2 (levels 3-4), and 30.2% of these cases were positive for MUC5AC (levels 1-4). In contrast, only one tenth of PDA cases (9.5%) were positive for MUC2 and 51.6% of these cases were positive for MUC5AC. PDA was more frequently negative for MUC2 and positive for MUC 5AC than was WMDA. Nearly all MUA cases (95.1%) were positive for MUC2, and over half of these cases (54.3%) aberrantly expressed MUC5AC. Although small in number, 71.5% of SRCC cases were positive for MUC2 and nearly half (46.7%) expressed MUC5AC. These results are summarized in Figure 3.
Figure 3.
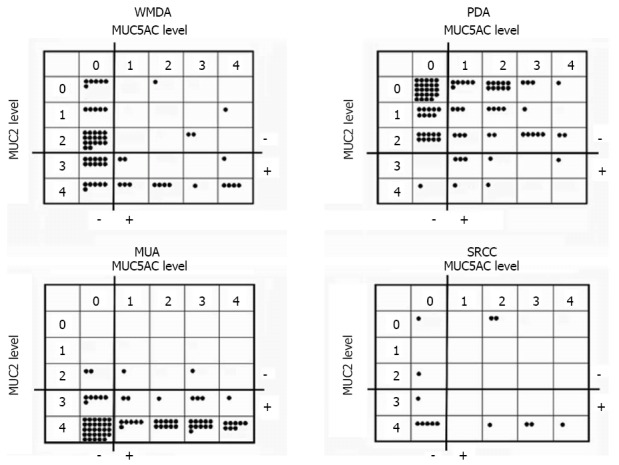
Expression profiles of mucin 2 and mucin 5AC in each colorectal carcinoma histological subtype. Each closed circle indicates one tumor. MUC: Mucin; WMDA: Well-to-moderately differentiated adenocarcinoma; PDA: Poorly differentiated adenocarcinoma; MUA: Mucinous adenocarcinoma; SRCC: Signet-ring cell carcinoma.
Expression of the mucin core proteins in the adenoma-carcinoma sequence
Among our study subjects, 21 tumors in 20 patients had an adenoma component indicative of originating from the adenoma-carcinoma sequence. We investigated the sequential expression status of the mucin core proteins. MUC2 expression was found in the adenoma component in all cases, except for one PDA case. MUC2 expression was relatively well maintained in the carcinoma component of WMDA and mildly decreased in some MUA cases. MUC2 expression significantly decreased in the carcinoma component of PDA. MUC5AC was aberrantly expressed in the tubular/tubulovillous adenoma components in all but three cases, and there was a significant decrease in MUC5AC expression in the carcinoma components as compared with the adenoma components in PDA and MUA (Table 2).
Table 2.
Expression levels of the mucin core proteins in the adenoma carcinoma sequence
|
Histology |
MUC2 |
MUC5AC |
||||||
| No. of patient | Adenoma | Carcinoma | Adenoma | Carcinoma | P value1 | Adenoma | Carcinoma | P value1 |
| 208 | TV | WMDA | 4 | 3 | ND | 4 | 1 | ND |
| 225 | T | WMDA | 4 | 4 | 3 | 2 | ||
| 237 | TV | WMDA | 4 | 4 | 2 | 4 | ||
| 243 | TV | WMDA | 4 | 4 | 4 | 4 | ||
| 84 | TV | PDA | 4 | 2 | 0.016 | 4 | 0 | 0.042 |
| 86 | TV | PDA | 4 | 0 | 1 | 0 | ||
| 87 | TV | PDA | 4 | 0 | 0 | 0 | ||
| 102 | TV | PDA | 4 | 0 | 4 | 0 | ||
| 116 | T | PDA | 2 | 0 | 2 | 0 | ||
| 138 | T | PDA | 4 | 0 | 3 | 0 | ||
| 148 | T | PDA | 3 | 0 | 0 | 0 | ||
| 15 | TV | MUA | 4 | 4 | 0.063 | 3 | 0 | 0.016 |
| 36 | TV | MUA | 4 | 4 | 3 | 3 | ||
| 37 | TV | MUA | 4 | 4 | 0 | 0 | ||
| 51 | TV | MUA | 4 | 4 | 3 | 3 | ||
| 131 | T | MUA | 4 | 2 | 3 | 1 | ||
| 148 | TV | MUA | 4 | 2 | 4 | 0 | ||
| 151 | TV | MUA | 4 | 3 | 3 | 1 | ||
| 155 | TV | MUA | 4 | 4 | 4 | 2 | ||
| 175 | TV | MUA | 4 | 4 | 4 | 2 | ||
| 191 | TV | MUA | 4 | 3 | 3 | 0 | ||
Wilcoxon signed-rank test. MUC: Mucin; T: Tubular adenoma; TV: Tubulovillous adenoma; WMDA: Well-to-moderately differentiated adenocarcinoma; PDA: Poorly differentiated adenocarcinoma; MUA: Mucinous adenocarcinoma; ND: Not determined.
Expression of the mucin core proteins and clinicopathologic variables
Expression status of the mucin core proteins was analyzed in association with clinicopathologic variables in each histological CRC subtype that had sufficient numbers for statistical analysis. In a contingency table analysis, no statistically significant associations were found between MUC2 expression and any clinicopathologic variables in any of the histological subtypes. In contrast, MUC5AC expression was significantly associated with right-sided location, absence of nodal metastasis, and lower TNM stage in PDA, and right-sided location in MUA. Furthermore, MUC5AC expression tended to be associated with older age in WMDA, PDA, and MUA, although the difference was not statistically significant. MUC5AC expression was not associated with any clinicopathologic variables in SRCC. These results are summarized in Table 3 (partly not shown).
Table 3.
Expression of the mucin core proteins and clinicopathologic variables n (%)
| WMDA | PDA | MUA | ||||||||
| MUC2 | MUC2 | MUC2 | ||||||||
| - | + | P value | - | + | P value | - | + | P value | ||
| Median age (range), yr | 64 (32-82) | 64 (34-87) | 0.783 | 64 (35-92) | 66.5 (55-86) | 0.633 | 65 (52-88) | 71 (26-90) | 0.842 | |
| Gender | Male | 21 (33.3) | 18 (28.6) | 0.537 | 41 (45.1) | 4 (4.4) | 1.000 | 1 (1.2) | 45 (55.6) | 0.311 |
| Female | 11 (17.5) | 13 (20.6) | 42 (46.1) | 4 (4.4) | 3 (3.7) | 32 (39.5) | ||||
| Locus | Right-sided | 10 (15.9) | 10 (15.9) | 0.932 | 47 (51.6) | 6 (6.6) | 0.461 | 1 (1.2) | 40 (49.4) | 0.359 |
| Left-sided | 22 (34.9) | 21 (33.3) | 36 (39.6) | 2 (2.2) | 3 (3.7) | 37 (45.7) | ||||
| Venous invasion | Yes | 24 (38.1) | 24 (38.1) | 1.000 | 71 (79.0) | 6 (6.6) | 0.325 | 3 (3.7) | 38 (46.9) | 0.616 |
| No | 8 (12.7) | 7 (11.1) | 11 (12.2) | 2 (2.2) | 1 (1.2) | 39 (48.1) | ||||
| Lymphatic invasion | Yes | 21 (33.3) | 14 (22.2) | 0.102 | 76 (84.4) | 6 (6.7) | 0.148 | 1 (1.2) | 51 (63.0) | 1.000 |
| No | 11 (17.5) | 17 (27.0) | 6 (6.7) | 2 (2.2) | 3 (3.7) | 26 (32.1) | ||||
| Nodal metastasis | Yes | 20 (31.7) | 14 (22.2) | 0.167 | 64 (72.7) | 5 (5.7) | 0.362 | 2 (2.5) | 35 (44.3) | 1.000 |
| No | 12 (19.0) | 17 (27.0) | 16 (18.2) | 3 (3.4) | 2 (2.5) | 40 (50.6) | ||||
| TNM stage | I/II | 12 (19.0) | 17 (27.0) | 0.167 | 14 (15.6) | 3 (3.3) | 0.171 | 2 (2.5) | 38 (48.1) | 1.000 |
| III/IV | 20 (31.7) | 14 (22.2) | 68 (75.5) | 5 (5.6) | 2 (2.5) | 37 (46.8) | ||||
| dMMR | Yes | ND | 16 (17.6) | 3 (3.3) | 0.356 | ND | ||||
| No | 67 (73.6) | 5 (5.5) | ||||||||
| MUC5AC | MUC5AC | MUC5AC | ||||||||
| - | + | P value | - | + | P value | - | + | P value | ||
| Median age (range), yr | 64 (32-83) | 69 (47-87) | 0.099 | 63 (36-87) | 70 (35-92) | 0.096 | 67 (34-87) | 72 (26-90) | 0.061 | |
| Gender | Male | 26 (41.3) | 13 (20.6) | 0.677 | 22 (24.2) | 23 (25.3) | 0.919 | 23 (28.4) | 23 (28.4) | 0.371 |
| Female | 18 (28.6) | 6 (9.5) | 22 (24.2) | 24 (26.3) | 14 (17.3) | 21 (25.9) | ||||
| Locus | Right-sided | 10 (15.9) | 10 (15.9) | 0.410 | 20 (22.0) | 33 (36.3) | 0.017 | 12 (14.8) | 29 (35.8) | 0.003 |
| Left-sided | 34 (54.0) | 9 (14.3) | 24 (26.4) | 14 (15.4) | 25 (30.9) | 15 (18.5) | ||||
| Venous invasion | Yes | 34 (54.0) | 14 (22.2) | 0.757 | 38 (42.2) | 39 (43.3) | 1.000 | 16 (19.8) | 25 (30.9) | 0.224 |
| No | 10 (15.9) | 5 (7.9) | 6 (6.7) | 7 (7.8) | 21 (25.9) | 19 (23.5) | ||||
| Lymphatic invasion | Yes | 26 (41.3) | 9 (14.3) | 0.560 | 41 (45.6) | 41 (45.6) | 0.714 | 25 (30.9) | 29 (35.8) | 0.875 |
| No | 18 (28.6) | 10 (15.9) | 3 (3.3) | 5 (5.6) | 12 (14.8) | 15 (18.5) | ||||
| Nodal metastasis | Yes | 23 (36.5) | 11 (17.5) | 0.892 | 40 (45.5) | 29 (33.0) | 0.010 | 17 (21.5) | 20 (25.3) | 0.950 |
| No | 21 (33.3) | 8 (12.7) | 4 (4.5) | 15 (17.0) | 19 (24.1) | 23 (29.1) | ||||
| TNM stage | I/II | 21 (33.3) | 8 (12.7) | 0.892 | 3 (3.3) | 14 (15.6) | 0.010 | 18 (22.8) | 22 (27.8) | 0.918 |
| III/IV | 23 (36.5) | 11 (17.5) | 41 (45.6) | 32 (35.6) | 18 (22.8) | 21 (26.6) | ||||
| dMMR | Yes | ND | 3 (3.3) | 16 (17.6) | 0.003 | ND | ||||
| No | 41 (45.1) | 31 (34.1) | ||||||||
MUC: Mucin; WMDA: Well-to-moderately differentiated adenocarcinoma; PDA: Poorly differentiated adenocarcinoma; MUA: Mucinous adenocarcinoma; dMMR: Mismatch-repair deficiency; ND: Not determined; TNM: Tumor node metastasis.
Expression of the MMR proteins and the mucin core proteins in PDA
In PDA cases, MUC5AC positivity was significantly associated with right-sided location and lower TNM stage, and marginally associated with older age. Survival curve analysis also suggested a better prognosis for MUC5AC-positive PDA cases than for negative ones as described below. These are clinical features associated with high levels of microsatellite instability (MSI; MSI-H)[17-19]. A subset of sporadic CRC cases (approximately 10%-15%) is MSI-H; this is caused by inactivation of the DNA MMR system. Identifying MSI previously required molecular testing, although immunostaining for hMLH1 and hMSH2 has come to be accepted as a practical test to detect MSI[20,21]. Therefore, we investigated MMR status in PDA cases using immunohistochemistry. Tumors that did not express either of these two were considered MMR deficiency (dMMR).
dMMR was found in 19 PDA cases, 16 of 47 MUC5AC-positive cases and 3 of 44 MUC5AC-negative cases (P = 0.003). In contrast, there was no significant association between MUC2 expression and dMMR. Thus, dMMR showed statistically significant association with MUC5AC positivity, although it should be noted that dMMR was found in only one third of MUC5AC-positive tumors and dMMR was also found in one tenth of MUC5AC-negative tumors (Table 3).
Expression of the mucin core proteins and prognosis
The effects of clinicopathologic variables on recurrence/metastasis-free survival (RFS) and overall survival (OS) were investigated using Cox regression analysis for each CRC subtype. For WMDA, TNM stage was the only significant prognostic factor for RFS by univariate and multivariate analysis, but no significant predictor for OS was identified. Next, we included dMMR in the survival analysis for PDA. Univariate Cox regression analysis showed that TNM stage, MUC5AC expression, and dMMR were significant prognostic factors for RFS; however none of these was significant by multivariate analysis. TNM stage and dMMR were also significant predictors for OS by univariate analysis but were not significant by multivariate analysis (Table 4).
Table 4.
Prognostic significance of clinicopathologic variables in well-to-moderately differentiated adenocarcinoma and poorly differentiated adenocarcinoma
|
Recurrence/metastasis |
Death |
|||||||
| Parameter | HR (95%CI) | P value | Parameter | HR (95%CI) | P value | |||
| WMDA | Univariate analysis | Univariate analysis | ||||||
| Age (over 65 yr) | 2.215 (0.974-5.037) | 0.058 | Age (over 65 yr) | 1.984 (0.794-4.955) | 0.142 | |||
| Gender (male) | 0.887 (0.398-1.975) | 0.769 | Gender (male) | 2.194 (0.727-6.617) | 0.163 | |||
| Location (right-sided) | 1.268 (0.559-2.875) | 0.570 | Location (right-sided) | 1.335 (0.524-3.402) | 0.545 | |||
| TNM stage (III/IV) | 3.642 (1.446-9.170) | 0.006 | TNM stage (III/IV) | 2.632 (0.990-6.996) | 0.052 | |||
| MUC2 positive | 0.477 (0.210-1.082) | 0.077 | MUC2 positive | 0.527 (0.207-1.341) | 0.179 | |||
| MUC5AC positive | 1.389 (0.613-3.145) | 0.431 | MUC5AC positive | 1.803 (0.723-4.497) | 0.206 | |||
| Multivariate analysis | Multivariate analysis | |||||||
| Age (over 65 yr) | 2.220 (0.965-5.017) | 0.061 | ||||||
| TNM stage (III/IV) | 3.473 (1.370-8.805) | 0.009 | ||||||
| MUC2 positive | 0.554 (0.243-1.265) | 0.161 | ||||||
| PDA | Univariate analysis | Univariate analysis | ||||||
| Age (over 65 yr) | 0.891 (0.495-1.602) | 0.700 | Age (over 65 yr) | 0.985 (0.955-1.016) | 0.331 | |||
| Gender (male) | 0.624 (0.343-1.138) | 0.124 | Gender (male) | 0.899 (0.484-1.667) | 0.734 | |||
| Location (right-sided) | 0.857 (0.474-1.549) | 0.609 | Location (right-sided) | 0.686 (0.371-1.270) | 0.231 | |||
| TNM stage (III/IV) | 2.647 (1.109-6.320) | 0.028 | TNM stage (III/IV) | 3.208 (1.236-8.330) | 0.017 | |||
| MUC2 positive | 0.936 (0.368-2.379) | 0.890 | MUC2 positive | 1.009 (0.357-2.850) | 0.987 | |||
| MUC5AC positive | 0.433 (0.237-0.793) | 0.007 | MUC5AC positive | 0.599 (0.323-1.112) | 0.105 | |||
| dMMR | 0.373 (0.164-0.847) | 0.018 | dMMR | 0.352 (0.153-0.810) | 0.014 | |||
| Multivariate analysis | Multivariate analysis | |||||||
| TNM stage (III/IV) | 1.698 (0.672-4.289) | 0.263 | TNM stage (III/IV) | 2.385 (0.886-6.421) | 0.085 | |||
| MUC5AC positive | 0.586 (0.307-1.117) | 0.104 | dMMR | 0.466 (0.195-1.111) | 0.085 | |||
| dMMR | 0.228 (0.260-1.308) | 0.175 | ||||||
WMDA: Well-to-moderately differentiated adenocarcinoma; PDA: Poorly differentiated adenocarcinoma; dMMR: Mismatch-repair deficiency; TNM: Tumor node metastasis; MUC: Mucin.
For MUA, MUC2 expression was excluded from the analysis because there were very few MUC2-negative MUA cases (n = 4 out of 81). The TNM stage was the only significant predictor for RFS, and no variable was a significant predictor for OS (data not shown).
Thus, no significant associations were found between mucin expression and the prognosis of each CRC subtype by multivariate Cox analysis. However, as the data suggested marginal associations between mucin expression and prognosis, and from the need for subsequent discussion, Kaplan-Meier survival curves for associations with mucin expression were generated and assessed by log-rank test for each CRC subtype.
For this analysis of WMDA, marginally better RFS and OS with MUC2-positive tumors were found as compared with negative tumors. The prognosis of MUC5AC-positive WMDA cases tended to be worse as compared to those that were negative, although this difference was not significant (Figure 4A-D). There was also no difference with respect to MUC2 status in PDA. However, MUC5AC expression in PDA was significantly and marginally associated with a better prognosis in terms of RFS and OS, respectively (Figure 4E-H). Because MUC2 positivity was very high in MUA (77 of 81 tumors), its clinical significance in these settings was not investigated. MUC5AC expression in MUA did not affect its prognosis (Figure 4I and J).
Figure 4.
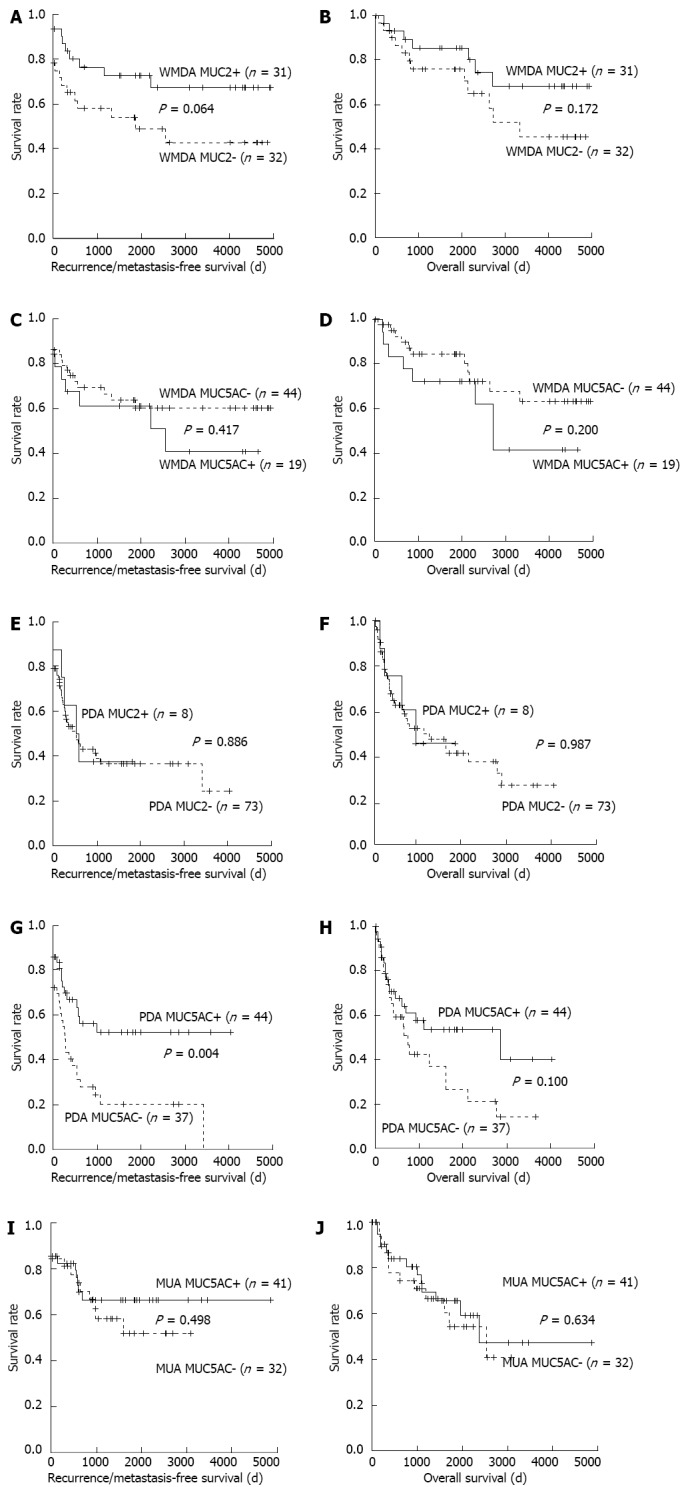
Survival curves. A-D: Well-to-moderately differentiated adenocarcinoma (WMDA); E-H: Poorly differentiated adenocarcinoma (PDA); I and J: Mucinous adenocarcinoma (MUA). A: Recurrence/metastasis-free; B: Overall survival curves with or without mucin (MUC) 2 expression of WMDA; C: Recurrence/metastasis-free; D: Overall survival curves with or without MUC5AC expression; E: Recurrence/metastasis-free; F: Overall survival curves with or without MUC2 expression; G: Recurrence/metastasis-free; H: Overall survival curves with or without MUC5AC expression; I: Recurrence/metastasis-free; J: Overall survival curves with or without MUC5AC expression. Curves were generated using the Kaplan-Meier method and compared by log-rank tests. P values were derived from comparing mucin-negative and -positive tumors.
DISCUSSION
MUC2 is normally expressed in the perinuclear cytoplasm of goblet cells in normal colonic mucosa. MUC2 is also expressed in adenomas and mucinous carcinomas[4]. MUC2 downregulation occurs in non-mucinous adenocar-cinomas that arise within adenomas, whereas cancers that are considered to develop de novo do not express MUC2[4]. Thus, MUC2 levels have been thought to be a predictor of malignant potential. However, despite a poor prognosis, higher levels of MUC2 expression were maintained in MUA and SRCC than in WMDA. This suggests the difficulty with using MUC2 levels as a differentiation marker in these subtypes.
Some investigators reported MUC2 expression in CRC in association with clinical significance, although the association between MUC2 expression and prognosis has been controversial. For example, Matsuda et al[9] analyzed 86 CRCs that included 82 WMDA tumors and 4 other variants and reported that the MUC2 expression level was not associated with advanced Dukes’ stage and liver metastasis. Baldus et al[10] investigated 243 CRCs that included 213 grade I-II tumors and 30 grade III tumors, which also included 22 MUA tumors, and reported that MUC2 reactivity was not a marker for worse survival. In contrast, Kang et al[8] investigated 301 patients with stage II-III CRCs, including 200 well-to-moderately differentiated and 101 poorly differentiated cancers (also 266 nonmucinous and 35 mucinous) and reported that a loss of MUC2 expression was associated with a worse overall survival with CRC of stages II and III. Hanski et al[7] showed that a loss of MUC2 expression in CRC owing to promoter methylation was associated with liver and nodal metastasis. On the other hand, a loss of MUC2 expression in CRCs was associated with peritumoral lymphocyte infiltration[22]. Host responses, such as peritumoral lymphocyte infiltration, are known to be associated with a favorable CRC prognosis[23]. MUC2 mucin harbors the sialosyl-Tn antigen that mediates the inhibition of natural killer cell cytotoxicity[24].
However, MUC5AC expression is usually absent in normal colonic mucosa and is only occasionally found in pericancerous normally appearing colonic mucosa. Aberrant MUC5AC expression can be observed in a subset of adenomas and adenocarcinomas[11,25-27]. Microscopically, MUC5AC was detected primarily as focal staining in the cytoplasm and mucous droplets in goblet cells in the normally appearing colonic mucosa but was diffuse in the cytoplasm of cancer cells. MUC5AC expression levels are highest in adenoma but decrease with increasing degrees of dysplasia, and the positive rates for MUC5AC expression were lower in CRC than in adenoma[11,25-27]. These results suggest that MUC5AC may play a role in early carcinogenesis and its expression status can be used to classify CRC from the viewpoint of pathogenesis.
Biemer-Hüttmann et al[28] and Losi et al[29] reported associations between MUC5AC expression and MSI-H, which is linked with histological subtypes like PDA and MUA. Kocer et al[12] analyzed MUC5AC expression in 41 CRCs that included 33 adenocarcinomas, 5 mucinous carcinomas, and 3 neuroendocrine carcinomas. They reported that MUC5AC-negative CRCs had lower rates of disease-free status and of overall survival, but thy did not investigate associations between MSI and MUC5AC expression.
It has come to be well recognized that CRC comprises various carcinomas that originate from distinct pathogenetic pathways. Owing to this CRC heterogeneity, the significance of mucin core protein expression in CRC remains controversial. Thus, we performed these analyses for each histological CRC subtype.
In the present study, the expression profiles of MUC2 and MUC5AC in conventional adenocarcinoma (WMDA and PDA), MUA, and SRCC were similar to those in previous reports. High MUC2 expression levels in MUA and SRCC suggested distinct histogenetic pathways for these cancer cells, which maintained the feature of mucin-producing goblet cells, from conventional adenocarcinoma. In addition, the expression of the both mucin core proteins tended to decrease during the course of disease progression, from adenoma to carcinoma or from WMDA to PDA. Disease progression is usually accompanied by a decrease in cells of the goblet lineage.
These results are consistent with the hypothesis that the levels of the mucin core proteins may be a marker of malignancy potential in the adenoma-carcinoma sequence in both non-mucinous and mucinous carcinomas. We found that the prognostic significance of the mucin core proteins was different between MUC2 and MUC5AC. In our survival curve analysis, a tendency for better prognosis with MUC2-positive cases than for negative cases was observed in WMDA. A difference in prognosis was not evident from the MUC2 status in PDA.
However, MUC5AC expression in PDA was significantly associated with a better RFS and marginally associated with a better OS. MUC5AC was a significant factor for RFS by univariate Cox regression analysis. Although no significant effects of MUC5AC expression on RFS or OS were found by multivariate analysis, these results may have been because of the limited number of cases or other unknown factors associated with this patient population. A larger study will be needed to clarify these points.
In comparison, MUC5AC expression was not a factor associated with better prognosis in WMDA and MUA. Aberrant MUC5AC expression is considered to be an early event in carcinogenesis. In addition, MUC5AC expression was significantly associated with right-sided location, absence of nodal metastasis, and a lower TNM stage and was marginally associated with older age in our PDA series. These clinical features as well as poor differentiation are characteristic of MSI-high tumors. MUC5AC expression in PDA was significantly associated with dMMR as shown by a loss of MMR protein expression (16 of 47 MUC5AC-positive cases vs 3 of 44 negative cases; P = 0.003). Unlike the report by Biemer-Hüttmann et al[28] stating that MUC2 expression was also associated with MSI-H, our PDA cases did not exhibit an association between the two. These results suggest that PDA also consists of heterogenous groups of cancers and that MUC5AC expression status may be one of the classification hallmarks.
To date, the mechanisms underlining aberrant MUC5AC expression in the colon have not been determined. During colon carcinogenesis, MUC5AC expression may be regarded as the re-expression of this fetal mucin. MUC5AC mucin is detected from the fourth month of gestation and is maximum during the sixth month[30]. On the other hand, the MUC5AC promoter was shown to be activated by various inflammation mediators[31]. It was also reported that tumor necrosis factor-α stimulated colon cancer HT-29 cells, which are a goblet cell line, to secrete MUC5AC mucin in a dose-dependent manner[32]. Forgue-Lafitte et al[33] reported that MUC5AC mucin was detectable in the mucus of ulcerative colitis patients who underwent surgery. In their series, 10 patients suffering from ulcerative colitis tested were positive for MUC5AC, which suggested that long-term chronic inflammation may induce the production of this mucin in the colonic epithelium. In addition, MUC5AC expression in the regenerating areas close to ulcerations in Crohn’s disease suggests its involvement in tissue repair mechanisms[34]. CRC with MUC5AC expression may originate from precancerous lesions owing to long-standing inflammation caused by bacterial infection, inflammatory bowel disease, or other reasons.
In our study, we sometimes observed aberrant MUC5AC expression in normally-looking colonic mucosa at the interface between non-tumor and tumor tissue, where a strong anti-tumor inflammatory reaction had been observed. We speculate that this may be suggestive of the origin of a neoplasm with aberrant MUC5AC expression. Furthermore, it was previously reported that long-standing inflammation due to ulcerative colitis resulted in CRC with dMMR[35,36]. Taken together, a hypothesis of inflammation-related carcinogenesis may explain the pathogenesis of CRC from MUC5AC-positive precancerous lesions and an association between dMMR and MUC5AC expression.
In conclusion, we investigated MUC2 and MUC5AC expression status in each of the histological CRC subtypes. MUC2 levels were decreased and MUC5AC levels were increased from WMDA to PDA. MUA and SRCC maintained high MUC2 levels. The expressions of these mucins in PDA and MUA decreased during disease progression in the adenoma-carcinoma sequence. MUC5AC expression was closely associated with MMR deficiency in PDA. MUC2 and MUC5AC expression tended to be associated with a better prognosis in WMDA and PDA, respectively, although these were not statistically significant. Thus, the mucin proteins show distinct clinical significance according to the histological subtypes, and this may also suggest different pathogeneses for these tumors.
ACKNOWLEDGMENTS
We greatly appreciate the staff members of the Department of Pathology, Dokkyo Medical University Koshigaya Hospital and the Department of Pathology, Tokyo Kosei Nenkin Hospital for their technical and clerical assistance.
COMMENTS
Background
Mucin (MUC)2 mucin is predominantly found in colorectal goblet cells and MUC5AC mucin is primarily expressed in gastric mucosa. Altered mucin expression may be correlated with the biological behavior of and, possibly, the prognosis for colorectal carcinoma (CRC).
Research frontiers
To date, MUC2 and MUC5AC expressions in CRC have been investigated for their association with clinicopathologic characteristics and prognosis. However, many contradictory results make it difficult to interpret the clinical significance of altered mucin expression, possibly owing to various CRC subtypes. The authors investigated mucin expression by immunohistochemistry in each of the histological CRC subtypes individually and assessed its significance.
Innovations and breakthroughs
MUC2 levels may be associated with malignant potential in conventional adenocarcinoma, whereas mucinous adenocarcinoma and signet-ring cell carcinoma retain high MUC2 levels, which suggests distinct pathogenesis. In comparison, MUC5AC expression may be an early event in tumorigenesis. MUC5AC expression in poorly differentiated adenocarcinoma (PDA) was closely associated with mismatch repair deficiency. MUC2 and MUC5AC expression tended to be associated with a better prognosis in well-to-moderately differentiated adenocarcinoma and PDA, respectively. Thus, the mucin proteins show different clinical significance according to the histological subtypes, and this may also suggest different pathogeneses of these tumors.
Applications
These results could be the basis for further studies to understand the pathogenesis of CRC. Immunohistochemical detection of MUC5AC may be useful for further subclassifications and for predicting a favorable prognosis for PDA.
Terminology
MUC2 and MUC5AC are the backbone proteins of secreted mucins. MUC2 is synthesized in goblet cells in the gastrointestinal tract and MUC5AC is normally expressed in gastric foveolar epithelium. The MUC2 and MUC5AC genes encode gel-forming mucins and are located in a cluster on chromosome 11p15.5.
Peer review
This study is important and interesting. Although there are a large number of publications on the roles of MUC2 and MUC5AC expression in CRC, the results are conflicting. This study adds some refinements to these issues, such as MUC2 and MUC5AC expressions in terms of CRC patient survival, adenoma-carcinoma sequence, and expression of MMR proteins, with regard to associations with clinicopathologic variables by offering its own evidence.
Footnotes
Supported by Haraguchi Memorial Trust Fund
P- Reviewers Khan WI, Sgourakis G S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 2.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Pigny P, Guyonnet-Duperat V, Hill AS, Pratt WS, Galiegue-Zouitina S, d’Hooge MC, Laine A, Van-Seuningen I, Degand P, Gum JR, et al. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996;38:340–352. doi: 10.1006/geno.1996.0637. [DOI] [PubMed] [Google Scholar]
- 4.Blank M, Klussmann E, Krüger-Krasagakes S, Schmitt-Gräff A, Stolte M, Bornhoeft G, Stein H, Xing PX, McKenzie IF, Verstijnen CP, et al. Expression of MUC2-mucin in colorectal adenomas and carcinomas of different histological types. Int J Cancer. 1994;59:301–306. doi: 10.1002/ijc.2910590302. [DOI] [PubMed] [Google Scholar]
- 5.Mizoshita T, Tsukamoto T, Inada KI, Hirano N, Tajika M, Nakamura T, Ban H, Tatematsu M. Loss of MUC2 expression correlates with progression along the adenoma-carcinoma sequence pathway as well as de novo carcinogenesis in the colon. Histol Histopathol. 2007;22:251–260. doi: 10.14670/HH-22.251. [DOI] [PubMed] [Google Scholar]
- 6.Hirano K, Nimura S, Mizoguchi M, Hamada Y, Yamashita Y, Iwasaki H. Early colorectal carcinomas: CD10 expression, mucin phenotype and submucosal invasion. Pathol Int. 2012;62:600–611. doi: 10.1111/j.1440-1827.2012.02850.x. [DOI] [PubMed] [Google Scholar]
- 7.Hanski C, Riede E, Gratchev A, Foss HD, Böhm C, Klussmann E, Hummel M, Mann B, Buhr HJ, Stein H, et al. MUC2 gene suppression in human colorectal carcinomas and their metastases: in vitro evidence of the modulatory role of DNA methylation. Lab Invest. 1997;77:685–695. [PubMed] [Google Scholar]
- 8.Kang H, Min BS, Lee KY, Kim NK, Kim SN, Choi J, Kim H. Loss of E-cadherin and MUC2 expressions correlated with poor survival in patients with stages II and III colorectal carcinoma. Ann Surg Oncol. 2011;18:711–719. doi: 10.1245/s10434-010-1338-z. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda K, Masaki T, Watanabe T, Kitayama J, Nagawa H, Muto T, Ajioka Y. Clinical significance of MUC1 and MUC2 mucin and p53 protein expression in colorectal carcinoma. Jpn J Clin Oncol. 2000;30:89–94. doi: 10.1093/jjco/hyd023. [DOI] [PubMed] [Google Scholar]
- 10.Baldus SE, Mönig SP, Hanisch FG, Zirbes TK, Flucke U, Oelert S, Zilkens G, Madejczik B, Thiele J, Schneider PM, et al. Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology. 2002;40:440–449. doi: 10.1046/j.1365-2559.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- 11.Bartman AE, Sanderson SJ, Ewing SL, Niehans GA, Wiehr CL, Evans MK, Ho SB. Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int J Cancer. 1999;80:210–218. doi: 10.1002/(SICI)1097-0215(19990118)80:2<210::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470–477. doi: 10.1046/j.1440-1827.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 13.Bozman FT, Carneiro F, Hruban RH, Theise N, editors . WHO classification of tumours. Pathology and genetics. Tumours of the digestive system. 4th ed. Berlin: Springer-Verlag; 2010. [Google Scholar]
- 14.O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69:866–872. [PubMed] [Google Scholar]
- 15.Fairley TL, Cardinez CJ, Martin J, Alley L, Friedman C, Edwards B, Jamison P. Colorectal cancer in U.S. adults younger than 50 years of age, 1998-2001. Cancer. 2006;107(5 Suppl):1153–1161. doi: 10.1002/cncr.22012. [DOI] [PubMed] [Google Scholar]
- 16.American Joint Committee on Cancer. Colon and Rectum Cancer Staging. 7th ed. 2009. Available from: http://www.cancerstaging.org/staging/index.html. [Google Scholar]
- 17.Kakar S, Burgart LJ, Thibodeau SN, Rabe KG, Petersen GM, Goldberg RM, Lindor NM. Frequency of loss of hMLH1 expression in colorectal carcinoma increases with advancing age. Cancer. 2003;97:1421–1427. doi: 10.1002/cncr.11206. [DOI] [PubMed] [Google Scholar]
- 18.Togo G, Toda N, Kanai F, Kato N, Shiratori Y, Kishi K, Imazeki F, Makuuchi M, Omata M. A transforming growth factor beta type II receptor gene mutation common in sporadic cecum cancer with microsatellite instability. Cancer Res. 1996;56:5620–5623. [PubMed] [Google Scholar]
- 19.Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91:2417–2422. [PubMed] [Google Scholar]
- 20.Marcus VA, Madlensky L, Gryfe R, Kim H, So K, Millar A, Temple LK, Hsieh E, Hiruki T, Narod S, et al. Immunohistochemistry for hMLH1 and hMSH2: a practical test for DNA mismatch repair-deficient tumors. Am J Surg Pathol. 1999;23:1248–1255. doi: 10.1097/00000478-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 22.Ajioka Y, Allison LJ, Jass JR. Significance of MUC1 and MUC2 mucin expression in colorectal cancer. J Clin Pathol. 1996;49:560–564. doi: 10.1136/jcp.49.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shunyakov L, Ryan CK, Sahasrabudhe DM, Khorana AA. The influence of host response on colorectal cancer prognosis. Clin Colorectal Cancer. 2004;4:38–45. doi: 10.3816/CCC.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 24.Ogata S, Maimonis PJ, Itzkowitz SH. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Res. 1992;52:4741–4746. [PubMed] [Google Scholar]
- 25.Buisine MP, Janin A, Maunoury V, Audié JP, Delescaut MP, Copin MC, Colombel JF, Degand P, Aubert JP, Porchet N. Aberrant expression of a human mucin gene (MUC5AC) in rectosigmoid villous adenoma. Gastroenterology. 1996;110:84–91. doi: 10.1053/gast.1996.v110.pm8536891. [DOI] [PubMed] [Google Scholar]
- 26.Bara J, Loisillier F, Burtin P. Antigens of gastric and intestinal mucous cells in human colonic tumours. Br J Cancer. 1980;41:209–221. doi: 10.1038/bjc.1980.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bara J, Languille O, Gendron MC, Daher N, Martin E, Burtin P. Immunohistological study of precancerous mucus modification in human distal colonic polyps. Cancer Res. 1983;43:3885–3891. [PubMed] [Google Scholar]
- 28.Biemer-Hüttmann AE, Walsh MD, McGuckin MA, Simms LA, Young J, Leggett BA, Jass JR. Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res. 2000;6:1909–1916. [PubMed] [Google Scholar]
- 29.Losi L, Scarselli A, Benatti P, Ponz de Leon M, Roncucci L, Pedroni M, Borghi F, Lamberti I, Rossi G, Marino M, et al. Relationship between MUC5AC and altered expression of MLH1 protein in mucinous and non-mucinous colorectal carcinomas. Pathol Res Pract. 2004;200:371–377. doi: 10.1016/j.prp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Bara J, Gautier R, Daher N, Zaghouani H, Decaens C. Monoclonal antibodies against oncofetal mucin M1 antigens associated with precancerous colonic mucosae. Cancer Res. 1986;46:3983–3989. [PubMed] [Google Scholar]
- 31.Van Seuningen I, Pigny P, Perrais M, Porchet N, Aubert JP. Transcriptional regulation of the 11p15 mucin genes. Towards new biological tools in human therapy, in inflammatory diseases and cancer? Front Biosci. 2001;6:D1216–D1234. doi: 10.2741/seuning. [DOI] [PubMed] [Google Scholar]
- 32.Smirnova MG, Birchall JP, Pearson JP. TNF-alpha in the regulation of MUC5AC secretion: some aspects of cytokine-induced mucin hypersecretion on the in vitro model. Cytokine. 2000;12:1732–1736. doi: 10.1006/cyto.2000.0763. [DOI] [PubMed] [Google Scholar]
- 33.Forgue-Lafitte ME, Fabiani B, Levy PP, Maurin N, Fléjou JF, Bara J. Abnormal expression of M1/MUC5AC mucin in distal colon of patients with diverticulitis, ulcerative colitis and cancer. Int J Cancer. 2007;121:1543–1549. doi: 10.1002/ijc.22865. [DOI] [PubMed] [Google Scholar]
- 34.Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:553–571. doi: 10.1016/j.gtc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Issa JP, Ahuja N, Toyota M, Bronner MP, Brentnall TA. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–3577. [PubMed] [Google Scholar]
- 36.Fleisher AS, Esteller M, Harpaz N, Leytin A, Rashid A, Xu Y, Liang J, Stine OC, Yin J, Zou TT, et al. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer Res. 2000;60:4864–4868. [PubMed] [Google Scholar]


