Abstract
OBJECTIVE
The purpose of our study was to evaluate tissue sampling methods used for MRI-detected suspicious contralateral breast lesions in the American College of Radiology Imaging Network (ACRIN) 6667 trial.
MATERIALS AND METHODS
Breast MRI was performed at 25 institutions in 969 women who had a recent diagnosis of unilateral breast cancer and negative contralateral mammography and clinical breast examinations. Biopsy was recommended for MRI findings in 135 women, and 121 underwent sampling. Frequencies and positive biopsy rates of sampling methods used for initial diagnosis and imaging guidance techniques were calculated and compared.
RESULTS
Sampling yielded 30 malignant and 91 benign results. Initial sampling used needle biopsy in 88 of 121 (72.7%) and surgical biopsy in 30 of 121 (24.8%) women. Surgical biopsy was excisional biopsy in 28 of 30 (93.3%) and mastectomy in two of 30 (6.7%). The remaining three of 121 (2.5%) women underwent mastectomy, but it was not documented whether this represented initial tissue sampling. Of imaging-guided procedures, 56 of 106 (52.8%) used MRI; 49 of 106 (46.2%), ultrasound; and one of 106 (1.0%), stereotaxis. MRI-guided sampling was with needle biopsy rather than wire-localized surgical biopsy in 33 of 56 (58.9%) women, whereas ultrasound used needle biopsy in 47 of 49 (95.9%). Positive biopsy rates of sampling methods were 20.5% for needle biopsy, 46.2% for excisional biopsy, and 0% for mastectomy.
CONCLUSION
The majority of initial biopsies for MRI-detected contralateral breast lesions used needle biopsy rather than surgical biopsy. Contralateral surgery could have been avoided in most cases had needle biopsy been performed because most excisional biopsy and all mastectomy results were benign. MRI-guided biopsy was significantly more likely than ultrasound-guided sampling to use wire-localized surgical biopsy rather than needle biopsy.
Keywords: breast MRI, contralateral breast lesions, tissue sampling methods
Breast MRI has become an important tool for the detection of breast carcinoma because of its high sensitivity [1–6] and ability to detect malignancy that is clinically and mammographically occult. These merits have resulted in the increasing use of breast MRI for clinical applications that include evaluation of the extent of ipsilateral malignancy [2, 6–13] and screening of the contralateral breast [6, 8, 13–20] in patients with newly diagnosed breast cancer, screening of asymptomatic women at high risk for breast cancer [21–30], and evaluation of patients with metastatic axillary adenopathy and an unknown primary cancer site [31–36].
Because there is overlap in the imaging features of benign and malignant lesions, variable and moderate positive biopsy rates have been reported for breast lesions identified with MRI and deemed to be suspicious [21–30]. Therefore, biopsy is required for definitive diagnosis of such breast MRI findings. However, there are many potential methods by which tissue sampling of MRI-detected findings can be performed. A lesion can be sampled by percutaneous needle biopsy, which has become the preferred method for initial sampling of findings detected on mammography or ultrasound. Alternatively, a more invasive surgical excisional biopsy can be performed after wire localization.
In addition, the imaging modalities used to guide tissue sampling can vary. Because lesions initially identified on breast MRI are not infrequently occult on other imaging tests, tissue sampling using MRI guidance may be necessary. However, if a suspicious MRI finding can be detected with targeted ultrasound, ultrasound-guided biopsy can be performed as a less costly and less time-consuming sampling method. The reported frequencies of sonographic depiction of MRI-detected findings range from 23% to 67%, with a mean of 51% across studies [37–42].
Despite the widespread dissemination of breast MRI, data are limited regarding how lesions identified with MRI undergo biopsy across practice sites. In addition, there are few data comparing the outcomes, including the positive biopsy rates, of different biopsy methods across a spectrum of practices. Further understanding of the current methods of tissue sampling and their outcomes is important to improve clinical decision making regarding effective biopsy techniques for MRI findings. The purpose of this investigation was to describe the tissue sampling methods used for MRI-detected contralateral breast lesions across a range of practice sites participating in the American College of Radiology Imaging Network (ACRIN) 6667 trial. In particular, we sought to ascertain and compare the frequencies of use and positive biopsy rates of initial biopsy techniques used for MRI lesions.
Materials and Methods
The ACRIN 6667 trial was a multiinstitution study funded by the National Cancer Institute. The primary aims of the study were to assess the diagnostic yield and accuracy of MRI in evaluating the contralateral breast of women with a recent unilateral diagnosis of breast cancer. Data collection for the ACRIN 6667 trial was prospective, with the protocol and data collection optimized to meet the primary study aims. The cancer yield and measures of diagnostic accuracy of MRI for this study have previously been reported [43].
Our study is a cross-sectional investigation using the available prospectively collected ACRIN data regarding the tissue sampling used for the MRI-detected suspicious contralateral breast lesions. We seek to provide a snapshot description of the varied biopsy methods during the study interval across the spectrum of practice sites. From April 1, 2003, through June 10, 2004, 987 patients meeting the inclusion criteria were enrolled at 25 practice sites encompassing a variety of clinical settings from academia to private practice. Each participating institution obtained institutional review board approval before patient accrual.
Study Participants
This study included the ACRIN 6667 trial participants found to have a suspicious MRI-detected contralateral breast lesion who underwent subsequent tissue sampling of the suspicious MRI finding. Eligible participants for the ACRIN 6667 trial were women 18 years or older with a diagnosis of unilateral breast cancer within 60 days before the study MRI. All participants were also required to have negative mammographic and clinical breast examinations of the study breast within 90 days before the MRI. Women were excluded from participation if they had undergone breast MRI within 12 months before the study MRI, had a contraindication to undergoing MRI (e.g., implanted magnetic device or severe claustrophobia), or were pregnant. Additional exclusion criteria were a remote breast cancer diagnosis, chemotherapy, or hormonal therapy for breast cancer within 6 months before the study MRI.
Data Collection
Breast MRI technique
All participants underwent dynamic contrast-enhanced breast MRI. Minimum standard criteria were required for each MRI performed: 1.5 T or greater magnet, dedicated breast surface coil, one unenhanced and two contrast-enhanced 3D T1-weighted gradient-echo sequences (TR/TE, < 60/ < 20). Initial and delayed contrast-enhanced images were obtained within 4 and 8 minutes of contrast injection. Spatial resolution criteria included voxel sizes less than 0.9 mm in the frequency encoding direction, less than 1.8 mm in the phase encoding direction, and less than or equal to 3 mm in the slice direction, providing full coverage of the breast of interest. Acquisition in the axial, sagittal, or coronal planes was acceptable, providing minimum specified spatial resolution requirements were met.
Breast MRI interpretation
Participating radiologists were required to have interpreted a minimum of 50 breast MRI studies and had to have performed at least five MRI-guided breast biopsies before the trial. All examinations were interpreted in accordance with the recommendations of the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) [44]. Tissue sampling was recommended for lesions assessed as BI-RADS category 4 (suspicious abnormality) or BI-RADS category 5 (highly suggestive of malignancy).
Tissue sampling methods and ascertainment of histopathology outcomes
The ACRIN protocol specified that all lesions identified as suspicious or highly suggestive of malignancy on MRI (category 4 or 5) undergo biopsy in the form of a core needle biopsy (CNB) or excisional biopsy. The methods of tissue sampling were not among the primary aims of the trial and were not specified by the study protocol. Thus, the techniques used for biopsy were at the discretion of the participating institutions. The criteria used by sites to determine their methods of biopsy were not reported, and are thus beyond the scope of our study. Participating sites were not required to perform targeted ultrasound of study MRI lesions, and sites did not uniformly report whether ultrasound was or was not performed.
Practice sites reported their biopsy techniques as a component of data collection for the primary investigation. For this secondary study, we recorded the available tissue sampling methods and categorized them on the basis of initial sampling techniques using the following definitions: needle biopsy was defined as initial sampling by CNB, fine-needle aspiration (FNA), or using percutaneous sampling by miscellaneous means. The category of CNB included both spring-loaded and vacuum-assisted breast biopsy methods. Surgical biopsy was defined as initial sampling by excisional biopsy or mastectomy. An additional category was defined for those cases in which mastectomy was performed, but it could not be confirmed that this was the initial sampling method or instead followed a needle biopsy. Imaging-guided procedures were classified as those using ultrasound, MRI, or stereotactic biopsy.
Cancer status was followed for 365 days after the study MRI as a component of the ACRIN 6667 trial. In accordance with the study protocol, results of all breast imaging tests, clinical examinations, and biopsies and surgeries were documented by medical record review and patient contact. Tissue sampling results were classified as positive for cancer if invasive carcinoma or ductal carcinoma in situ (DCIS) was histologically verified within 365 days after the initial study MRI and negative for cancer if the study records showed no diagnosis of cancer within that period.
Statistical Methods
The ACRIN Biostatistics Center at the Center for Statistical Sciences at Brown University performed the data analysis. We calculated frequencies of initial tissue sampling methods and imaging guidance techniques, estimated the corresponding rates, and derived 95% exact CIs for rates of interest. The positive biopsy rate for a patient group undergoing biopsy was defined as the percentage of patients in the group whose biopsy resulted in a finding of malignancy. Cases of atypical ductal hyperplasia at initial needle biopsy that were upgraded to malignancy at subsequent surgical excision were counted as negative at needle biopsy. We derived 95% exact CIs for each positive biopsy rate and used the exact test to compare positive biopsy rates for tissue sampling methods. Computations were carried out using SAS software (version 9.2, SAS Institute).
Results
Patients and Overall Histopathology Outcomes
Biopsy was recommended for contralateral breast MRI findings in 135 women. The 121 patients who underwent tissue sampling comprise our study population. The mean age of the 121 women was 53 years (SD, 11.1 years). The MRI maximum lesion size was available for 76 of 121 cases and ranged from 3 to 72 mm with a mean size of 10 mm. Histopathology outcomes were malignant in 30 of 121 (24.8%) and benign in 91 of 121 (75.2%) women. Malignancies were invasive ductal carcinoma (IDC) not otherwise specified (NOS) in 12 of 30 (40.0%), DCIS in 12 of 30 (40.0%), invasive lobular carcinoma (ILC) in four of 30 (13.3%), and IDC specified as tubular type in two of 30 (6.7%) patients. Two of the 30 malignancies were atypical ductal hyperplasia at initial needle biopsy but were upgraded to cancer at subsequent surgery (one case was upgraded to ILC and one case to DCIS).
Tissue Sampling Methods, Positive Biopsy Rates, and False-Negative Rates
Initial tissue sampling was performed using needle biopsy in 88 of 121 (72.7%; 95% CI, 63.9–80.4%) and surgical biopsy in 30 of 121 (24.8%; 17.4–33.5%) women. The remaining three of 121 (2.5%; 0.5–7.1%) women underwent mastectomy, but it could not be ascertained whether this was the initial tissue sampling method (Table 1). The needle biopsy techniques were ultrasound-guided CNB in 47 of 88 (53.4%; 42.5–64.1%), MRI-guided CNB in 33 of 88 (37.5%; 27.4– 48.5%), and miscellaneous other needle biopsy methods without other details specified in eight of 88 (9.1%; 4.0–17.1%) patients (one by stereotactic biopsy, two by vacuum-assisted breast biopsy NOS, two by CNB NOS, one by percutaneous biopsy NOS, and two by FNA). Among the 33 MRI-guided CNB cases, data were not available regarding whether the technique was spring-loaded or vacuumassisted breast biopsy. However, data regarding needle gauge were reported for the 33 cases. There were 24 of 33 (72.7%) 9-gauge, one of 33 (3.0%) 10-gauge, three of 33 (9.1%) 11-gauge, two of 33 (6.1%) 14-gauge, one of 33 (3.0%) 18-gauge, and two of 33 (6.1%) gauge-unknown biopsies. Assuming that the 9-, 10- and 11-gauge biopsies were vacuum- assisted breast biopsies, they comprised 84.8% of all MRI-guided CNB cases. Initial surgical biopsy was via excisional biopsy in 28 of 30 (93.3%; 77.9–99.2%) and mastectomy in two of 30 (6.7%; 0.8–22.1%) cases.
TABLE 1.
Frequencies of Use of Tissue Sampling Methods
| Biopsy Type | No./Total | 95% CI |
|---|---|---|
| Initial needle biopsy | 88/121 (72.7) | 63.9–80.4 |
| Ultrasound core needle biopsy | 47/88 (53.4) | 42.5–64.1 |
| MRI core needle biopsy | 33/88 (37.5) | 27.4–48.5 |
| Miscellaneousa | 8/88 (9.1) | 4.0–17.1 |
| Initial surgical biopsy | 30/121 (24.8) | 17.4–33.5 |
| Excisional biopsy | 28/30 (93.3) | 77.9–99.2 |
| Mastectomy | 2/30 (6.7) | 0.8–22.1 |
| Unspecified mastectomyb | 3/121 (2.5) | 0.5–7.1 |
Note—Data in parentheses are percentages.
One by stereotactic biopsy, two by vacuum-assisted breast biopsy not otherwise specified (NOS), two by core needle biopsy NOS, one by percutaneous biopsy NOS, and two by fine-needle aspiration.
Mastectomy performed but unspecified if after needle biopsy.
Imaging guidance was reported to have been used in 106 of the 121 participants undergoing tissue sampling. Of these, 56 of 106 (52.8%; 42.9–62.6%) used MRI, 49 of 106 (46.2%; 36.5–56.2%) used ultrasound, and one of 106 (1.0%; 0.0–5.1%) used stereotactic guidance (Table 2). MRI-guided sampling was performed with needle biopsy rather than surgical biopsy in 33 of 56 (58.9%; 45.0–71.9%), whereas ultrasoundguided sampling was performed with needle biopsy in 47 of 49 (95.9%; 86.0–99.5%) patients. Wire-localized surgical excisional biopsy accounted for the remaining 23 MRI- and two ultrasound-guided procedures.
TABLE 2.
Frequencies of Use of Imaging-Guidance Techniques
| Biopsy Type | No./Total | 95% CI |
|---|---|---|
| MRI-guided biopsy | 56/106 (52.8) | 42.9–62.6 |
| Needle biopsy | 33/56 (58.9) | 45.0–71.9 |
| Excisional biopsy | 23/56 (41.1) | 28.1–55.0 |
| Ultrasound-guided biopsy | 49/106 (46.2) | 36.5–56.2 |
| Needle biopsy | 47/49 (95.9) | 86.0–99.5 |
| Excisional biopsy | 2/49 (4.1) | 0.5–14.0 |
| Stereotactic biopsy | 1/106 (0.9) | 0.0–5.1 |
Note—Data in parentheses are percentages.
Positive biopsy rates of initial sampling methods were 20.5% for needle biopsy (18 malignancies in 88 biopsies) compared with 42.9% (12 malignancies in 28 biopsies) for excisional biopsy (p = 0.03) and 0% (zero malignancies in two biopsies) for mastectomy. Examples of lesions sampled with varied biopsy methods and their benign versus malignant results are shown in Figures 1–3.
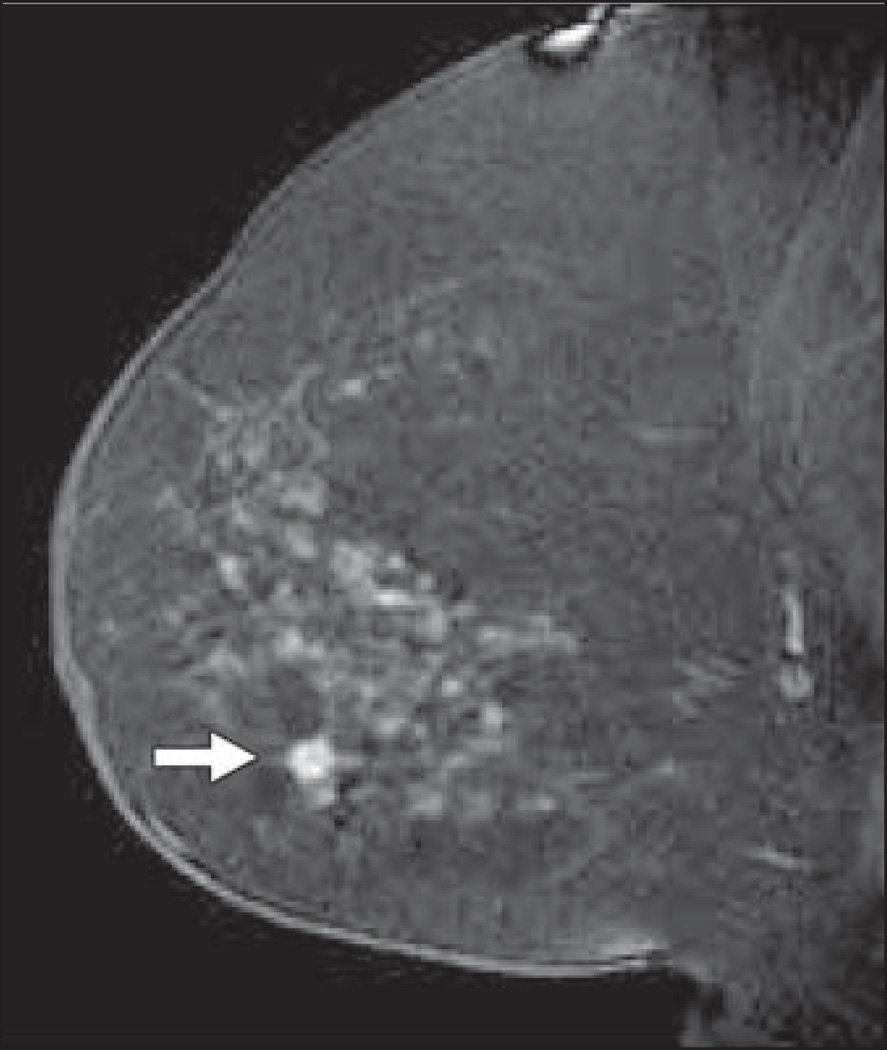
Fig. 1.
Sagittal T1-weighted fat-suppressed immediate contrast-enhanced MR image of right breast in 78-year-old woman with newly diagnosed left breast cancer shows irregular mass measuring 6 mm in lower breast (arrow). MRI-guided core needle biopsy showed invasive ductal carcinoma.
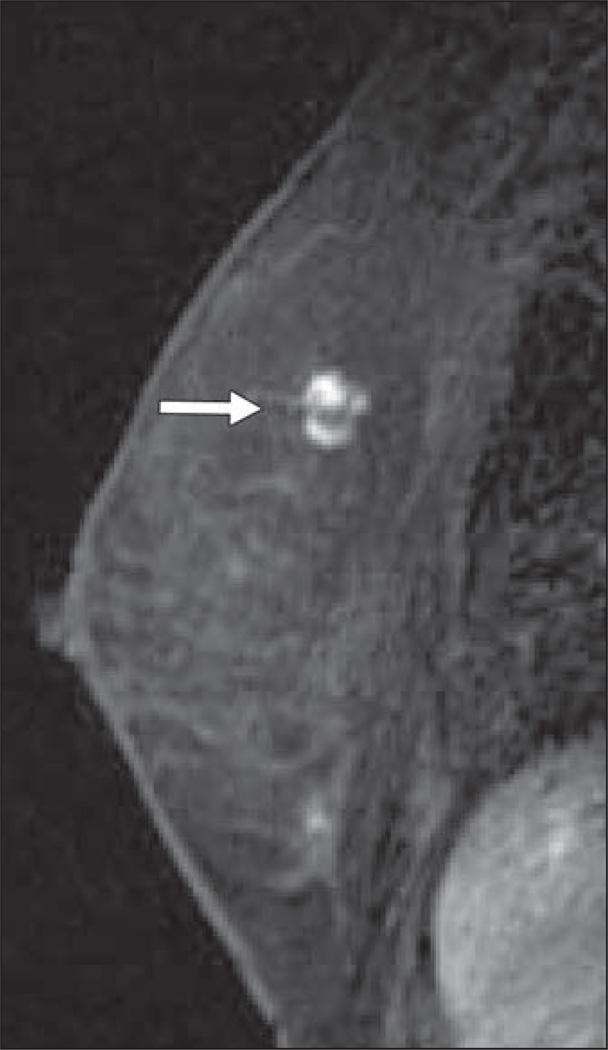
Fig. 3.
Sagittal T1-weighted fat-suppressed immediate contrast-enhanced MR image of right breast in 60-year-old woman with newly diagnosed left breast cancer shows nonmasslike enhancement in anterior breast (arrow). MRI-guided core needle biopsy showed benign sclerosing adenosis and fibrocystic changes.
Discussion
The ACRIN 6667 trial was performed to evaluate the ability of MRI to detect otherwise occult contralateral breast malignancy in women with a recent unilateral breast cancer diagnosis. Data were obtained from 25 participating sites encompassing a variety of clinical settings from academia to private practice. Our study used the available prospectively collected data regarding tissue sampling methods used for the suspicious MRI-detected breast lesions found in the ACRIN 6667 trial. This secondary cross-sectional investigation describes the varied biopsy methods and positive biopsy rate outcomes during the study interval across the spectrum of practice sites. There are many potential biopsy methods for MRI-detected lesions, but sparse data are available regarding their frequencies of use and outcomes across varied practice sites. To our knowledge, our investigation is the first to study biopsy methods and results across a broad array of centers.
In our study, we found that the initial tissue sampling method was needle biopsy rather than surgical biopsy in the majority of women (72.7% vs 24.8%). The use of needle biopsy as an alternative to surgical biopsy for sampling of lesions detected using mammography or ultrasound has been thoroughly studied and is well established. Needle biopsy is the preferred method of initial tissue sampling for such findings because compared with surgical biopsy it is faster, less expensive, less invasive, and improves surgical management in cases found to be malignant. In particular, diagnosis by needle biopsy has been shown to increase the frequency of negative margins and decrease the number of required surgical procedures [45–47] for breast malignancies. Thus, it is reassuring that most women in the study underwent needle biopsy rather than surgical biopsy for their MRI-detected findings. Multiple investigations have now confirmed the safety and accuracy of needle biopsy for MRI-detected breast lesions [48–52], and it is reasonable to expect that the evident cost and outcome advantages of needle biopsy for mammographic or sonographic findings are also true for initial diagnosis of MRI-detected lesions. Further, needle biopsy yielding a benign and concordant diagnosis allows women to avoid an unwarranted surgery.
In our study, the overall positive biopsy rate for concerning MRI findings was 24.8%. A spectrum of positive biopsy rates have previously been described for suspicious contralateral breast MRI findings in this patient population. Ours is within the range of 18.8–61.5% for positive biopsy rates that have been reported in the largest prior studies [6, 8, 12, 14–17, 20, 53–62]. Although we found that sampling by excisional biopsy had a higher positive biopsy rate than that by needle biopsy (42.9% vs 20.5%), the majority of surgical biopsy results, including excisional biopsy, were benign. Thus, most women who underwent initial surgical biopsy could potentially have avoided surgery in the contralateral breast had needle biopsy been performed.
Of particular note, neither of the MRI-detected lesions in the two of 121 (1.7%) women in the study who underwent mastectomy without prior tissue sampling was found to be malignant. In the published Comparative Effectiveness of MRI in Breast Cancer trial [63], 16 of 50 (32.0%) participants with suspicious MRI-detected lesions underwent pathologically avoidable mastectomy for false-positive MRI findings, and at least six of 16 (37.5%) of these women did not undergo tissue sampling before mastectomy. This constituted a considerable limitation in study methodology and patient impact. A much smaller proportion of women in our study underwent mastectomy without antecedent tissue sampling of their MRI findings. Although women with breast cancer may decide to undergo contralateral mastectomy for a variety of reasons, our results regarding the lack of malignancy at mastectomy underscore the importance of counselling women that a suspicious MRI finding is not definitively a malignancy and to make surgical decisions accordingly.
MRI and ultrasound were used with similar frequencies to guide biopsies in our study. However, sampling using MRI guidance was significantly more likely than ultrasound guidance to use wire-localized surgical biopsy rather than needle biopsy. The preponderance of MRI-guided localization for surgical biopsy compared with MRI-guided needle biopsy may reflect a lack of training or equipment for needle biopsy at the time of the study, given the enrollment period of 2003 through 2004. It is also possible that some MRI-detected lesions were thought to be inaccessible for needle biopsy because of factors such as far posterior location, which could prompt sites to instead perform wire localization as close to the lesion as possible. The recently opened ACR Breast MRI Accreditation Program requires that facilities perform MRI-guided intervention or create a referral arrangement with a cooperating facility that could provide these services. Given the benefits of needle biopsy over surgical biopsy previously discussed, our results suggest that MRI-guided needle biopsy is the more desirable MRI-directed intervention, in particular given improvements in lesion accessibility with newer breast biopsy coils.
Our study has limitations. Although it is the only investigation to assess biopsy methods and outcomes of suspicious MRI lesions across multiple practice sites, the tissue sampling techniques used were at the discretion of the participating institutions. Considerations influencing biopsy decisions may have included BIRADS assessment categories, patient or practitioner preferences, or technical considerations but are beyond the scope of this cross-sectional study. However, the lack of standardized criteria regarding selection of sampling methods may make our descriptive results more generalizable across practice types. Alternatively, factors that may decrease broad generalizability include our particular study cohort of women with a known contralateral breast cancer. Furthermore, although this was a multiinstitutional study of both academic and nonacademic sites, there may be a selection bias in that sites that have chosen to participate in an ACRIN trial may have higher relative expertise compared with nonparticipating sites.
In summary, our study showed a variety of sampling methods, and imaging-guidance techniques were used for suspicious MRI-detected contralateral breast lesions. Most lesions were sampled using needle biopsy rather than surgical biopsy, an encouraging result given the multiple advantages of needle biopsy over surgery even in patients undergoing surgery for the opposite breast. We found that most initial excisional biopsy and all mastectomy results were benign, indicating that contralateral surgery could potentially have been avoided had needle sampling been performed. Sampling guided by MRI was more likely to be used for surgical biopsy than for needle biopsy when compared with biopsy guided by ultrasound, revealing an opportunity for practice sites to improve patient care by increasing the proportion of MRI-guided procedures that use needle biopsy.
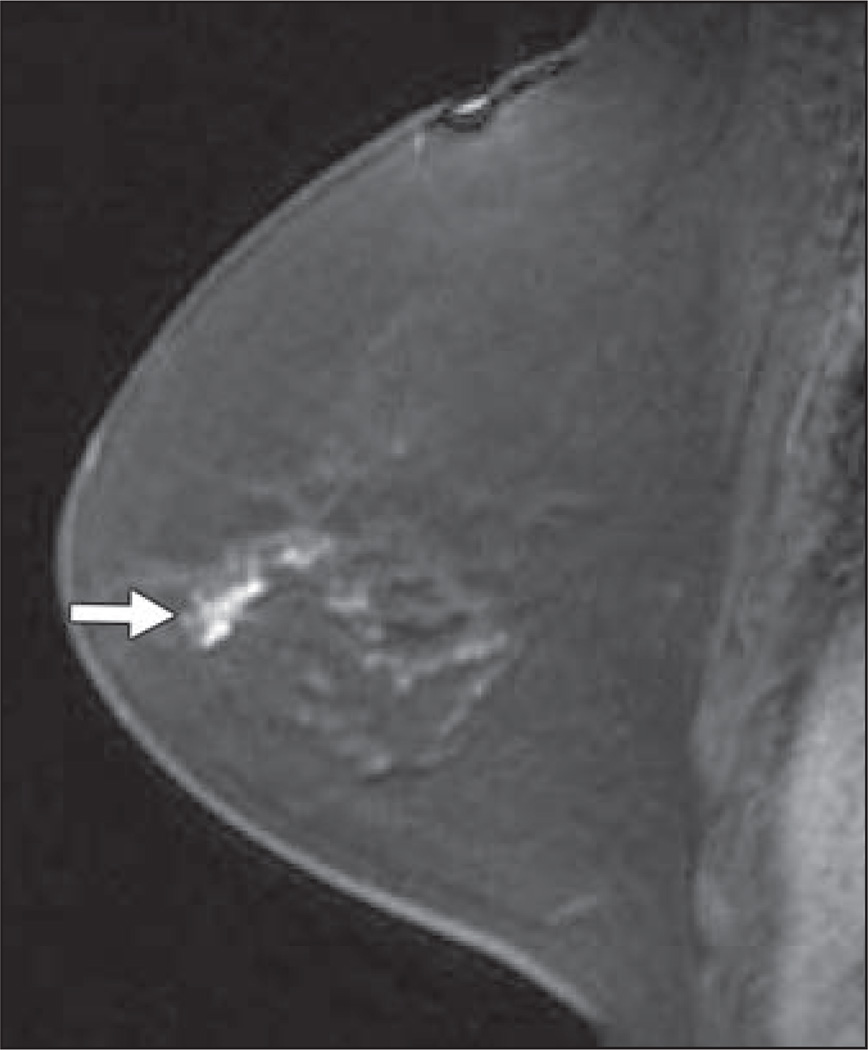
Fig. 2.
Sagittal T1-weighted fat-suppressed immediate contrast-enhanced MR image of right breast in 54-year-old woman with newly diagnosed left breast cancer shows lobular mass measuring 15 mm in upper breast (arrow). Ultrasound-guided core needle biopsy showed invasive ductal carcinoma.
Acknowledgments
The American College of Radiology Imaging Network receives funding from the National Cancer Institute through grants U01 CA079778 and U10 CA080098.
References
- 1.Kaiser WA, Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA: preliminary observations. Radiology. 1989;170:681–686. doi: 10.1148/radiology.170.3.2916021. [DOI] [PubMed] [Google Scholar]
- 2.Harms SE, Flamig DP, Hesley KL, et al. MR imaging of the breast with rotating delivery of excitation off resonance: clinical experience with pathologic correlation. Radiology. 1993;187:493–501. doi: 10.1148/radiology.187.2.8475297. [DOI] [PubMed] [Google Scholar]
- 3.Boetes C, Barentsz JO, Mus RD, et al. MR characterization of suspicious breast lesions with a gadolinium-enhanced turboFLASH subtraction technique. Radiology. 1994;193:777–781. doi: 10.1148/radiology.193.3.7972823. [DOI] [PubMed] [Google Scholar]
- 4.Heywang-Köbrunner SH, Viehweg P, Heinig A, Kuchler C. Contrast-enhanced MRI of the breast: accuracy, value, controversies, solutions. Eur J Radiol. 1997;24:94–108. doi: 10.1016/s0720-048x(96)01142-4. [DOI] [PubMed] [Google Scholar]
- 5.Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001;220:13–30. doi: 10.1148/radiology.220.1.r01jl3113. [DOI] [PubMed] [Google Scholar]
- 6.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–849. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 7.Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468–473. doi: 10.1002/cncr.11490. [DOI] [PubMed] [Google Scholar]
- 8.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999;213:881–888. doi: 10.1148/radiology.213.3.r99dc01881. [DOI] [PubMed] [Google Scholar]
- 9.Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR. 2003;180:901–910. doi: 10.2214/ajr.180.4.1800901. [DOI] [PubMed] [Google Scholar]
- 10.Mumtaz H, Hall-Craggs MA, Davidson T, et al. Staging of symptomatic primary breast cancer with MR imaging. AJR. 1997;169:417–424. doi: 10.2214/ajr.169.2.9242745. [DOI] [PubMed] [Google Scholar]
- 11.Orel SG, Schnall MD, Powell CM, et al. Staging of suspected breast cancer: effect of MR imaging and MR-guided biopsy. Radiology. 1995;196:115–122. doi: 10.1148/radiology.196.1.7784554. [DOI] [PubMed] [Google Scholar]
- 12.Schelfout K, Van Goethem M, Kersschot E, et al. Contrast-enhanced MR imaging of breast lesions and effect on treatment. Eur J Surg Oncol. 2004;30:501–507. doi: 10.1016/j.ejso.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Schnall MD, Blume J, Bluemke DA, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J Surg Oncol. 2005;92:32–38. doi: 10.1002/jso.20381. [DOI] [PubMed] [Google Scholar]
- 14.Rieber A, Merkle E, Bohm W, Brambs HJ, Tomczak R. MRI of histologically confirmed mammary carcinoma: clinical relevance of diagnostic procedures for detection of multifocal or contralateral secondary carcinoma. J Comput Assist Tomogr. 1997;21:773–779. doi: 10.1097/00004728-199709000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Slanetz PJ, Edmister WB, Yeh ED, Talele AC, Kopans DB. Occult contralateral breast carcinoma incidentally detected by breast magnetic resonance imaging. Breast J. 2002;8:145–148. doi: 10.1046/j.1524-4741.2002.08304.x. [DOI] [PubMed] [Google Scholar]
- 16.Liberman L, Morris EA, Kim CM, et al. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR. 2003;180:333–341. doi: 10.2214/ajr.180.2.1800333. [DOI] [PubMed] [Google Scholar]
- 17.Lee SG, Orel SG, Woo IJ, et al. MR imaging screening of the contralateral breast in patients with newly diagnosed breast cancer: preliminary results. Radiology. 2003;226:773–778. doi: 10.1148/radiol.2263020041. [DOI] [PubMed] [Google Scholar]
- 18.Viehweg P, Rotter K, Laniado M, et al. MR imaging of the contralateral breast in patients after breast-conserving therapy. Eur Radiol. 2004;14:402–408. doi: 10.1007/s00330-003-2086-2. [DOI] [PubMed] [Google Scholar]
- 19.Pediconi F, Venditti F, Padula S, et al. CE-magnetic resonance mammography for the evaluation of the contralateral breast in patients with diagnosed breast cancer. Radiol Med (Torino) 2005;110:61–68. [PubMed] [Google Scholar]
- 20.Lehman CD, Blume JD, Thickman D, et al. Added cancer yield of MRI in screening the contralateral breast of women recently diagnosed with breast cancer: results from the International Breast Magnetic Resonance Consortium (IBMC) trial. J Surg Oncol. 2005;92:9–15. doi: 10.1002/jso.20350. discussion, 15–16. [DOI] [PubMed] [Google Scholar]
- 21.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23:8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 22.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 23.Podo F, Sardanelli F, Canese R, et al. The Italian multi-centre project on evaluation of MRI and other imaging modalities in early detection of breast cancer in subjects at high genetic risk. J Exp Clin Cancer Res. 2002;21:115–124. [PubMed] [Google Scholar]
- 24.Tilanus-Linthorst MM, Obdeijn IM, Bartels KC, de Koning HJ, Oudkerk M. First experiences in screening women at high risk for breast cancer with MR imaging. Breast Cancer Res Treat. 2000;63:53–60. doi: 10.1023/a:1006480106487. [DOI] [PubMed] [Google Scholar]
- 25.Morris EA, Liberman L, Ballon DJ, et al. MRI of occult breast carcinoma in a high-risk population. AJR. 2003;181:619–626. doi: 10.2214/ajr.181.3.1810619. [DOI] [PubMed] [Google Scholar]
- 26.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 27.Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103:1898–1905. doi: 10.1002/cncr.20971. [DOI] [PubMed] [Google Scholar]
- 28.Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244:381–388. doi: 10.1148/radiol.2442060461. [DOI] [PubMed] [Google Scholar]
- 29.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 30.Sardanelli F, Podo F, D’Agnolo G, et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology. 2007;242:698–715. doi: 10.1148/radiol.2423051965. [DOI] [PubMed] [Google Scholar]
- 31.Stomper PC, Waddell BE, Edge SB, Klippenstein DL. Breast MRI in the evaluation of patients with occult primary breast carcinoma. Breast J. 1999;5:230–234. doi: 10.1046/j.1524-4741.1999.99004.x. [DOI] [PubMed] [Google Scholar]
- 32.Henry-Tillman RS, Harms SE, Westbrook KC, Korourian S, Klimberg VS. Role of breast magnetic resonance imaging in determining breast as a source of unknown metastatic lymphadenopathy. Am J Surg. 1999;178:496–500. doi: 10.1016/s0002-9610(99)00221-4. [DOI] [PubMed] [Google Scholar]
- 33.Orel SG, Weinstein SP, Schnall MD, et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999;212:543–549. doi: 10.1148/radiology.212.2.r99au40543. [DOI] [PubMed] [Google Scholar]
- 34.Obdeijn IM, Brouwers-Kuyper EM, Tilanus-Linthorst MM, Wiggers T, Oudkerk M. MR imaging-guided sonography followed by fine-needle aspiration cytology in occult carcinoma of the breast. AJR. 2000;174:1079–1084. doi: 10.2214/ajr.174.4.1741079. [DOI] [PubMed] [Google Scholar]
- 35.Olson JA, Jr, Morris EA, Van Zee KJ, Linehan DC, Borgen PI. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 2000;7:411–415. doi: 10.1007/s10434-000-0411-4. [DOI] [PubMed] [Google Scholar]
- 36.Buchanan CL, Morris EA, Dorn PL, Borgen PI, Van Zee KJ. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol. 2005;12:1045–1053. doi: 10.1245/ASO.2005.03.520. [DOI] [PubMed] [Google Scholar]
- 37.LaTrenta LR, Menell JH, Morris EA, Abramson AF, Dershaw DD, Liberman L. Breast lesions detected with MR imaging: utility and histopathologic importance of identification with US. Radiology. 2003;227:856–861. doi: 10.1148/radiol.2273012210. [DOI] [PubMed] [Google Scholar]
- 38.DeMartini WB, Eby PR, Peacock S, Lehman CD. Utility of targeted sonography for breast lesions that were suspicious on MRI. AJR. 2009;192:1128–1134. doi: 10.2214/AJR.07.3987. [DOI] [PubMed] [Google Scholar]
- 39.Sim LS, Hendriks JH, Bult P, Fook-Chong SM. US correlation for MRI-detected breast lesions in women with familial risk of breast cancer. Clin Radiol. 2005;60:801–806. doi: 10.1016/j.crad.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Wiratkapun C, Duke D, Nordmann AS, et al. Indeterminate or suspicious breast lesions detected initially with MR imaging: value of MRI-directed breast ultrasound. Acad Radiol. 2008;15:618–625. doi: 10.1016/j.acra.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Abe H, Schmidt RA, Shah RN, et al. MR-directed (“second-look”) ultrasound examination for breast lesions detected initially on MRI: MR and sonographic findings. AJR. 2010;194:370–377. doi: 10.2214/AJR.09.2707. [DOI] [PubMed] [Google Scholar]
- 42.Candelaria R, Fornage BD. Second-look US examination of MR-detected breast lesions. J Clin Ultrasound. 2011;39:115–121. doi: 10.1002/jcu.20784. [DOI] [PubMed] [Google Scholar]
- 43.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 44.American College of Radiology. Breast imaging reporting and data system (BI-RADS. 4th ed. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 45.Yim JH, Barton P, Weber B, et al. Mammographically detected breast cancer: benefits of stereotactic core versus wire localization biopsy. Ann Surg. 1996;223:688–697. doi: 10.1097/00000658-199606000-00007. discussion, 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verkooijen HM, Borel Rinkes IH, Peeters PH, et al. Impact of stereotactic large-core needle biopsy on diagnosis and surgical treatment of nonpalpable breast cancer. Eur J Surg Oncol. 2001;27:244–249. doi: 10.1053/ejso.2000.1102. [DOI] [PubMed] [Google Scholar]
- 47.White RR, Halperin TJ, Olson JA, Jr, Soo MS, Bentley RC, Seigler HF. Impact of core-needle breast biopsy on the surgical management of mammographic abnormalities. Ann Surg. 2001;233:769–777. doi: 10.1097/00000658-200106000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberman L, Morris EA, Dershaw DD, Thornton CM, Van Zee KJ, Tan LK. Fast MRI-guided vacuum-assisted breast biopsy: initial experience. AJR. 2003;181:1283–1293. doi: 10.2214/ajr.181.5.1811283. [DOI] [PubMed] [Google Scholar]
- 49.Lehman CD, Deperi ER, Peacock S, McDonough MD, DeMartini WB, Shook J. Clinical experience with MRI-guided vacuum-assisted breast biopsy. AJR. 2005;184:1782–1787. doi: 10.2214/ajr.184.6.01841782. [DOI] [PubMed] [Google Scholar]
- 50.Liberman L, Bracero N, Morris E, Thornton C, Dershaw DD. MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. AJR. 2005;185:183–193. doi: 10.2214/ajr.185.1.01850183. [DOI] [PubMed] [Google Scholar]
- 51.Mahoney MC. Initial clinical experience with a new MRI vacuum-assisted breast biopsy device. J Magn Reson Imaging. 2008;28:900–905. doi: 10.1002/jmri.21549. [DOI] [PubMed] [Google Scholar]
- 52.Peters NH, Meeuwis C, Bakker CJ, et al. Feasibility of MRI-guided large-core-needle biopsy of suspicious breast lesions at 3 T. Eur Radiol. 2009;19:1639–1644. doi: 10.1007/s00330-009-1310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buxant F, Scuotto F, Hottat N, Noel JC, Simon P. Does preoperative magnetic resonance imaging modify breast cancer surgery? Acta Chir Belg. 2007;107:288–291. doi: 10.1080/00015458.2007.11680058. [DOI] [PubMed] [Google Scholar]
- 54.Pediconi F, Catalano C, Roselli A, et al. Contrastenhanced MR mammography for evaluation of the contralateral breast in patients with diagnosed unilateral breast cancer or high-risk lesions. Radiology. 2007;243:670–680. doi: 10.1148/radiol.2433060838. [DOI] [PubMed] [Google Scholar]
- 55.Lee JM, Orel SG, Czerniecki BJ, Solin LJ, Schnall MD. MRI before reexcision surgery in patients with breast cancer. AJR. 2004;182:473–480. doi: 10.2214/ajr.182.2.1820473. [DOI] [PubMed] [Google Scholar]
- 56.Bilimoria KY, Cambic A, Hansen NM, Bethke KP. Evaluating the impact of preoperative breast magnetic resonance imaging on the surgical management of newly diagnosed breast cancers. Arch Surg. 2007;142:441–445. doi: 10.1001/archsurg.142.5.441. discussion, 445–447. [DOI] [PubMed] [Google Scholar]
- 57.Hollingsworth AB, Stough RG. Preoperative breast MRI for locoregional staging. J Okla State Med Assoc. 2006;99:505–515. [PubMed] [Google Scholar]
- 58.Rieber A, Schirrmeister H, Gabelmann A, et al. Pre-operative staging of invasive breast cancer with MR mammography and/or PET: boon or bunk? Br J Radiol. 2002;75:789–798. doi: 10.1259/bjr.75.898.750789. [DOI] [PubMed] [Google Scholar]
- 59.Bagley FH. The role of magnetic resonance imaging mammography in the surgical management of the index breast cancer. Arch Surg. 2004;139:380–383. doi: 10.1001/archsurg.139.4.380. discussion, 383. [DOI] [PubMed] [Google Scholar]
- 60.Deurloo EE, Peterse JL, Rutgers EJ, Besnard AP, Muller SH, Gilhuijs KG. Additional breast lesions in patients eligible for breast-conserving therapy by MRI: impact on preoperative management and potential benefit of computerised analysis. Eur J Cancer. 2005;41:1393–1401. doi: 10.1016/j.ejca.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 61.Hlawatsch A, Teifke A, Schmidt M, Thelen M. Preoperative assessment of breast cancer: sonography versus MR imaging. AJR. 2002;179:1493–1501. doi: 10.2214/ajr.179.6.1791493. [DOI] [PubMed] [Google Scholar]
- 62.Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009;27:5640–5649. doi: 10.1200/JCO.2008.21.5756. [DOI] [PubMed] [Google Scholar]
- 63.Turnbull L, Brown S, Harvey I, et al. Comparative Effectiveness of MRI in Breast Cancer (COM-ICE) trial: a randomised controlled trial. Lancet. 2010;375:563–571. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]