Abstract
AIM: To investigate control of two different types of massive presacral bleeding according to the anatomy of the presacral venous system.
METHODS: A retrospective review was performed in 1628 patients with middle or low rectal carcinoma who were treated surgically in the Department of Colorectal Surgery, Changhai Hospital, Shanghai, China from January 2008 to December 2012. In four of these patients, the presacral venous plexus (n = 2) or basivertebral veins (n = 2) were injured with massive presacral bleeding during mobilization of the rectum. The first two patients with low rectal carcinoma were operated upon by a junior associate professor and the source of bleeding was the presacral venous plexus. The other two patients with recurrent rectal carcinoma were both women and the source of bleeding was the basivertebral veins.
RESULTS: Two different techniques were used to control the bleeding. In the first two patients with massive bleeding from the presacral venous plexus, we used suture ligation around the venous plexus in the area with intact presacral fascia that communicated with the site of bleeding (surrounding suture ligation). In the second two patients with massive bleeding from the basivertebral veins, the pelvis was packed with gauze, which resulted in recurrent bleeding as soon as it was removed. Following this, we used electrocautery applied through one epiploic appendix pressed with a long Kelly clamp over the bleeding sacral neural foramen where was felt like a pit Electrocautery adjusted to the highest setting was then applied to the clamp to “weld” closed the bleeding point. Postoperatively, the blood loss was minimal and the drain tube was removed on days 4-7.
CONCLUSION: Surrounding suture ligation and epiploic appendices welding are effective techniques for controlling massive presacral bleeding from presacral venous plexus and sacral neural foramen, respectively.
Keywords: Massive presacral bleeding, Rectal surgery, Suture ligation, Welding
Core tip: Massive presacral bleeding is an uncommon but potentially life-threatening complication of rectal surgery. It is difficult to control the bleeding and several alternative techniques for hemostasis have been proposed. We described the use of two simple and effective techniques for controlling two different types of massive presacral bleeding, classified according to the anatomy of the presacral venous system.
INTRODUCTION
Massive presacral bleeding is a potentially life-threatening complication of rectal surgery and remains one of the most challenging intraoperative emergencies to colorectal surgeons[1,2]. The incidence and the mortality have been reported as high as 9.4% and 4.3%, respectively[3,4]. Total mesorectal excision was introduced in 1982 and is considered the gold standard with an acceptable intraoperative risk for rectal carcinoma[5]. However, massive presacral bleeding remains inevitable, especially in recurrent rectal carcinoma or in operations performed by junior colorectal surgeons. Several hemostatic techniques for controlling this intraoperative emergency have been proposed, such as the use of thumbtacks, bone wax, balloon tamponade, and endoscopic stapling[6-8]. However, some techniques fail to arrest the bleeding[9], resulting in shock and even death.
Based on the anatomy of presacral venous system, massive presacral bleeding can be divided into two different types. In our opinion, the key feature in controlling massive presacral bleeding is correct judgment of the bleeding type. Here, we report our experience with massive presacral bleeding and describe the use of two simple and effective techniques (surrounding suture ligation and epiploic appendices welding) for controlling two different types of massive presacral bleeding according to the anatomy of the presacral venous system. To the best of our knowledge, there are no reports of this hemostatic strategy in the literature.
MATERIALS AND METHODS
This was a retrospective review of 1628 patients with middle or low rectal carcinoma who were treated surgically in the Department of Colorectal Surgery, Changhai Hospital, Shanghai, China from January 2008 to December 2012 (Table 1). All the patients who sustained massive presacral bleeding during mobilization of the rectum were recorded.
Table 1.
Adjuvant therapy for low rectal dissection in 1628 patients n (%)
| Adjuvant therapy | Patients | Patients with bleeding |
| Rectal carcinoma | 1606 | 2 (0.12) |
| Without neoadjuvant therapy | 1463 | 2 (0.14) |
| With neoadjuvant radiotherapy | 89 | 0 (0.00) |
| With neoadjuvant chemotherapy | 29 | 0 (0.00) |
| With neoadjuvant radiochemotherapy | 25 | 0 (0.00) |
| Recurrent rectal carcinoma | 22 | 2 (9.00) |
| With preoperative radiotherapy | 6 | 0 (0.00) |
| With preoperative chemotherapy | 1 | 0 (0.00) |
| With preoperative radiochemotherapy | 3 | 0 (0.00) |
| Without preoperative radiotherapy | 12 | 2 (16.7) |
In four of these patients, the presacral venous plexus (n = 2) or basivertebral veins (n = 2) were injured. The first two patients (a 63-year-old woman and a 58-year-old man) with low rectal carcinoma were operated upon by a junior associate professor and the source of bleeding was the presacral venous plexus. The other two patients with recurrent rectal carcinoma were both women (aged 69 and 72 years, respectively). The rectal stumps were found to be densely adherent to the surrounding structures and the source of bleeding was the basivertebral veins. Two different techniques were used to control the bleeding.
RESULTS
In the first patient with massive bleeding from the presacral venous plexus, suture ligation was used initially to control the bleeding, which exacerbated the bleeding. As an alternative, the pelvis was packed with gauze, which resulted in recurrent bleeding as soon as the packing was removed. Following this, we tried to perform suture ligation around the venous plexus in the area with intact presacral fascia that communicated with the bleeding site (surrounding suture ligation). The bleeding stopped after 11 attempts at suture ligation. The patient underwent a super-low anterior resection with protective ileostomy. The estimated blood loss was 2000 mL. In the second patient with massive bleeding from the presacral venous plexus, we used the same technique to control bleeding with eight attempts at surrounding suture ligation. The estimated blood loss was 800 mL.
In the following two patients with massive bleeding from the basivertebral veins, the pelvis was packed with gauze, which resulted in recurrent bleeding as soon as it was removed. In the first of these patients, we tried to control bleeding by surrounding suture ligation initially. However, this technique was unsuccessful. Following this, we used electrocautery applied through one epiploic appendix pressed with a long Kelly clamp over the bleeding sacral neural foramen where was felt like a pit. First, fingertip pressure was applied directly to the sacral neural foramen to control the bleeding. Then, one epiploic appendix, 1-2 cm in diameter, was excised and mounted on a long Kelly clamp. The finger was rapidly withdrawn and the epiploic appendices pressed directly over the sacral neural foramen. Electrocautery adjusted to the highest setting was applied to the clamp to “weld” closed the bleeding point. The bleeding stopped after 3 min. In this patient, blood loss was 6000 mL. In the last patient with massive bleeding from the basivertebral veins, we used the same technique to control bleeding within 10 min. The estimated blood loss was 600 mL. Intraoperative data are shown in Table 2.
Table 2.
Patients with massive presacral bleeding
| Patient | Sex | Age (yr) | TNM stage | Surgical procedure | Blood loss (mL) | Procedures used to control bleeding | Postoperative complication |
| 1 | Female | 63 | T4N0M0 | AR + ileostomy | 2000 | Surrounding suture ligation | None |
| 2 | Male | 58 | T4N1M0 | AR + ileostomy | 800 | Surrounding suture ligation | None |
| 3 | Female | 69 | Recurrent | AR + ileostomy | 6000 | Epiploic appendices welding | None |
| 4 | Female | 72 | Recurrent | APR | 600 | Epiploic appendices welding | None |
TNM: Tumor-node-metastasis; AR: Anterior resection; APR: Abdominoperineal resection.
Postoperatively, the blood loss was minimal and the drain tube was removed on days 4-7. Patients were discharged on day 8 and they all returned for ileostomy reversal 3 mo later.
DISCUSSION
Massive presacral bleeding is considered to be an intraoperative emergency during rectal surgery. The anatomy of the presacral venous system makes it vulnerable to serious bleeding that can often be difficult to control[10]. The presacral venous plexus runs into the pelvic fascia that covers the anterior aspect of the sacrum. It is formed by the two lateral sacral veins, the middle sacral vein, and the in-between communicating veins. These veins are avalvular and communicate via the basivertebral veins with the internal vertebral venous system (Figure 1)[11]. Massive presacral bleeding can be divided into two different types according to the anatomy. The first type of bleeding arises from the presacral venous plexus. It may be massive, but can be stopped by suture ligation. The other type is massive, high-pressure bleeding that can be controlled only by pressing the sacrum with the finger or gauze. This type of bleeding originates from the sacral neural foramen where the basivertebral vein is injured[12]. When the patient is in the lithotomy position, the hydrostatic pressure is increased 2-3 times above the pressure in the inferior vena cava[13]. This avalvular system communicates with the vertebral veins, which explains why it is difficult to stop the bleeding.
Figure 1.
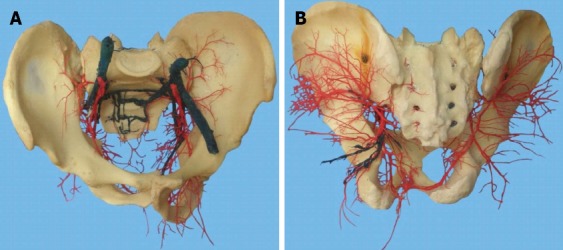
Presacral vascular cast. A: Front view; B: Dorsal view.
Intraoperative massive bleeding may be more common during difficult operations in patients with large and fixed tumors, neoadjuvant radiotherapy, and recurrent rectal carcinoma[14]. The rate of massive presacral bleeding was higher in patients with recurrent rectal carcinoma than in those with rectal carcinoma (9.0% vs 0.12%, P = 0.001). The higher incidence of this emergency in patients with recurrent rectal carcinoma might be related to the more difficult dissection that results from fibrosis and anatomical disruption in this area. The expectation that resection of locally advanced tumors carries a higher risk of presacral vessel lesions was not confirmed in our study because we studied a small number of cases in a single institution.
Incorrect pelvic contraction or inappropriate manipulation is the most common cause of injury to the presacral venous plexus in patients without the above common contributing factors. In this series, two operations with massive presacral bleeding were performed by a junior associate professor. Initial suture ligation was performed incorrectly, which caused more massive bleeding and blood loss was estimated at 2000 mL. Massive bleeding was eventually controlled using or surrounding suture ligation technique. Blood loss was likely related to the extent and site of intraoperative vessel injury, the specific management of the bleeding, and the expertise of the surgeon[1].
The main clinical characteristics of massive presacral bleeding include: (1) bleeding that occurs suddenly during mobilization of the rectum, which can quickly lead to hemorrhagic shock and even result in death; (2) gushing of blood from the pelvic floor, which makes the bleeding site undetectable; (3) ligation of the internal iliac vessel is futile; and (4) bleeding does not stop, even in hemorrhagic shock. According to previous reports, blood loss in presacral bleeding ranges between 300 and 7800 mL[12]. Most of these patients need blood transfusion. In our study, blood loss ranged between 600 and 6000 mL (mean, 2350 mL), and three patients needed blood transfusions. In patients with colorectal carcinoma undergoing surgery, blood transfusion is associated with adverse clinical outcomes, including increased mortality[15]. Therefore, it is important to use a simple and effective procedure to control massive presacral bleeding in rectal surgery.
In our experience, whenever massive presacral bleeding occurs, the first step is direct pressure with the finger at the bleeding point. At the same time, surgeons should inform an anesthetist to prepare sufficient blood. When the bleeding point cannot be exposed clearly, gauze should be pressed directly over the presacral area and the pressure maintained for 15-20 min. The blood surrounding the gauze should be removed by suction. If possible, the specimen should be removed to achieve better exposure. Next, the surgeon should remove the packing gauze, slowly exposing the bleeding point. The bleeding type should been distinguished as soon as possible and an appropriate hemostatic technique can be deployed.
In the first type of massive presacral bleeding, the bleeding point originates from the presacral venous plexus. In our experience, appropriate suture ligation remains an effective method to control this type of bleeding. It should be performed by an experienced surgeon, maintaining continuous pressure over the bleeding site using a gauze nut at the tip of a long Kelly clamp. Surgeons should continue to mobilize the rectum to achieve better exposure if possible. In cases in which the venous branches surrounding the gauze nut can be identified, they are suture ligated one by one with suture thread (VCP772D; Ethicon). Importantly, the suture-ligated tissues should include the presacral fascia, presacral veins, and deep connective tissues. Suture ligation should be performed where the presacral fascia is intact. Jiang et al[16] have reported that circular suture ligation of the venous plexus in the area with intact presacral fascia that surrounds the bleeding site is an effective and simple technique to control presacral venous bleeding. In the present study, this type of bleeding was successfully controlled in two patients by the surrounding suture ligation technique (Figure 2A and B).
Figure 2.
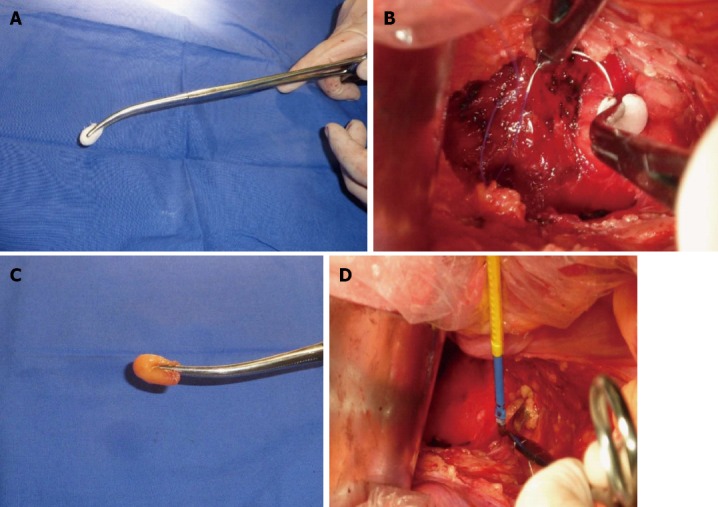
Bleeding point originated from the presacral venous plexus and a sacral neural foramen where the basivertebral veins were injured. A: Continuous pressure over the bleeding site using a gauze nut at the tip of a long Kelly clamp; B: Venous branches surrounding the gauze nut could be identified, and were suture ligated one by one with 3-0 suture thread; C: Continuous pressure over the bleeding site using the epiploic appendices at the tip of a long Kelly clamp; D: Electrocautery applied through the epiploic appendices pressed with a long Kelly clamp over the bleeding vessel.
However, there are several limitations to the surrounding suture ligation technique. First, it can be difficult for bleeding occurring at the bottom of a narrow pelvis, which is typical in patients with obesity[16]. Second, previous rectal surgery can lead to fibrosis of the presacral area, which increases the difficulty in identification of presacral vein distribution and suture ligation. Assessment of the vein locations according to the typical pattern of vein distribution could be wrong, leading to failure of bleeding control. We performed surgery for recurrent rectal carcinoma in two cases. Surrounding suture ligation technique was ineffective because vessel distribution was difficult to identify. Lastly, for bleeding coming from a retracted vein inside the sacrum, other techniques may be used to control the massive bleeding.
In the second type of massive presacral bleeding, the bleeding point originates from a sacral neural foramen where the basivertebral veins are injured.
Harrison et al[17] have reported a technique of muscle fragment welding to control presacral bleeding during rectal mobilization. We performed this technique in two cases whose bleeding points originated from a sacral neural foramen during surgery for recurrent rectal carcinoma. This type of bleeding was effectively controlled using electrocautery applied through the epiploic appendices pressed with a long Kelly clamp over the bleeding vessel (Figure 2C and D). Compared with the technique of muscle fragment welding, it is easier to excise one epiploic appendix than a muscle fragment. Because of the round shape of the epiploic appendices, it is easier to fill the sacral neural foramen. The cauterized epiploic appendices usually adhere to the presacral tissue as a charred coagulum. Hemostasis was immediate and permanent, and no major complications were noted. This technique is intended to deliver heat energy through the forceps to the epiploic appendices. The epiploic appendices act primarily as a fluid-containing electrode that allows conduction of energy and heat to the basivertebral veins. The temperature increases gradually and coagulation is achieved. As in a previous study, necrosis and subsequent abscess development were not seen in our patients, and this may be related to the hypervascular nature of the presacral area and revascularization of the small segment of the epiploic appendices[18].
Alternative methods have been described in the literature. Pelvic packing effectively controls massive presacral bleeding. Intra-abdominal packing should be familiar to colorectal surgeons because when other attempts to provide hemostasis fail, it can be the last resort to control life-threatening bleeding[19]. Packing gauze must be carefully removed at a planned second laparotomy when the patient has stabilized hemodynamically. However, there is a risk of infection or secondary complication from foreign bodies.
Nowadays hemostatic agents are readily available. Some authors have reported that they are effective in stopping bleeding from presacral veins[20-30]. However, in our experience, they are ineffective in stopping massive presacral bleeding. Hemostatic agents may be considered in cases of little bleeding when other techniques have failed.
In conclusion, surrounding suture ligation and epiploic appendices welding are safe, readily available, and highly effective techniques for controlling massive presacral bleeding from the presacral venous plexus and sacral neural foramen, respectively (Figure 3).
Figure 3.
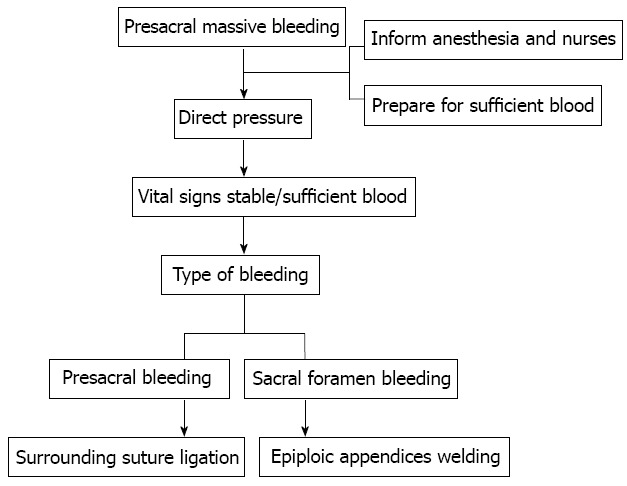
Process in the management of massive presacral bleeding.
COMMENTS
Background
Massive presacral bleeding is a potentially life-threatening complication of rectal surgery and remains one of the most challenging intraoperative emergencies. The incidence and mortality have been reported to be as high as 9.4% and 4.3%, respectively. Total mesorectal excision was introduced in 1982 and is considered a gold standard with an acceptable intraoperative risk during surgery for rectal carcinoma. However, massive presacral bleeding remains inevitable, especially in surgery for recurrent rectal carcinoma or during operations performed by junior colorectal surgeons.
Research frontiers
Several hemostatic techniques for controlling this intraoperative emergency have been proposed, such as the use of thumbtacks, bone wax, balloon tamponade, and endoscopic stapling. However, some techniques fail to arrest the bleeding, resulting in shock and even death.
Innovations and breakthroughs
Based on the anatomy of the presacral venous system, massive presacral bleeding can be divided into two different types. The key factor in controlling massive presacral bleeding is correct assessment of the bleeding type. This article reports two simple and effective techniques for controlling two different types of massive presacral bleeding, classified according to the anatomy of the presacral venous system. According to the authors, there are no reports of this hemostatic strategy in the literature.
Applications
Surrounding suture ligation and epiploic appendices welding are safe, readily available, and highly effective techniques for controlling massive presacral bleeding from the presacral venous plexus and sacral neural foramen, respectively.
Terminology
Presacral bleeding is considered to be an intraoperative emergency in rectal surgery. The anatomy of the presacral venous system makes it vulnerable to serious bleeding that can often be difficult to control.
Peer review
The literature review indicates a large body of work on presacral bleeding already. Often a greater number of techniques described equates to a lack of a gold standard of care, which can be problematic in whatever field. This article outlines two useful and effective techniques to deal with this severe, although not frequent, complication of rectal surgery. The results are interesting and suggest that surrounding suture ligation and epiploic appendices welding for controlling two different types of massive presacral bleeding are simple and effective techniques.
Footnotes
Supported by Changhai Hospital 1255 Project Fund, No. CH125542500; and Shanghai Natural Science Foundation, No. 134119a3800
P- Reviewers Virk JS, Zorcolo L S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Li YY, Chen Y, Xu HC, Wang D, Liang ZQ. A new strategy for managing presacral venous hemorrhage: bipolar coagulation hemostasis. Zhonghua Yixve Zazhi. 2010;123:3486–3488. [PubMed] [Google Scholar]
- 2.Wang LT, Feng CC, Wu CC, Hsiao CW, Weng PW, Jao SW. The use of table fixation staples to control massive presacral hemorrhage: a successful alternative treatment. Report of a case. Dis Colon Rectum. 2009;52:159–161. doi: 10.1007/DCR.0b013e3181972242. [DOI] [PubMed] [Google Scholar]
- 3.van der Vurst TJ, Bodegom ME, Rakic S. Tamponade of presacral hemorrhage with hemostatic sponges fixed to the sacrum with endoscopic helical tackers: report of two cases. Dis Colon Rectum. 2004;47:1550–1553. doi: 10.1007/s10350-004-0614-2. [DOI] [PubMed] [Google Scholar]
- 4.Pollard CW, Nivatvongs S, Rojanasakul A, Ilstrup DM. Carcinoma of the rectum. Profiles of intraoperative and early postoperative complications. Dis Colon Rectum. 1994;37:866–874. doi: 10.1007/BF02052590. [DOI] [PubMed] [Google Scholar]
- 5.Petronella P, Scorzelli M, Manganiello A, Nunziata L, Ferretti M, Campitiello F, Santoriello A, Freda F, Canonico S. Our experience of total mesorectal excision for rectal cancers. Hepatogastroenterology. 2010;57:482–486. [PubMed] [Google Scholar]
- 6.Civelek A, Yeğen C, Aktan AO. The use of bonewax to control massive presacral bleeding. Surg Today. 2002;32:944–945. doi: 10.1007/s005950200189. [DOI] [PubMed] [Google Scholar]
- 7.Basso L. Balloon tamponade for control of massive presacral haemorrhage. Br J Surg. 1996;83:866–867. doi: 10.1002/bjs.1800830641. [DOI] [PubMed] [Google Scholar]
- 8.Hill AD, Menzies-Gow N, Darzi A. Methods of controlling presacral bleeding. J Am Coll Surg. 1994;178:183–184. [PubMed] [Google Scholar]
- 9.Suh M, Shaikh JR, Dixon AM, Smialek JE. Failure of thumbtacks used in control of presacral hemorrhage. Am J Forensic Med Pathol. 1992;13:324–325. doi: 10.1097/00000433-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 10.McPartland KJ, Hyman NH. Damage control: what is its role in colorectal surgery? Dis Colon Rectum. 2003;46:981–986. doi: 10.1097/01.DCR.0000075206.70623.E4. [DOI] [PubMed] [Google Scholar]
- 11.Baqué P, Karimdjee B, Iannelli A, Benizri E, Rahili A, Benchimol D, Bernard JL, Sejor E, Bailleux S, de Peretti F, et al. Anatomy of the presacral venous plexus: implications for rectal surgery. Surg Radiol Anat. 2004;26:355–358. doi: 10.1007/s00276-004-0258-7. [DOI] [PubMed] [Google Scholar]
- 12.Wang QY, Shi WJ, Zhao YR, Zhou WQ, He ZR. New concepts in severe presacral hemorrhage during proctectomy. Arch Surg. 1985;120:1013–1020. doi: 10.1001/archsurg.1985.01390330025005. [DOI] [PubMed] [Google Scholar]
- 13.Germanos S, Bolanis I, Saedon M, Baratsis S. Control of presacral venous bleeding during rectal surgery. Am J Surg. 2010;200:e33–e35. doi: 10.1016/j.amjsurg.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Bhangu A, Brown G, Akmal M, Tekkis P. Outcome of abdominosacral resection for locally advanced primary and recurrent rectal cancer. Br J Surg. 2012;99:1453–1461. doi: 10.1002/bjs.8881. [DOI] [PubMed] [Google Scholar]
- 15.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Li X, Wang Y, Qu H, Jin Z, Dai Y. Circular suture ligation of presacral venous plexus to control presacral venous bleeding during rectal mobilization. J Gastrointest Surg. 2013;17:416–420. doi: 10.1007/s11605-012-2028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison JL, Hooks VH, Pearl RK, Cheape JD, Lawrence MA, Orsay CP, Abcarian H. Muscle fragment welding for control of massive presacral bleeding during rectal mobilization: a review of eight cases. Dis Colon Rectum. 2003;46:1115–1117. doi: 10.1007/s10350-004-7289-3. [DOI] [PubMed] [Google Scholar]
- 18.Remzi FH, Oncel M, Fazio VW. Muscle tamponade to control presacral venous bleeding: report of two cases. Dis Colon Rectum. 2002;45:1109–1111. doi: 10.1007/s10350-004-6369-8. [DOI] [PubMed] [Google Scholar]
- 19.Cirese E, Larciprete G. Emergency pelvic packing to control intraoperative bleeding after a Piver type-3 procedure. An unusual way to control gynaecological hemorrhage. Eur J Gynaecol Oncol. 2003;24:99–100. [PubMed] [Google Scholar]
- 20.Chen Y, Chen F, Xie P, Qiu P, Zhou J, Deng Y. Combined oxidized cellulose and cyanoacrylate glue in the management of severe presacral bleeding. Surg Today. 2009;39:1016–1017. doi: 10.1007/s00595-009-4012-y. [DOI] [PubMed] [Google Scholar]
- 21.Zhang CH, Song XM, He YL, Han F, Wang L, Xu JB, Chen CQ, Cai SR, Zhan WH. Use of absorbable hemostatic gauze with medical adhesive is effective for achieving hemostasis in presacral hemorrhage. Am J Surg. 2012;203:e5–e8. doi: 10.1016/j.amjsurg.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Kandeel A, Meguid A, Hawasli A. Controlling difficult pelvic bleeding with argon beam coagulator during laparoscopic ultra low anterior resection. Surg Laparosc Endosc Percutan Tech. 2011;21:e21–e23. doi: 10.1097/SLE.0b013e3182054f13. [DOI] [PubMed] [Google Scholar]
- 23.Joseph P, Perakath B. Control of presacral venous bleeding with helical tacks on PTFE pledgets combined with pelvic packing. Tech Coloproctol. 2011;15:79–80. doi: 10.1007/s10151-010-0650-8. [DOI] [PubMed] [Google Scholar]
- 24.Karaman K, Bostanci EB, Ercan M, Kurt M, Teke Z, Reyhan E, Akoglu M. Topical Ankaferd application to presacral bleeding due to total mesorectal excision in rectal carcinoma. J Invest Surg. 2010;23:175. doi: 10.3109/08941930903564134. [DOI] [PubMed] [Google Scholar]
- 25.Losanoff JE, Richman BW, Jones JW. Cyanoacrylate adhesive in management of severe presacral bleeding. Dis Colon Rectum. 2002;45:1118–1119. doi: 10.1007/s10350-004-6372-0. [DOI] [PubMed] [Google Scholar]
- 26.Delaney CP. Hemostatic step-by-step procedure to control presacral bleeding after laparoscopic TME. World J Surg. 2009;33:816. doi: 10.1007/s00268-008-9911-3. [DOI] [PubMed] [Google Scholar]
- 27.Ng X, Chiou W, Chang S. Controlling a presacral hemorrhage by using a saline bag: report of a case. Dis Colon Rectum. 2008;51:972–974. doi: 10.1007/s10350-007-9189-9. [DOI] [PubMed] [Google Scholar]
- 28.Becker A, Koltun L, Shulman C, Sayfan J. Bone cement for control of massive presacral bleeding. Colorectal Dis. 2008;10:409–410. doi: 10.1111/j.1463-1318.2007.01373.x. [DOI] [PubMed] [Google Scholar]
- 29.Filippakis GM, Leandros M, Albanopoulos K, Genetzakis M, Lagoudianakis E, Pararas N, Konstandoulakis MM. The use of spray electrocautery to control presacral bleeding: a report of four cases. Am Surg. 2007;73:410–413. [PubMed] [Google Scholar]
- 30.Papalambros E, Sigala F, Felekouras E, Prassas E, Giannopoulos A, Aessopos A, Bastounis E, Hepp W. Management of massive presacral bleeding during low pelvic surgery -- an alternative technique. Zentralbl Chir. 2005;130:267–269. doi: 10.1055/s-2005-836528. [DOI] [PubMed] [Google Scholar]


