Abstract
Primary hepatic leiomyoma is a neoplasm of mesenchymal origin and occurs only rarely. Secondary to benign smooth muscle proliferation, it is usually found in adult women and is associated with Epstein-Barr virus (EBV) infection. Here, we report the 29th case of primary hepatic leiomyoma with its unique features related to diagnosis, treatment and developmental biology. A 48-year-old man, with an immunocompromised status, complained of pain in the upper quadrant of the abdomen. Serological analysis indicated no presence of hepatitis virus, no human immunodeficiency virus, and no EBV infection. The levels of α-fetoprotein and carcinoembryonic antigen were normal. A mass was detected in segment III of the hepatic lobe by ultrasonography and an abdominal computed tomography scan. Endoscopy had negative findings. Exploratory laparotomy found no existing extrahepatic tumor and left lateral lobectomy was performed. Pathological examination showed the mass to be a typical leiomyoma. The cells were positive for α-smooth muscle actin and desmin, and negative for the makers of gastrointestinal stromal tumor (GIST), including CD117, CD34 and DOG1 (discovered on GIST1). In situ hybridization revealed negative status for EBV-encoded small RNA. After left lateral lobectomy, the patient was not given chemotherapy or radiotherapy. During a 2-year follow-up, no sign of local recurrence or distant metastasis was observed. In conclusion, we report a rare case of primary hepatic leiomyoma in a male patient without EBV infection. Hepatic resection was curative. This case presents data to expand our knowledge concerning the complex and heterogeneous nature of primary liver leiomyoma, indicating that EBV infection is important but neither necessary nor sufficient for the development of primary liver leiomyoma.
Keywords: Epstein-Barr virus, Primary hepatic leiomyoma, Cancer diagnosis, Tumor resection, Developmental biology
Core tip: Primary hepatic leiomyoma is usually found in adult women and is associated with Epstein-Barr virus (EBV) infection. We report the 29th case worldwide in a 48-year-old kidney allograft recipient without EBV infection and extrahepatic tumor. He achieved clinical cure by mass resection. The leiomyoma was positive for α-smooth muscle actin and desmin, and negative for gastrointestinal stromal tumor markers, including CD117, CD34 and DOG1 (discovered on gastrointestinal stromal tumor 1). The tumor was negative for EBV-encoded small RNA. The data indicate that EBV infection is important but neither necessary nor sufficient for development of primary liver leiomyoma.
INTRODUCTION
Primary hepatic leiomyoma occurs rarely. The first case was described by Demel[1] in a 42-year-old woman. To date, only 28 cases have been reported worldwide in the literature (Table 1). Secondary to benign smooth muscle proliferation, primary hepatic leiomyoma is usually found in adult women, and is associated with Epstein-Barr virus (EBV) infection. Due to its low prevalence, the diagnosis, treatment and biological behavior remain elusive and require further investigation[2-5]. More data to provide essential information concerning this disease are keenly awaited.
Table 1.
Summary of the published cases of primary liver leiomyoma
| Author | Age/sex | EBV infection | Symptoms | Location/size (cm) | Immunosuppression | Treatment |
| Demel[1] | 42/F | Unknown | RUQ pain | RL/12 | NS | Laparotomy |
| Rios-Dalenz et al | 87/F | Unknown | RUQ pain/bleeding | LL/- | NS | Autopsy |
| Ishak et al | 64/M | Unknown | Abdominal mass | RL/- | NS | Laparotomy |
| Hawkins et al[2] | 66/M | Unknown | Abdominal mass | LH/13 | NS | Left hepatectomy |
| Rummeny et al | 46/F | Unknown | RUQ pain | NS | NS | NS |
| Hollands et al[6] | 17/M | Unknown | Abdominal pain | LH/9 | NS | Left hepatectomy |
| Herzberg et al | 30/F | Unknown | RUQ fullness | RL/19 | NS | Partial right hepatectomy |
| Doyle et al[11] | 1.5/F | Positive | Incidental | LL/3 | Yes | LL segmentectomy |
| Reinertson et al | 32/F | Unknown | RUQ pain | LH/10 | NS | Left hepatectomy |
| Hailer et al | 9/M | Unknown | Incidental | LH/5.6 | Yes | Partial hepatectomy |
| Davidoff et al[12] | 5/M | Positive | Incidental | RR/15 | Yes | Right trisegmentectomy |
| Yoon et al | 41/F | Unknown | RUQ discomfort | RL/19 | No | Right hepatectomy |
| Yanase et al | 59/F | Unknown | Liver dysfunction | RL/13 | NS | Right hepatectomy |
| Mesenas et al | 59/M | Unknown | NS | RL/3.6 | NS | Segmentectomy (S5) |
| Belli et al[7] | 67/F | Unknown | Abdominal mass | RL/30 | NO | Right extended resection |
| Sclabas et al[13] | 30/F | Positive | Epigastric pain | LL/4.4, 0.6 | Yes | LL sectionectomy |
| Cheuk et al[14] | 37/M | Positive | Abdominal discomfort | LH/3.5, 1 | Yes | Conservative management |
| Kanazawa et al | 31/M | Unknown | None | LL/3.5 | No | LL sectionectomy |
| Beuzen et al | 36/F | Unknown | RUQ pain | LL/5 | No | LL sectionectomy |
| Imasato et al[3] | 61/F | Unknown | None | S1/4.5 | No | Right hepatectomy |
| Urizono et al | 71/M | Unknown | NS | S1/3 | No | Partial hepatectomy |
| Marin et al | 64/F | Unknown | None | RL | No | Right hepatectomy |
| Sousa et al | 61/F | Unknown | Dyspepsia | LL/9.5 | No | Left hepatectomy |
| Kalil et al | 44/F | Unknown | Abdominal mass | RL/7 | No | Atypical resection |
| Santos et al | 28/F | Unknown | Incidental | RL (S6)/5.5 | No | Segmentectomy |
| Raber et al | 46/F | Unknown | Incidental | RL/2.8 | Yes | Conservative management |
| Perini et al[5] | 45/M | Positive | Epigastric pain | LL/4.3 | Yes | LL sectionectomy |
| Perini et al[5] | 45/F | Unknown | RUQ pain | RL (S6)/16.5 | No | Segmentectomy |
LL: Left lateral; NS: Not stated; RL: Right lobe; RUQ: Right upper quadrant; S: Segment; LH: Left hepatic lobe; EBV: Epstein-Barr virus.
CASE REPORT
We report a 48-year-old man who complained of pain in the upper quadrant of the abdomen for 1 year and was admitted 2 years ago. He had received a renal graft 9 years before with the immunosuppressive regimens of cyclosporine A, mycophenolate mofetil and prednisone. Due to the calcineurin inhibitor nephrotoxicity found by fine-needle aspiration biopsy 3 years ago, cyclosporine was changed to tacrolimus. Two years ago, mycophenolate mofetil was replaced by azathioprine due to persistent diarrhea. On the day of admission, he was receiving tacrolimus (4.1 ng/dL), azathioprine (50 mg/d), and prednisone (5 mg/d). Routine blood analysis showed a white blood cell count of 7.2 × 109/L and lymphocyte count of 2.4 × 109/L. His liver function was normal and graded as A (score: 6) by Child-Turcotte-Pugh classification. There was no evidence of hepatitis B or hepatitis C virus infection. Human immunodeficiency virus (HIV) testing was negative. Serological testing for EBV was also negative. α-fetoprotein was 5.27 ng/mL (range: 1.09-8.04 ng/mL), and carcinoembryonic antigen was 2.55 ng/mL (normal range: 0-5 ng/mL). Ultrasonography revealed a mass in the left region of the liver, and an abdominal computed tomography (CT) scan showed a tumor of 3.7 cm × 4.9 cm in segment III of the hepatic lobe (Figure 1). No tumor was found by esophagogastroduodenscopy and colonoscopy.
Figure 1.
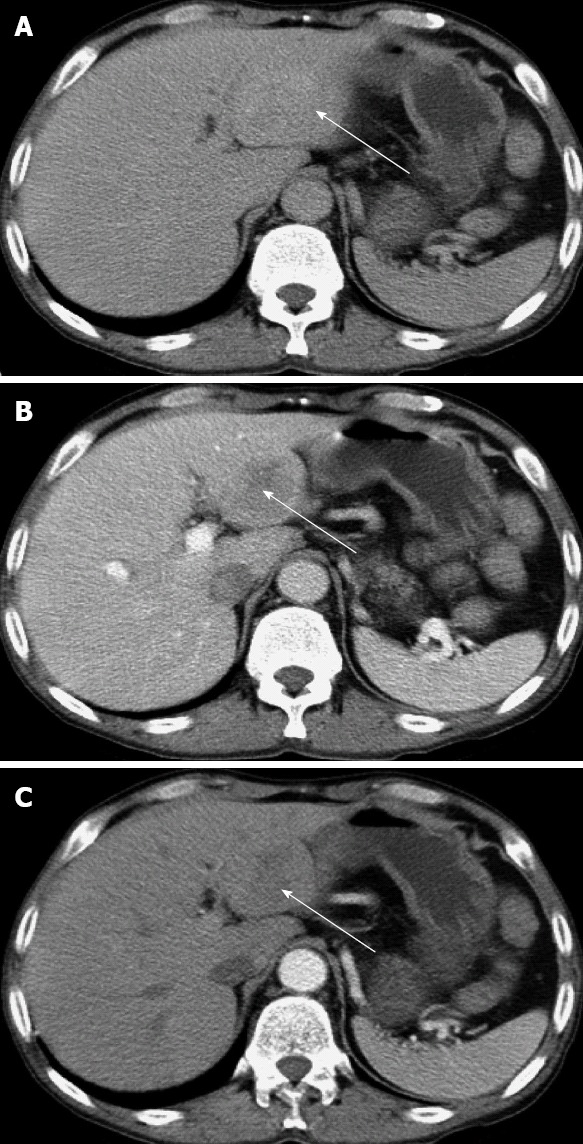
Abdominal computed tomography scan shows a mass in segment III of the liver. A: Hepatic equilibrium phase; B: Portal venous phase; C: Hepatic arterial phase. The arrows indicate the tumor in the liver.
After diagnosis with a liver tumor, the patient underwent exploratory laparotomy. A solitary tumor was found in segment III of the liver (Figure 2A). No tumors were present at extrahepatic sites; particularly in the pelvis. Left lateral hepatectomy was performed. The patient recovered with an uneventful postoperative course and abdominal pain disappeared.
Figure 2.
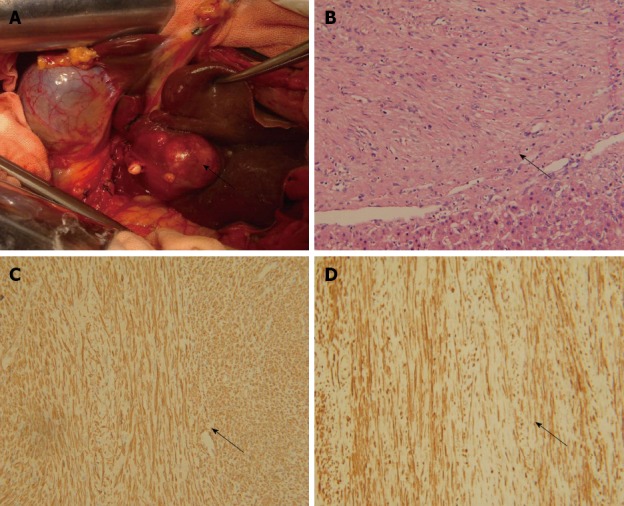
Pathological characteristics of the primary liver leiomyoma. A: Tumor (arrow) located in segment III of the liver; B: Tumor (arrow) and normal liver tissue, hematoxylin and eosin staining, × 200; C: α-smooth muscle actin staining (arrow) of tumor tissues, immunohistochemical staining, × 200; D: Desmin staining (arrow) of tumor tissues, immunohistochemical staining, × 200.
Histopathological examination of the resected specimen showed that the tumor consisted of spindle cells with scarce mitotic figures. The cells had elongated nuclei and eosinophilic cytoplasm forming a fabric-like structure, and neither giant cells nor anaplasia were present (Figure 2B). Immunohistochemical staining showed that the cells were positive for α-smooth muscle actin and desmin (Figure 2C and D), and negative for the gastrointestinal stromal tumor (GIST) markers, including CD117, CD34 and DOG1 (discovered on GIST1). In situ hybridization revealed that the nuclei of the tumor cells were negative for EBV-encoded small RNA (EBER) (Figure 3).
Figure 3.
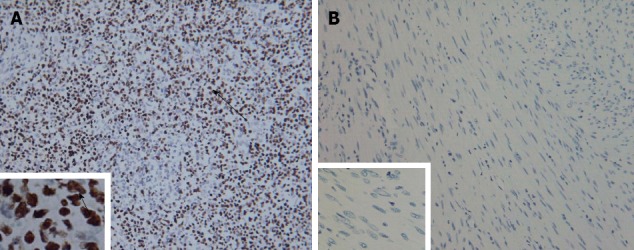
Tumor cells stained negative by in situ hybridization with Epstein-Barr virus-encoded small RNA. A: Positive control staining × 200, × 1000; B: Tumor cell staining × 200, × 1000. Arrows indicate positive staining of the nuclei.
Diagnosis of primary hepatic leiomyoma was then made. As a benign tumor, neither chemotherapy nor radiotherapy was administered to the patient. During a 24-mo postoperative follow-up, no sign of local recurrence or distant metastasis was observed, indicating a clinical cure in this case.
DISCUSSION
Primary hepatic leiomyoma occurs rarely. The first case was described by Demel[1] in a 42-year-old woman. To date, only 28 cases have been reported worldwide (Table 1). Secondary to benign smooth muscle proliferation, primary hepatic leiomyoma is usually found in adult women, and is associated with EBV infection. Due to its low prevalence, diagnosis, treatment and biological behavior remain elusive and require further investigation.
Leiomyoma is relatively common and tends to originate from the muscularis of the gut or the media of the blood vessels, and usually develops in the urogenital and gastrointestinal tracts. Primary hepatic leiomyoma is rare and has its own particular clinical and biological features.
To diagnose primary hepatic leiomyoma, Hawkins et al[2] has proposed the following criteria: (1) the tumor is composed of leiomyocytes; and (2) the presence of a leiomyomatous tumor at other sites can be excluded. Moreover, this liver tumor must be distinguished from GIST[3,4]. In the present case, we excluded the presence of hepatocellular carcinoma, and laboratory tests and histopathological examination were the first step in this process. Then, the diagnosis of leiomyoma was established on the basis of its pathological features. GIST makers (CD117, CD34 and DOG1) were also negative. Combining the findings of ultrasonography, abdominal CT scan, esophagogastroduodenscopy, colonoscopy and exploratory laparotomy, the final diagnosis of primary hepatic leiomyoma was made.
Although no standard therapy is available at present, consistent with the existing reports (Table 1), the tumor was successfully excised and neither chemotherapy nor radiotherapy was applied. Our experience supports that hepatic resection is both diagnostic and curative for primary hepatic leiomyoma.
Some unique characteristics should be noted in this case. First, the patient was male, and primary hepatic leiomyoma is more likely to be found in adult women (18 out of the total 28 cases were female) (Table 1). The relevance of sex may partly be due to the activity of the smooth muscle cells in female urogenital tissue in tumorigenesis and progenesis. The cellular origin of primary hepatic leiomyoma remains unclear and may arise from vessels or the biliary tree[6-8]. In this report, the patient had negative findings in the pelvis and for detection of GIST markers. More observations are required to explore the cellular source of primary hepatic leiomyoma. Second, this case was an adult patient. To date, a total of four pediatric cases (< 18 years) have been identified with primary hepatic leiomyoma (Table 1). Whether or not the developmental mechanisms are different between children and adults requires further investigation. Third, it could be deduced that EBV infection plays a critical role in development of primary hepatic leiomyoma[9,10]. Based on the reported literature, five patients were examined for EBV infection and all of them were positive[11-14]. The relationship between development of primary hepatic and EBV infection and immunocompromised status is also interesting. Seven out of the 28 patients (25%) were immunocompromised (6 transplanted and 1 HIV infection), and five of the seven cases (71.4%) were EBV-positive (4 transplanted and 1 HIV infection). However, in the present case, EBER in situ hybridization, which is the gold standard for detection and localization of latent EBV in tissues, showed that the patient did not have EBV infection, which was different from the status of other patients currently being studied.
Our data indicate that EBV infection is important but neither necessary nor sufficient for the development of primary liver leiomyoma. This observation highlights the complex and heterogeneous nature of the disease and raises the question whether EBV is a passenger rather than a causative agent for this tumor. Due to the rare occurrence of the tumor, an international primary hepatic leiomyoma sample bank, which needs worldwide cooperation of the involved institutions, will contribute to untangling the complex pathogenesis using omics- and system-based methodologies, and therefore to clarify the underlying mechanism behind this interesting tumor.
In conclusion, this report of the 29th case of primary hepatic leiomyoma with its unique features related to diagnosis, treatment and developmental biology contributes to our knowledge of the tumor.
Footnotes
Supported by Grants from the National Natural Science Foundation of China, No. 81072441, to Gong NG; and grants from the National High-Tech Research and Development Program (Program 863) of the Ministry of Science and Technology of China, 2012AA021010, to Ming CS
P- Reviewers Imashuku S, Miles JJ S- Editor Gou SX L- Editor A E- Editor Li JY
References
- 1.Demel R. Ein operierter fall von leber-myom. Virchows Arch. 1926;261:881–884. doi: 10.1007/BF01892215. [DOI] [Google Scholar]
- 2.Hawkins EP, Jordan GL, McGavran MH. Primary leiomyoma of the liver. Successful treatment by lobectomy and presentation of criteria for diagnosis. Am J Surg Pathol. 1980;4:301–304. doi: 10.1097/00000478-198006000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Imasato M, Tono T, Kano T, Kimura Y, Iwazawa T, Ohnishi T, Nakano Y, Yano H, Okamoto S, Monden T. Primary leiomyoma of the liver: a case report. Nihon Geka Gakkai Zasshi. 2005;106:725–729. [PubMed] [Google Scholar]
- 4.Iwatsuki S, Todo S, Starzl TE. Excisional therapy for benign hepatic lesions. Surg Gynecol Obstet. 1990;171:240–246. [PMC free article] [PubMed] [Google Scholar]
- 5.Perini MV, Fink MA, Yeo DA, Carvalho CA, Morais CF, Jones RM, Christophi C. Primary liver leiomyoma: a review of this unusual tumour. ANZ J Surg. 2013;83:230–233. doi: 10.1111/j.1445-2197.2012.06257.x. [DOI] [PubMed] [Google Scholar]
- 6.Hollands MJ, Jaworski R, Wong KP, Little JM. A leiomyoma of the liver. HPB Surg. 1989;1:337–343. doi: 10.1155/1989/45978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belli G, Ciciliano F, Lannelli A, Marano I. Hepatic resection for primary giant leiomyoma of the liver. HPB (Oxford) 2001;3:11–12. doi: 10.1080/136518201753173692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prévot S, Néris J, de Saint Maur PP. Detection of Epstein Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1-infected patient. Virchows Arch. 1994;425:321–325. doi: 10.1007/BF00196156. [DOI] [PubMed] [Google Scholar]
- 9.Morel D, Merville P, Le Bail B, Berger F, Saric J, Potaux L. Epstein-Barr virus (EBV)-associated hepatic and splenic smooth muscle tumours after kidney transplantation. Nephrol Dial Transplant. 1996;11:1864–1866. doi: 10.1093/oxfordjournals.ndt.a027689. [DOI] [PubMed] [Google Scholar]
- 10.Lee ES, Locker J, Nalesnik M, Reyes J, Jaffe R, Alashari M, Nour B, Tzakis A, Dickman PS. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med. 1995;332:19–25. doi: 10.1056/NEJM199501053320104. [DOI] [PubMed] [Google Scholar]
- 11.Doyle H, Tzakis AG, Yunis E, Starzl TE. Smooth muscle tumor arising de novo in a liver allograft: A case report. Clin Transplant. 1991;5:60–62. [PMC free article] [PubMed] [Google Scholar]
- 12.Davidoff AM, Hebra A, Clark BJ, Tomaszewski JE, Montone KT, Ruchelli E, Lau HT. Epstein-Barr virus-associated hepatic smooth muscle neoplasm in a cardiac transplant recipient. Transplantation. 1996;61:515–517. doi: 10.1097/00007890-199602150-00036. [DOI] [PubMed] [Google Scholar]
- 13.Sclabas GM, Maurer CA, Wente MN, Zimmermann A, Büchler MW. Case report: hepatic leiomyoma in a renal transplant recipient. Transplant Proc. 2002;34:3200–3202. doi: 10.1016/S0041-1345(02)03563-7. [DOI] [PubMed] [Google Scholar]
- 14.Cheuk W, Li PC, Chan JK. Epstein-Barr virus-associated smooth muscle tumour: a distinctive mesenchymal tumour of immunocompromised individuals. Pathology. 2002;34:245–249. doi: 10.1080/00313020220131309. [DOI] [PubMed] [Google Scholar]


