Abstract
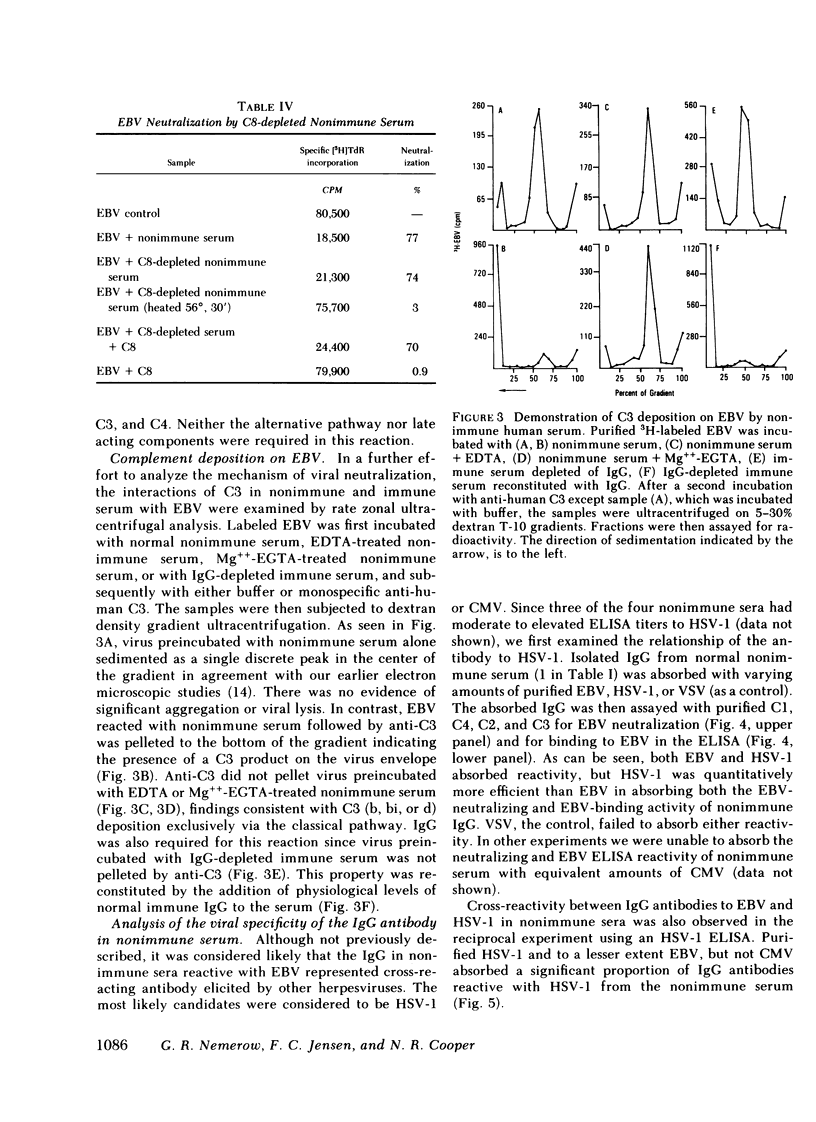
These studies were carried out to investigate the mechanism of neutralization of purified Epstein-Barr virus (EBV) by fresh human serum from normal individuals lacking antibody to the EBV viral capsid (VCA) and nuclear antigens (EBNA). Such individuals thus lack serological evidence of immunity to EBV. Although an enzyme-linked immunosorbent assay (ELISA) with highly purified immobilized EBV detected low levels of IgG antibody reactive with EBV in these normal nonimmune sera, this antibody failed to neutralize EBV in the absence of complement. Studies with depleted sera and mixtures of purified complement proteins at physiologic concentrations showed that the IgG antibody and C1, C4, C2, and C3 of the classical pathway were able to fully neutralize EBV. Mixtures of the purified components of the alternative pathway at physiologic concentrations failed to neutralize purified EBV in the presence or absence of the antibody and the alternative pathway did not potentiate classical pathway-mediated neutralization. No evidence for a requirement for C8 was obtained, precluding lysis as the mechanism of neutralization. Since C3 deposition on the viral surface accompanied classical pathway activation, viral neutralization is most likely secondary to the accumulation of complement protein on the viral surface. A coating of protein on the virus could interfere with attachment to, or penetration of potentially susceptible cells.
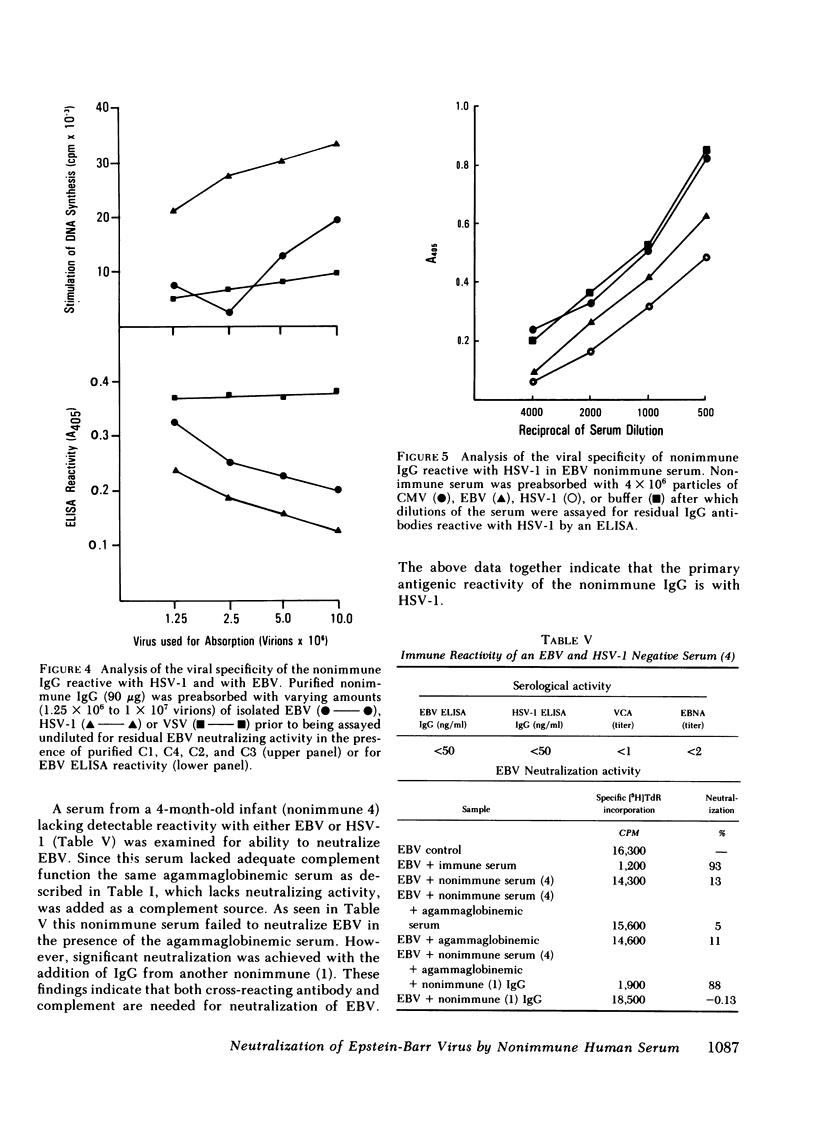
Experiments were undertaken to determine the specificity of the IgG antibody in the sera of EBV nonimmune individuals which, together with complement, neutralized EBV. Both purified EBV and herpes simplex I (HSV-1) absorbed the EBV ELISA reactivity and EBV-neutralizing activity of nonimmune sera, whereas another member of the herpesvirus group, cytomegalovirus, was inactive in this regard. HSV-1 was quantitatively more efficient than EBV in absorbing reactivity, a finding that indicates that the antibody has a higher affinity for HSV-1 than for EBV. Further absorption studies indicated that the cross-reaction occurred in both directions as EBV also absorbed HSV-1 reactive antibodies as tested in an HSV-1 ELISA. EBV was also less efficient than HSV-1 in absorbing reactivity with HSV-1. A serum lacking detectable antibodies to both EBV and HSV-1 failed to neutralize EBV. These studies cumulatively indicate that fresh serum from EBV nonimmune individuals neutralizes EBV by the combined action of a previously undescribed cross-reacting antibody apparently elicited by HSV-1 and C1, C4, C2, and C3 of the classical complement pathway.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Lint T. F., Mortensen R. F., Gewurz H. C567-initiated cytolysis of lymphoid cells: description of the phenomenon and studies on its control by C567 inhibitors. J Immunol. 1977 Jan;118(1):198–202. [PubMed] [Google Scholar]
- Beebe D. P., Cooper N. R. Neutralization of vesicular stomatitis virus (VSV) by human complement requires a natural IgM antibody present in human serum. J Immunol. 1981 Apr;126(4):1562–1568. [PubMed] [Google Scholar]
- Berry D. M., Almeida J. D. The morphological and biological effects of various antisera on avian infectious bronchitis virus. J Gen Virol. 1968 Jul;3(1):97–102. doi: 10.1099/0022-1317-3-1-97. [DOI] [PubMed] [Google Scholar]
- Britton S., Andersson-Anvret M., Gergely P., Henle W., Jondal M., Klein G., Sandstedt B., Svedmyr E. Epstein-Barr-virus immunity and tissue distribution in a fatal case of infectious mononucleosis. N Engl J Med. 1978 Jan 12;298(2):89–92. doi: 10.1056/NEJM197801122980208. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Curtiss L. K., Jensen F. C. Physiologic concentrations of normal human plasma lipoproteins inhibit the immortalization of peripheral B lymphocytes by the Epstein-Barr virus. J Clin Invest. 1981 Aug;68(2):329–336. doi: 10.1172/JCI110260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Polley M. J., Müller-Eberhard H. J. The second component of human complement (C2): quantitative molecular analysis of its reactions in immune hemolysis. Immunochemistry. 1970 Apr;7(4):341–356. doi: 10.1016/0019-2791(70)90237-5. [DOI] [PubMed] [Google Scholar]
- Daniels C. A., Borsos T., Rapp H. J., Snyderman R., Notkins A. L. Neutralization of sensitized virus by purified components of complement. Proc Natl Acad Sci U S A. 1970 Mar;65(3):528–535. doi: 10.1073/pnas.65.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. A., Borsos T., Rapp H. J., Snyderman R., Notkins A. L. Neutralization of sensitized virus by the fourth component of complement. Science. 1969 Aug 1;165(3892):508–509. doi: 10.1126/science.165.3892.508. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., ACHONG B. G., BARR Y. M. VIRUS PARTICLES IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. Lancet. 1964 Mar 28;1(7335):702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Graham B. J., Minamishima Y., Dresman G. R., Haines H. G., Benyesh-Melnick M. Complement-requiring neutralizing antibodies in hyperimmune sera to human cytomegaloviruses. J Immunol. 1971 Dec;107(6):1618–1630. [PubMed] [Google Scholar]
- Henle G., Henle W. Observations on childhood infections with the Epstein-Barr virus. J Infect Dis. 1970 Mar;121(3):303–310. doi: 10.1093/infdis/121.3.303. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W. Serum IgA antibodies of Epstein-Barr virus (EBV)-related antigens. A new feature of nasopharyngeal carcinoma. Bibl Haematol. 1975 Oct;(43):322–325. doi: 10.1159/000399157. [DOI] [PubMed] [Google Scholar]
- Hewetson J. F., Rocchi G., Henle W., Henle G. Neutralizing antibodies to Epstein-Barr virus in healthy populations and patients with infectious mononucleosis. J Infect Dis. 1973 Sep;128(3):283–289. doi: 10.1093/infdis/128.3.283. [DOI] [PubMed] [Google Scholar]
- Jacob R. J., Roizman B. Anatomy of herpes simplex virus DNA VIII. Properties of the replicating DNA. J Virol. 1977 Aug;23(2):394–411. doi: 10.1128/jvi.23.2.394-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy J. P., Simons R. L., Douglas R. G. Effect of selective complement deficiency on the rate of neutralization of enveloped viruses by human sera. J Immunol. 1977 Jan;118(1):28–34. [PubMed] [Google Scholar]
- Linscott W. D., Levinson W. E. Complement components required for virus neutralization by early immunoglobulin antibody. Proc Natl Acad Sci U S A. 1969 Oct;64(2):520–527. doi: 10.1073/pnas.64.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell I., Klein G., Lint T. F., Lachmann P. J. Activation of the alternative complement pathway by human B cell lymphoma lines is associated with Epstein-Barr virus transformation of the cells. Eur J Immunol. 1978 Jul;8(7):453–458. doi: 10.1002/eji.1830080702. [DOI] [PubMed] [Google Scholar]
- Miller G., Niederman J. C., Stitt D. A. Infectious mononucleosis: appearance of neutralizing antibody to Epstein-Barr virus measured by inhibition of formation of lymphoblastoid cell lines. J Infect Dis. 1972 Apr;125(4):403–406. doi: 10.1093/infdis/125.4.403. [DOI] [PubMed] [Google Scholar]
- Mills B. J., Cooper N. R. Antibody-independent neutralization of vesicular stomatitis virus by human complement. I. Complement requirements. J Immunol. 1978 Oct;121(4):1549–1557. [PubMed] [Google Scholar]
- Nemerow G. R., Cooper N. R. Isolation of Epstein Barr-virus and studies of its neutralization by human IgG and complement. J Immunol. 1981 Jul;127(1):272–278. [PubMed] [Google Scholar]
- Niederman J. C., Miller G., Pearson H. A., Pagano J. S., Dowaliby J. M. Infectious mononucleosis. Epstein-Barr-virus shedding in saliva and the oropharynx. N Engl J Med. 1976 Jun 17;294(25):1355–1359. doi: 10.1056/NEJM197606172942501. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Rosenthal J., Johnson B. Rate-zonal centrifugation of herpes simplex virus-antibody complexes. Virology. 1971 Jan;43(1):321–325. doi: 10.1016/0042-6822(71)90253-4. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Cooper N. R., Larson D. L. Formation and biologic role of polyoma virus-antibody complexes. A critical role for complement. J Exp Med. 1974 Aug 1;140(2):549–565. doi: 10.1084/jem.140.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced fc-binding receptors. J Virol. 1982 Jan;41(1):129–136. doi: 10.1128/jvi.41.1.129-136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purtilo D. T., Hutt L., Bhawan J., Yang J. P., Cassel C., Allegra S., Rosen F. S. Immunodeficiency to the Epstein-Barr virus in the X-linked recessive lymphoproliferative syndrome. Clin Immunol Immunopathol. 1978 Feb;9(2):147–156. doi: 10.1016/0090-1229(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Radwan A. I., Crawford T. B. The mechanisms of neutralization of sensitized equine arteritis virus by complement components. J Gen Virol. 1974 Nov;25(2):229–237. doi: 10.1099/0022-1317-25-2-229. [DOI] [PubMed] [Google Scholar]
- Reboul A., Arlaud G. J., Sim R. B., Colomb M. G. A simplified procedure for the purification of C1-inactivator from human plasma. Interaction with complement subcomponents C1r and C1s. FEBS Lett. 1977 Jul 1;79(1):45–50. doi: 10.1016/0014-5793(77)80347-5. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Robinson J. Assay for Epstein-Barr virus based on stimulation of DNA synthesis in mixed leukocytes from human umbilical cord blood. J Virol. 1975 May;15(5):1065–1072. doi: 10.1128/jvi.15.5.1065-1072.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Müller-Eberhard H. J. Fourth component of human complement: description of a three polypeptide chain structure. J Exp Med. 1974 Nov 1;140(5):1324–1335. doi: 10.1084/jem.140.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Pangburn M. K., Lesavre P. H., Müller-Eberhard H. J. Initiation of the alternative pathway of complement: recognition of activators by bound C3b and assembly of the entire pathway from six isolated proteins. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3948–3952. doi: 10.1073/pnas.75.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Pangburn M. K., Medicus R. G., Müller-Eberhard H. J. Raji cell injury and subsequent lysis by the purified cytolytic alternative pathway of human complement. Clin Immunol Immunopathol. 1980 Mar;15(3):384–396. doi: 10.1016/0090-1229(80)90050-1. [DOI] [PubMed] [Google Scholar]
- Shope T. C., Kaplan J. Inhibition of the in vitro outgrowth of Epstein-Barr virus-infected lymphocytes by TG lymphocytes. J Immunol. 1979 Nov;123(5):2150–2155. [PubMed] [Google Scholar]
- Shope T., Dechairo D., Miller G. Malignant lymphoma in cottontop marmosets after inoculation with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2487–2491. doi: 10.1073/pnas.70.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. B., Myrup A. C., Dutta S. K. Complement requirement for virus neutralization by antibody and reduced serum complement levels associated with experimental equine herpesvirus 1 infection. Infect Immun. 1981 Feb;31(2):636–640. doi: 10.1128/iai.31.2.636-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedmyr E., Jondal M. Cytotoxic effector cells specific for B Cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Thorley-Lawson D. A. Characterization of cross-reacting antigens on the Epstein-Barr virus envelope and plasma membranes of producer cells. Cell. 1979 Jan;16(1):33–42. doi: 10.1016/0092-8674(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Chess L., Strominger J. L. Suppression of in vitro Epstein-Barr virus infection. A new role for adult human T lymphocytes. J Exp Med. 1977 Aug 1;146(2):495–508. doi: 10.1084/jem.146.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A. The transformation of adult but not newborn human lymphocytes by Epstein Barr virus and phytohemagglutinin is inhibited by interferon: the early suppression by T cells of Epstein Barr infection is mediated by interferon. J Immunol. 1981 Mar;126(3):829–833. [PubMed] [Google Scholar]
- Tischendorf P., Shramek G. J., Balagtas R. C., Deinhardt F., Knospe W. H., Noble G. R., Maynard J. E. Development and persistence of immunity to Epstein-Barr virus in man. J Infect Dis. 1970 Nov;122(5):401–409. doi: 10.1093/infdis/122.5.401. [DOI] [PubMed] [Google Scholar]
- Tsoukas C. D., Fox R. I., Slovin S. F., Carson D. A., Pellegrino M., Fong S., Pasquali J. L., Ferrone S., Kung P., Vaughan J. H. T lymphocyte-mediated cytotoxicity against autologous EBV-genome-bearing B cells. J Immunol. 1981 May;126(5):1742–1746. [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1s subunit of the first complement component. J Immunol. 1974 Jan;112(1):339–350. [PubMed] [Google Scholar]
- Vestergaard B. F., Hesse J., Norrild B., Klein G. Production of rabbit antibodies against the viral capsid antigen (VCA) of the Epstein-Barr virus (EBV). Int J Cancer. 1978 Mar 15;21(3):323–328. doi: 10.1002/ijc.2910210312. [DOI] [PubMed] [Google Scholar]
- Westmoreland D., St Jeor S., Rapp F. The development by cytomegalovirus-infected cells of binding affinity for normal human immunoglobulin. J Immunol. 1976 Jun;116(6):1566–1570. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]



