Abstract
AIM: To investigate bone mineral density (BMD) in obese children with and without nonalcoholic fatty liver disease (NAFLD); and the association between BMD and serum adipokines, and high-sensitivity C-reactive protein (HSCRP).
METHODS: A case-control study was performed. Cases were 44 obese children with NAFLD. The diagnosis of NAFLD was based on magnetic resonance imaging (MRI) with high hepatic fat fraction (≥ 5%). Other causes of chronic liver disease were ruled out. Controls were selected from obese children with normal levels of aminotransferases, and without MRI evidence of fatty liver as well as of other causes of chronic liver diseases. Controls were matched (1- to 1-basis) with the cases on age, gender, pubertal stage and as closely as possible on body mass index-SD score. All participants underwent clinical examination, laboratory tests, and whole body (WB) and lumbar spine (LS) BMD by dual energy X-ray absorptiometry. BMD Z-scores were calculated using race and gender specific LMS curves.
RESULTS: Obese children with NAFLD had a significantly lower LS BMD Z-score than those without NAFLD [mean, 0.55 (95%CI: 0.23-0.86) vs 1.29 (95%CI: 0.95-1.63); P < 0.01]. WB BMD Z-score was also decreased in obese children with NAFLD compared to obese children with no NAFLD, though borderline significance was observed [1.55 (95%CI: 1.23-1.87) vs 1.95 (95%CI: 1.67-2.10); P = 0.06]. Children with NAFLD had significantly higher HSCRP, lower adiponectin, but similar leptin levels. Thirty five of the 44 children with MRI-diagnosed NAFLD underwent liver biopsy. Among the children with biopsy-proven NAFLD, 20 (57%) had nonalcoholic steatohepatitis (NASH), while 15 (43%) no NASH. Compared to children without NASH, those with NASH had a significantly lower LS BMD Z-score [mean, 0.27 (95%CI: -0.17-0.71) vs 0.75 (95%CI: 0.13-1.39); P < 0.05] as well as a significantly lower WB BMD Z-score [1.38 (95%CI: 0.89-1.17) vs 1.93 (95%CI: 1.32-2.36); P < 0.05]. In multiple regression analysis, NASH (standardized β coefficient, -0.272; P < 0.01) and HSCRP (standardized β coefficient, -0.192; P < 0.05) were significantly and independently associated with LS BMD Z-score. Similar results were obtained when NAFLD (instead of NASH) was included in the model. WB BMD Z-scores were significantly and independently associated with NASH (standardized β coefficient, -0.248; P < 0.05) and fat mass (standardized β coefficient, -0.224; P < 0.05).
CONCLUSION: This study reveals that NAFLD is associated with low BMD in obese children, and that systemic, low-grade inflammation may accelerate loss of bone mass in patients with NAFLD.
Keywords: Bone mineralization, Dual energy X-ray absorptiometry, Adipokines, C-reactive protein, Nonalcoholic fatty liver disease, Children
Core tip: Understanding the mechanisms underlying the relationship between nonalcoholic fatty liver disease (NAFLD) and low bone mineral density (BMD) is important to prevent poor bone mineralization in obese children. We showed that obese children with NAFLD have decreased BMD compared to obese children without liver involvement independently of adiposity, and that children with more severe histology have worse mineral status than children with more mild abnormalities. We also found a significant independent association of high sensitivity C-reactive protein with BMD scores, supporting the role of an inflammatory state which may accelerate loss of bone mass in patients with NAFLD.
INTRODUCTION
Concurrent with the increasing rates of childhood obesity, nonalcoholic fatty liver disease (NAFLD) has emerged as the leading cause of chronic liver disease in pediatric populations worldwide[1,2]. NAFLD comprises a disease spectrum ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), with varying degrees of inflammation and fibrosis, progressing to end-stage liver disease with cirrhosis and hepatocellular carcinoma[3]. NAFLD is strongly associated with obesity, insulin resistance, hypertension, and dyslipidemia, and is now regarded as the liver manifestation of the metabolic syndrome (MetS)[4]. Recently it has been suggested that NAFLD can be a cause of low bone mineral density (BMD) in obese children and adolescents[5-7]. However, the mechanisms explaining this relationship are not completely understood[8]. Obesity-induced low-grade systemic inflammation, a key component in the pathogenesis of insulin resistance and NAFLD, may negatively influence bone health[9,10]. Expanded and inflamed visceral adipose tissue releases a wide array of molecules potentially involved in the development of insulin resistance, including free fatty acids, tumor necrosis factor (TNF)-α, and other proinflammatory cytokines[11-14]. In the presence of increased free fatty acid flux and chronic, low-grade inflammation, the liver is both the target of and a contributor to systemic inflammatory changes[15]. Indeed, in a number of case-control studies, circulating levels of several inflammatory markers [i.e., C-reactive protein (CRP), interleukin (IL)-6, monocyte chemotactic protein 1 and TNF-α], procoagulant factors, and oxidative stress markers were found to be highest in patients with NASH, intermediate in those with simple steatosis, and lowest in control subjects without steatosis, and the differences were independent of obesity and other potentially confounding factors[16].
Adipose tissue also produces adipokines, which are pleiotropic molecules that not only regulate food intake and energy metabolism but also are implicated in the complex interactions between fat and bone[17,18]. Leptin, produced in bone marrow adipocytes and osteoblastic cells, regulates appetite and weight, osteoblast proliferation and differentiation in vitro[19-21], and osteoclasts[19,22,23]. Its receptor is expressed in osteoblasts[19,24]. Adiponectin, exclusively expressed by adipocytes, is inversely related to visceral fat mass and body mass index (BMI)[25] and regulates metabolism and inflammatory pathways[26]. Adiponectin affects osteoblast directly and osteoclast indirectly. It stimulates the proliferation and differentiation of human osteoblasts via the p38 mitogen-activated protein kinase signaling pathway[27]. In contrast, adiponectin indirectly influences osteoclasts by stimulating the receptor activator of nuclear factor-ĸB ligand (RANKL) and inhibiting osteoprotegerin production in osteoblasts[28]. Some studies have shown a negative association between adiponectin and BMD, independent of fat mass or BMI[29].
The aims of this study were to evaluate: (1) BMD in obese children with and without NAFLD; and (2) the association between BMD and the serum adipokines, leptin and adiponectin, and a circulating marker of systemic inflammation, high-sensitivity C-reactive protein (HSCRP), using multiple regression.
MATERIALS AND METHODS
Study design and patients
A case-control study was performed. Cases were Caucasian obese children (BMI above the 95th percentile for age and gender) seen at the Hepatology outpatient Clinic of the Department of Pediatrics, Sapienza University of Rome, Italy. The diagnosis of NAFLD was based on magnetic resonance imaging (MRI) with high hepatic fat fraction (HFF ≥ 5%). Other causes of chronic liver disease, including hepatic virus infections (hepatitis A-E and G, cytomegalovirus, and Epstein-Barr virus), autoimmune hepatitis, metabolic liver disease, α-1-antitrypsin deficiency, cystic fibrosis, Wilson’s disease, hemochromatosis, and celiac disease were ruled out with appropriate tests. Exclusion criteria were also smoking habits, and history of type 1 or 2 diabetes, renal disease, total parenteral nutrition, use of hepatotoxic medications, and chronic alcohol intake. Finally, children were excluded for conditions that could have adversely influenced BMD including glucocorticoid therapy, hypothyroidism, Cushing’s disease; history of long bone fractures; indwelling hardware; and abnormality of the skeleton or spine[30,31].
Controls were selected from Caucasian obese children with normal levels of aminotransferases, and without MRI evidence of fatty liver (HFF < 5%) as well as of other causes of chronic liver diseases (see above). Controls were also excluded if they had smoking habits, history of type 1 or 2 diabetes, renal disease, chronic alcohol intake, and any condition known to influence BMD[30,31]. Controls were then matched (1- to 1-basis) with the cases on age, gender, pubertal stage and as closely as possible on BMI-SD score (SDS).
The research protocol was approved by the Hospital Ethics Committee, and informed consent was obtained from subjects’ parents before assessment.
Clinical and laboratory data
All participants underwent physical examination including measurements of weight, standing height, BMI and determination of the stage of puberty, and laboratory tests. The pubertal stage was categorized into two groups (prepubertal: boys with pubic hair and gonadal stage I, and girls with pubic hair stage and breast stage I; pubertal: boys with pubic hair and gonadal stage ≥ II and girls with pubic hair stage and breast stage ≥ II). The degree of obesity was quantified using Cole’s least mean-square method, which normalizes the skewed distribution of BMI and expresses BMI as SDS[32]. Blood samples were taken, after an overnight fast, for estimation of glucose, insulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), HSCRP, leptin, and adiponectin.
Analyses of glucose, insulin, ALT, AST, and HSCRP were conducted by COBAS 6000 (Roche Diagnostics). Insulin concentrations were measured on cobas e 601 module (Electrochemiluminescence Technology, Roche Diagnostics), while the remaining analytes on cobas e 501 clinical chemistry module (Photometric Technology), according to the instructions of the manufacturer. The degree of insulin resistance was determined by a homeostasis model assessment of insulin resistance (HOMA-IR)[33]. Scores were calculated as the product of the fasting serum insulin level (μU/mL) and the fasting serum glucose level (mmol/L), divided by 22.5. A RIA was used to measure human (total) leptin (DRG Diagnostica, Marburg, Germany; detection limit, 0.5 ng/mL; inter- and intra-assay CVs, 3.0%-6.2% and 3.4%-8.3%, respectively), and adiponectin (DRG Diagnostica, Marburg, Germany; detection limit, 1 ng/mL; inter- and intra-assay CVs, 6.9%-9.2% and 1.8%-6.2%, respectively).
MRI for liver fat quantification
The amount of hepatic fat content (% HFF) was measured by MRI using the two-point Dixon method as modified by Fishbein[34], as previously described and validated[35]. MRI results were interpreted by an experienced radiologist who was blinded to clinical and laboratory findings.
Lumbar spine and whole body dual energy X-ray absorptiometry scans
Anteroposterior lumbar spine (L1-L4), and whole body scans were obtained from all cases and controls using a Hologic QDR-4500W (Waltham, MA, United States) in the fan beam mode with a multidetector system. All subjects were measured on the same machine. The measurements were performed by using standard positioning techniques. Quality control was performed daily using the Hologic anthropomorphic spine, and weekly with the whole body phantom. In our department, the precision error for BMD measurements is less than 1% for the spine phantom, and less than 2.5% for the whole body phantom. The data were analyzed using the software version 11.2. Spine scans were analyzed with low-density software[36]. BMD Z-scores for whole body (WB) and for lumbar spine (LS) were calculated using race and gender specific LMS curves[37]. Whole body DXA results (BMD, fat mass and lean mass) shown in this study represent values excluding the skull[38].
Liver biopsy
The clinical indication for biopsy was either to assess the presence of NASH and degree of fibrosis or other likely independent or competing liver diseases. Percutaneous needle liver biopsy was performed as previously described[35]. The main histologic features of NAFLD were scored according to the scoring system developed by the NASH Clinical Research Network (CRN)[39]. Features of steatosis, lobular inflammation, and hepatocyte ballooning were combined to obtain the NAFLD activity score. As recommended by a recent NASH CRN article[40], a microscopic diagnosis, based on overall injury pattern (i.e., steatosis, hepatocyte ballooning, and inflammation), as well as the presence of additional lesions (e.g., zonality of lesions, portal inflammation, and fibrosis), has been assigned to each case[41]. Accordingly, biopsies were subdivided into not-NASH and definite NASH subcategories[41].
Statistical analysis
Statistical analyses were performed using the SPSS package. The data are expressed either as frequencies or as means with 95%CI. Insulin, leptin and adiponectin levels were distributed with a long tail to the right (positive skew), but their logarithms were approximately normally distributed. Mean differences in anthropometric, laboratory and body composition variables between subjects were assessed by using the t test. Linear regression analysis was used to identify variables associated with BMD. Then, a stepwise multiple linear regression analysis (including all variables significantly associated with BMD) was used to determine the independent variables associated with BMD. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
Study subjects
Forty four obese children with MRI-diagnosed NAFLD were matched to 44 obese children without evidence of liver disease. By study design cases and controls were matched for age, gender, pubertal stage and BMI-SDS. The mean age of cases and controls was 12.5 (SD 1.8) years. Both cases and controls included 20 girls and 24 boys, and five prepubertal children. The mean BMI-SDS of cases and controls was 2.19 (SD 0.16) and 2.17 (SD 0.16), respectively. The clinical and laboratory characteristics for cases and controls are shown in Table 1. There were no differences between children with and without NAFLD with respect to lean and fat mass. Compared to the non-NAFLD group, children with NAFLD had significantly higher ALT, AST, insulin concentrations, HOMA-IR values, and HSCRP levels, but lower adiponectin concentrations. There were no significant differences between the two groups with respect to glucose as well as leptin.
Table 1.
Characteristics of obese children by liver status
| Variables | NAFLD (n = 44) | Non-NAFLD (n = 44) | P value |
| Lean mass, kg | 25.8 (24.0-30.0) | 26.5 (24.0-29.0) | NS |
| Fat mass, kg | 18.7 (17.0-21.0) | 16.8 (15.1-19.0) | NS |
| Percentage body fat | 40.2% (39.0%-41.0%) | 38.0% (36.0%-40.0%) | NS |
| Aspartate aminotransferase, U/L | 34 (30-38) | 24 (22-26) | < 0.0010 |
| Alanine aminotransferase, U/L | 45 (35-55) | 20 (18-22) | < 0.0001 |
| Glucose, mmol/L | 4.89 (4.69-5.10) | 4.88 (4.77-5.02) | NS |
| Insulin, μU/mL | 31.2 (21.9-40.6) | 20.1 (16.2-24.1) | < 0.0100 |
| HOMA-IR values | 4.27 (3.40-5.10) | 3.45 (2.97-4.01) | < 0.0100 |
| Leptin, μg/L | 19.5 (15.8-23.1) | 20.8 (18.2-23.4) | NS |
| Adiponectin, μg/L | 9.0 (7.3–11.0) | 12.9 (10.6–15.4) | < 0.0500 |
| HSCRP, μg/L | 3310 (2785-3836) | 2165 (1710-2620) | < 0.0100 |
| Hepatic fat fraction (%) | 17.0 (11.8-22.3) | 1.6 (1.0-3.1) | < 0.0001 |
Results are expressed as n (%), mean (95%CI), or geometric mean (95%CI) for log-transformed variables. NS: Not significant; HOMA-IR: Homeostasis model assessment of insulin resistance; HSCRP: High-sensitivity C-reactive protein; NAFLD: Nonalcoholic fatty liver disease.
Histological findings in children with NAFLD
Liver biopsy was obtained in 35 of the 44 children with MRI-diagnosed NAFLD, with parental refusal in 9 cases. The 35 children did not differ from those having only liver MRI with respect to age, gender, body composition, metabolic parameters, and bone measures.
Among patients with biopsy-proven NAFLD, 20 (57%) had definite NASH, while 15 (43%) no NASH. No statistically significant differences in body composition as well as in laboratory parameters such as glucose, insulin, leptin, adiponectin levels, and HOMA-IR values were found between children with NASH and those with simple steatosis. AST [mean, 41 U/L (95%CI: 34-48) vs 26 U/L (95%CI: 22-29); P < 0.001)], ALT [mean, 58 U/L (95%CI: 41-75) vs 30 U/L (95%CI: 20-45); P < 0.001)] as well as HFF [mean, 24.8% (95%CI: 19.5-30.2) vs 15.7% (95%CI: 5.6-28.8); P < 0.001)] were significantly higher in patients with NASH compared to children without NASH. HSCRP was also higher [mean, 4055 μg/L (95%CI: 2690-5419) vs 2870 μg/L (95%CI: 1794-3936); P = 0.07], although did not reach statistically significance.
Bone measures
Obese children with NAFLD had a significantly lower LS BMD Z-score than those without NAFLD [mean, 0.55 (95%CI: 0.23-0.86) vs 1.29 (95%CI: 0.95-1.63); P < 0.01] (Figure 1A). WB BMD Z-score was also decreased in obese children with NAFLD compared to obese children with no NAFLD, though borderline significance was observed [1.55 (95%CI: 1.23-1.87) vs 1.95 (95%CI: 1.67-2.10); P = 0.06] (Figure 1B). Among children with biopsy-proven NAFLD, those with NASH had a significantly lower LS BMD Z-score than children without NASH [mean, 0.27 (95%CI: -0.17-0.71) vs 0.75 (95%CI: 0.13-1.39); P < 0.05] (Figure 1C). Moreover, children with NASH had a significantly lower WB BMD Z-score than children without NASH [1.38 (95%CI: 0.89-1.17) vs 1.93 (95%CI: 1.32-2.36); P < 0.05] (Figure 1D).
Figure 1.
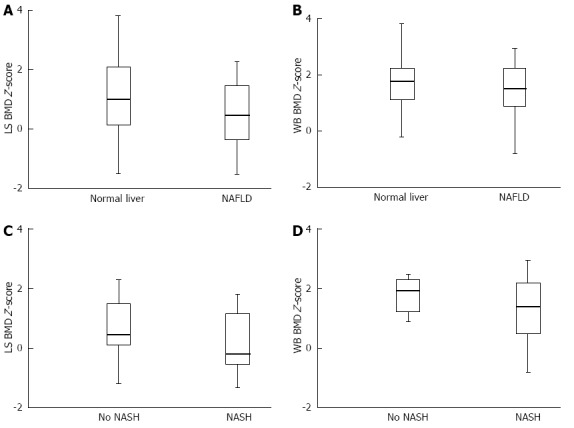
Bone measures. A: Lumbar spine bone mineral density Z-score (LS BMD Z-score) for obese children with and without nonalcoholic fatty liver disease (NAFLD). Box-plots give the median value (bold), 25th and 75th percentiles (lower and upper limits of the box), and lower and upper adjacent values (whiskers); B: Whole body bone mineral density Z-score (WB BMD Z-score) for obese children with and without NAFLD. Box-plots give the median value (bold), 25th and 75th percentiles (lower and upper limits of the box), and lower and upper adjacent values (whiskers); C: LS BMD Z-score for obese children with biopsy-proven NAFLD subdivided into those with and without nonalcoholic steatohepatitis (NASH). Box-plots give the median value (bold), 25th and 75th percentiles (lower and upper limits of the box), and lower and upper adjacent values (whiskers); D: WB BMD Z-score for obese children with biopsy-proven NAFLD subdivided into those with and without NASH. Box-plots give the median value (bold), 25th and 75th percentiles (lower and upper limits of the box), and lower and upper adjacent values (whiskers).
In univariate analysis, LS BMD Z-score correlated negatively with NAFLD (standardized β coefficient, -0.202; P < 0.01) and HSCRP (standardized β coefficient, -0.212; P <0.05). In contrast, leptin was positively associated with lumbar BMD (standardized β coefficient, -0.204; P < 0.05). No correlation was found between LS BMD Z-score and insulin as well as HOMA-IR. Likewise, neither BMI-SDS nor lean mass nor fat mass were correlated with LS BMD Z-score. After including in the model all the significant variables as well as age, gender, pubertal status, NAFLD (standardized β coefficient, -0.230; P < 0.01) and HSCRP (standardized β coefficient, -0.195; P < 0.05) remained significantly and independently associated with LS BMD Z-score (Table 2).
Table 2.
Multivariate analysis of the variables associated with lumbar spine and whole body bone mineral density Z-score in obese children
| Variables | Standardized coefficient1 | P value |
| LS BMD Z-score | ||
| NAFLD | -0.230 | < 0.01 |
| HSCRP, μg/L | -0.195 | < 0.05 |
| WB BMD Z-score | ||
| NAFLD | -0.218 | < 0.05 |
| Fat mass, kg | -0.225 | < 0.05 |
Included in the model were age, gender, pubertal stage, nonalcoholic fatty liver disease (NAFLD), and all variables significantly associated with lumbar spine or whole body bone mineral density (BMD) Z-score in univariate analysis [i.e., high-sensitivity C-reactive protein (HSCRP) and leptin levels or fat mass].
WB BMD Z-score was negatively associated with NAFLD (standardized β coefficient, -0.207; P < 0.05), fat mass (standardized β coefficient, -0.222; P < 0.05), and HSCRP (standardized β coefficient, -0.216; P < 0.05). No correlation was found between WB BMD Z-score and insulin as well as HOMA-IR. Likewise, neither BMI-SDS nor lean mass were correlated with WB BMD Z-score. After including in the model all the significant variables as well as age, gender, pubertal status, NAFLD (standardized β coefficient, -0.218; P < 0.05) and fat mass (standardized β coefficient, -0.225; P < 0.05) remained significantly and independently associated with WB BMD Z-score (Table 2).
DISCUSSION
In this study, we showed that obese children with NAFLD had decreased LS BMD and WB BMD compared to obese children without liver involvement independently of adiposity, and that children with more severe histology had worse bone mineral status than children with more mild abnormalities. Furthermore, we found a significant independent association of HSCRP with BMD scores, supporting the role of an inflammatory state which may accelerate loss of bone mass in patients with NAFLD.
Growing evidence suggests the presence of a complex interplay between the skeleton and numerous homeostatic processes, including energy balance, insulin resistance, obesity and MetS[8]. Recent years have also witnessed an increased awareness of the clinical and epidemiological association between NAFLD and bone health, both in terms of reduced BMD and an increased risk of osteoporosis[8]. To our knowledge, such an association has been so far independently reported by five studies in both children and adults[5-7,42,43].
With respect to studies in adults, Moon et al[42] showed that in postmenopausal women ultrasound-diagnosed NAFLD was significantly associated with low lumbar BMD and this significance was maintained after adjusting for the concerned variables including age, BMI, ALT, smoking status, and alcohol consumption, and even after taking the presence of MetS into account. However, in premenopausal women, there was no such relationship. Yet, in the study by Purnak et al[43] involving 102 adult patients with ultrasound-diagnosed NAFLD and 54 healthy controls, there were no statistically significant differences in BMD measurements between the two groups. However, in a subgroup of patients with NAFLD, the presence of elevated serum ALT and HSCRP levels, which were suggestive of NASH, was associated with lower BMD.
With respect to studies in children, Pirgon et al[5] reported a negative association between BMD and insulin resistance in obese adolescents both with (n = 42) and without (n = 40) ultrasound-diagnosed NAFLD, although the obese adolescents with NAFLD had lower spine BMD Z-scores than their non-NAFLD counterparts. The Authors suggested that NAFLD could exert a negative impact on BMD in obese adolescents, probably via an increased insulin resistance. In the study by Pardee et al[6], poor bone mineralization was common among the 38 obese children with biopsy-proven NAFLD, but not among the 38 obese children without evidence of liver disease. Cases and controls were matched for age, gender, race, ethnicity, height and weight. Among children with NAFLD, 17 (45%) had BMD Z-scores ≤ -2.0, compared to none of the controls (P < 0.0001). Importantly, among those children with NAFLD, children with NASH had a significantly (P < 0.05) lower BMD Z-score (-2.37) than children with NAFLD who did not have NASH (-1.58)[6]. These differences persisted after controlling for total per cent body fat. In the study by Campos et al[7], a 1-year interdisciplinary weight loss therapy was able to promote changes in the metabolic profile of 40 obese adolescents with (n = 18) or without (n = 22) ultrasound-diagnosed NAFLD, including a decrease in the BMI, body fat, visceral and subcutaneous fat, insulin concentration, HOMA-IR, and an increase in lean mass. At baseline, NAFLD group presented statistically lower values of bone mineral content (BMC); however, after one year of interdisciplinary therapy, there was an increase of BMC, reaching similar values of non-NAFLD group. Campos et al[7] suggest the importance of this kind of intervention to regulate bone mineral metabolism as result of an increased BMC and improved inflammatory state. Together, these studies indicate that NAFLD, in particular NASH, is associated with poor bone health.
Obesity and bone mineralization in children remains a topic of great interest, as data are conflicting regarding whether obesity in this age group is detrimental or protective to bone. Previous studies have suggested that body weight might improve bone mineralization in overweight adolescents by increasing the mechanical load on weight-bearing bones[44,45]. In terms of which component(s) of body weight underlie this association, the association between bone and lean mass has been found to be strongest[46]. Some studies have also suggested that fat mass may stimulate bone accrual in growing children, but these results have remained inconsistent showing both positive[47,48] and negative associations[49-51]. In multiple regression analysis, we found that fat mass had a negative association with WB BMD Z-score, while none of the anthropometric variables had an effect on LS BMD Z-scores. The basis for the negative effect of fat on WB BMD Z-score observed in the present study is unknown. We found that serum adipokines such as leptin and adiponectin were not significantly correlated with BMD Z-scores. In that vein, a recent systematic review of the literature concerning the influence of adipokines on BMD, rarely identified leptin as an independent predictor of BMD when BMI or fat mass parameters were included in the multivariate regression models[29]. Yet, in that systematic review, results were discordant for adiponectin[29]. Some studies showed a negative association between adiponectin and BMD, independent of fat mass or BMI[29]. Nevertheless, other studies did not find such associations[29]. There are possible explanations for this apparent discrepancy. Many variables, such as estrogen levels, proinflammatory cytokines, and preanalytical variability of adipokine dosage may interfere with adiponectin and bone.
Systemic inflammation is well known to contribute to low BMD in several diseases states[52-54]. CRP is a sensitive systemic marker of inflammation and tissue damage[55]. It is only produced by hepatocytes, predominantly under transcriptional control by IL-6, although other sites of local CRP synthesis and possible secretion have been suggested. Raised CRP levels are associated with many features of insulin resistance or MetS[56]. This may reflect, in part, the fact that adipocytes are the source of a substantial portion of IL-6 production[57]. On the other hand, inflammatory cytokines up-regulate the RANKL, leading to increased bone resorption and reduced BMD[58]. Some studies have suggested that an elevated CRP is associated with osteoporosis and non-traumatic fractures[9,10]. Our study suggests that HSCRP level is independently associated with LS BMD Z-scores in obese children with NAFLD. This finding is consistent with the hypothesis of a tight interplay between low-grade inflammation and bone turnover, even in patients with NAFLD.
COMMENTS
Background
In parallel with epidemic obesity, nonalcoholic fatty liver disease (NAFLD) has emerged as the leading cause of chronic liver disease in both pediatric and adult patients worldwide. Liver disease can be cause of low bone mineral density (BMD). However, the mechanisms explaining this relationship are still not completely understood.
Research frontiers
A better understanding of the factors that may influence bone mineral status in NAFLD may open a new frontier to fight two highly prevalent conditions like NAFLD and osteoporosis.
Innovations and breakthroughs
Recent years have witnessed an increased awareness of the clinical and epidemiological association between NAFLD and bone health, both in terms of reduced BMD and an increased risk of osteoporosis. Given the high prevalence of NAFLD and the adverse consequences of low BMD in childhood, understanding the mechanisms underlying the relationship between NAFLD and low BMD is important to prevent poor bone mineralization in this potentially vulnerable population. In this study, authors showed that obese children with NAFLD have decreased BMD compared to obese children without liver involvement independently of adiposity, and that children with more severe histology have worse mineral status than children with more mild abnormalities. They also found a significant independent association of high sensitivity C-reactive protein with BMD scores, supporting the role of an inflammatory state which may accelerate loss of bone mass in patients with NAFLD.
Applications
The presence of systemic inflammation may have important implications for the long-term skeletal health of children with NAFLD, and particularly those with nonalcoholic steatohepatitis (NASH).
Terminology
NAFLD comprises a disease spectrum ranging from simple fatty liver to NASH, with varying degrees of inflammation and fibrosis, progressing to end-stage liver disease with cirrhosis and hepatocellular carcinoma. Bone density (or BMD) is a medical term normally referring to the amount of mineral matter per square centimeter of bones. Bone density (or BMD) is used in clinical medicine as an indirect indicator of osteoporosis and fracture risk.
Peer review
In this paper, authors compared lumbar spine (LS) and whole body (WB) BMD measured by dual energy X-ray absorptiometry scans between 44 pediatric patients with magnetic resonance imaging diagnosed NAFLD and controls matched 1:1 for age, gender, and pubertal stage and body mass. They found that LS-BMD Z score was lower in NAFLD than in controls; Thirty three NAFLD patients were biopsied; LS and WB BMD Z score were lower in NASH than in non-NASH children. At multivariate analysis LS-BMD was independently associated with NASH and C-reactive protein levels. They conclude that NAFLD is associated with low BMD in obese children, and systemic low grade inflammation may play a role in such a relationship.
Footnotes
Supported by A Grant from Sapienza University of Rome, Progetti di Ricerca Universitaria 2010-2011
P- Reviewer Valenti LV S- Editor Gou SX L- Editor A E- Editor Ma S
References
- 1.Ovchinsky N, Lavine JE. A critical appraisal of advances in pediatric nonalcoholic Fatty liver disease. Semin Liver Dis. 2012;32:317–324. doi: 10.1055/s-0032-1329905. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Bhangoo A, Matthews NA, Anhalt H, Matta Y, Lamichhane B, Malik S, Narwal S, Wetzler G, Ten S. The prevalence of non-alcoholic fatty liver disease and metabolic syndrome in obese children. J Pediatr Endocrinol Metab. 2011;24:907–911. doi: 10.1515/JPEM.2011.282. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 4.Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 5.Pirgon O, Bilgin H, Tolu I, Odabas D. Correlation of insulin sensitivity with bone mineral status in obese adolescents with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) 2011;75:189–195. doi: 10.1111/j.1365-2265.2011. [DOI] [PubMed] [Google Scholar]
- 6.Pardee PE, Dunn W, Schwimmer JB. Non-alcoholic fatty liver disease is associated with low bone mineral density in obese children. Aliment Pharmacol Ther. 2012;35:248–254. doi: 10.1111/j.1365-2036.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos RM, de Piano A, da Silva PL, Carnier J, Sanches PL, Corgosinho FC, Masquio DC, Lazaretti-Castro M, Oyama LM, Nascimento CM, et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: effects of long-term interdisciplinary therapy. Endocrine. 2012;42:146–156. doi: 10.1007/s12020-012-9613. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz Y. Review article: non-alcoholic fatty liver disease and osteoporosis--clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012;36:345–352. doi: 10.1111/j.1365-2036.2012.05196.x. [DOI] [PubMed] [Google Scholar]
- 9.Ganesan K, Teklehaimanot S, Tran TH, Asuncion M, Norris K. Relationship of C-reactive protein and bone mineral density in community-dwelling elderly females. J Natl Med Assoc. 2005;97:329–333. [PMC free article] [PubMed] [Google Scholar]
- 10.Schett G, Kiechl S, Weger S, Pederiva A, Mayr A, Petrangeli M, Oberhollenzer F, Lorenzini R, Redlich K, Axmann R, et al. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch Intern Med. 2006;166:2495–2501. doi: 10.1001/archinte.166.22.2495. [DOI] [PubMed] [Google Scholar]
- 11.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–210. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007. [DOI] [PubMed] [Google Scholar]
- 13.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 14.Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 16.Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009;35:277–287. doi: 10.1055/s-0029-1222606. [DOI] [PubMed] [Google Scholar]
- 17.Zaidi M, Buettner C, Sun L, Iqbal J. Minireview: The link between fat and bone: does mass beget mass? Endocrinology. 2012;153:2070–2075. doi: 10.1210/en.2012-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magni P, Dozio E, Galliera E, Ruscica M, Corsi MM. Molecular aspects of adipokine-bone interactions. Curr Mol Med. 2010;10:522–532. doi: 10.2174/1566524011009060522. [DOI] [PubMed] [Google Scholar]
- 19.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 20.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/en.140.4.1630. [DOI] [PubMed] [Google Scholar]
- 21.Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 22.Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 23.Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–3553. doi: 10.1210/en.142.8.3546. [DOI] [PubMed] [Google Scholar]
- 24.Morroni M, De Matteis R, Palumbo C, Ferretti M, Villa I, Rubinacci A, Cinti S, Marotti G. In vivo leptin expression in cartilage and bone cells of growing rats and adult humans. J Anat. 2004;205:291–296. doi: 10.1111/j.0021-8782.2004.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999. Biochem Biophys Res Commun. 2012;425:560–564. doi: 10.1016/j.bbrc.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99–109. doi: 10.1016/j.yexcr.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21:1648–1656. doi: 10.1359/jbmr.060707. [DOI] [PubMed] [Google Scholar]
- 29.Biver E, Salliot C, Combescure C, Gossec L, Hardouin P, Legroux-Gerot I, Cortet B. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2703–2713. doi: 10.1210/jc.2011-0047. [DOI] [PubMed] [Google Scholar]
- 30.Fewtrell MS. Bone densitometry in children assessed by dual x ray absorptiometry: uses and pitfalls. Arch Dis Child. 2003;88:795–798. doi: 10.1136/adc.88.9.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 32.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Fishbein MH, Gardner KG, Potter CJ, Schmalbrock P, Smith MA. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15:287–293. doi: 10.1016/S0730-725X(96)00224-X. [DOI] [PubMed] [Google Scholar]
- 35.Pacifico L, Martino MD, Catalano C, Panebianco V, Bezzi M, Anania C, Chiesa C. T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:3012–3019. doi: 10.3748/wjg.v17.i25.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard MB, Feldman HI, Zemel BS, Berlin JA, Barden EM, Stallings VA. Evaluation of low density spine software for the assessment of bone mineral density in children. J Bone Miner Res. 1998;13:1687–1690. doi: 10.1359/jbmr.1998.13.11.1687. [DOI] [PubMed] [Google Scholar]
- 37.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 40.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 42.Moon SS, Lee YS, Kim SW. Association of nonalcoholic fatty liver disease with low bone mass in postmenopausal women. Endocrine. 2012;42:423–429. doi: 10.1007/s12020-012-9639-6. [DOI] [PubMed] [Google Scholar]
- 43.Purnak T, Beyazit Y, Ozaslan E, Efe C, Hayretci M. The evaluation of bone mineral density in patients with nonalcoholic fatty liver disease. Wien Klin Wochenschr. 2012;124:526–531. doi: 10.1007/s00508-012-0211-4. [DOI] [PubMed] [Google Scholar]
- 44.Stettler N, Berkowtiz RI, Cronquist JL, Shults J, Wadden TA, Zemel BS, Leonard MB. Observational study of bone accretion during successful weight loss in obese adolescents. Obesity (Silver Spring) 2008;16:96–101. doi: 10.1038/oby.2007.17. [DOI] [PubMed] [Google Scholar]
- 45.Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80:514–523. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- 46.Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, Boivin CM, Shaw NJ. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone. 2004;35:965–972. doi: 10.1016/j.bone.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–2541. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorentzon M, Swanson C, Andersson N, Mellström D, Ohlsson C. Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD study. J Bone Miner Res. 2005;20:1334–1341. doi: 10.1359/JBMR.050404. [DOI] [PubMed] [Google Scholar]
- 49.Petit MA, Beck TJ, Hughes JM, Lin HM, Bentley C, Lloyd T. Proximal femur mechanical adaptation to weight gain in late adolescence: a six-year longitudinal study. J Bone Miner Res. 2008;23:180–188. doi: 10.1359/JBMR.071018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 51.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86:1530–1538. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 52.Compeyrot-Lacassagne S, Tyrrell PN, Atenafu E, Doria AS, Stephens D, Gilday D, Silverman ED. Prevalence and etiology of low bone mineral density in juvenile systemic lupus erythematosus. Arthritis Rheum. 2007;56:1966–1973. doi: 10.1002/art.22691. [DOI] [PubMed] [Google Scholar]
- 53.Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136:123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leonard MB. Glucocorticoid-induced osteoporosis in children: impact of the underlying disease. Pediatrics. 2007;119 Suppl 2:S166–S174. doi: 10.1542/peds.2006-2023J. [DOI] [PubMed] [Google Scholar]
- 55.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/S0065-2776(08)60379-X. [DOI] [PubMed] [Google Scholar]
- 56.Fröhlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 57.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 58.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.ATV.19.4.972. [DOI] [PubMed] [Google Scholar]


