Abstract
Background:
Rapid and specific diagnosis of gastrointestinal tuberculosis (GITB) is of utmost importance.
Aim:
To evaluate Multiplex PCR (MPCR) using MPB64 and IS6110 primers specific for M. tuberculosis for rapid diagnosis of GITB.
Materials and Methods:
MPCR was performed on colonoscopy biopsy specimens on 11 GITB confirmed (culture/AFB/histopathology was positive), 29 GITB suspected and 30 Non GITB (control group) patients.
Results:
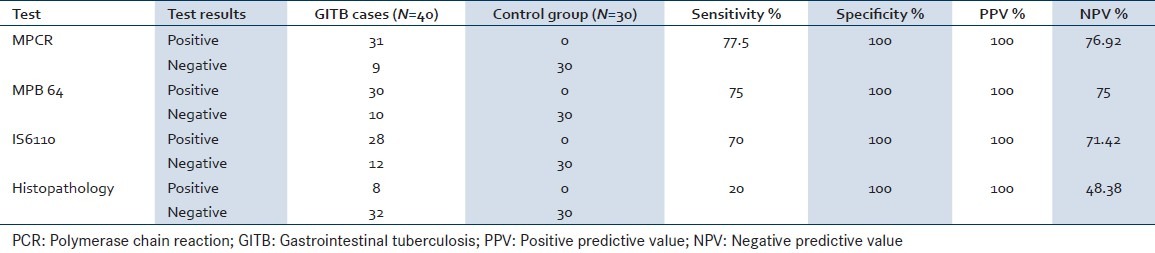
MPB64 PCR had sensitivity and specificity of 90% and 100% for confirmed GITB cases. In 29 clinically diagnosed but unconfirmed GITB cases, MPCR was positive in 72.41%. MPCR was negative in all control group patients. The overall sensitivity and specificity of microscopy, culture, histopathology and MPCR was 5%, 2% 20% and 77.5% and 100%, 100%, 100% and 100% respectively.
Conclusion:
MPCR has good sensitivity and specificity in diagnosing gastrointestinal tuberculosis.
Keywords: Gastrointestinal tract, Multiplex PCR, Tuberculosis
INTRODUCTION
Gastrointestinal Tuberculosis (GITB) is a major health problem in developing countries like India and is a rising threat in countries heavily infected with AIDS and due to trans global migration.[1] GITB mimics many other conditions like inflammatory bowel disease such as Crohn’s disease (CD) and malignancy.[2] There is increase in incidence of CD in TB endemic countries such as India and Southeast Asian countries.[3] The diagnosis is often difficult to establish immediately and accurately. This is primarily due to its widely-varying colonoscopic profiles, limitation of traditional microbiological methods and often non-conclusive histopathology reports.[4] The course of these two disorders is different, as intestinal TB is entirely curable, if diagnosed and treated early whereas, in CD is a progressive disease and treated by steroids.[5] The treatment modalities for both the conditions are poles apart, it is mandatory to establish the diagnosis as accurately as possible.
Nucleic acid based amplification tests (NAA) emerged as promising tools for diagnosing extra-pulmonary TB. A meta-analysis of NAA test used in diagnosis of TB concludes that commercial tests yielded results with high specificity and low sensitivity while heterogeneity and low diagnostic accuracy were concern with the in-house PCR test. Conventional microbiological methods like AFB smear and culture are quite inadequate for rapid diagnosis of GITB.[6,7] Most of the PCR based studies have used single target for amplification.[8] However, single gene target can result in false negative results and more reliable results are obtained by using more than one target gene for amplification.[9,10] The sensitivity of studies using amplification of two target genes is much better.[11] Most of the studies have used IS6110 as target for amplification in PCR with varying degree of success. However, IS6110 is absent in 10–15% of M. tuberculosis isolates from India,[12] which argues against its utility as a sole target for amplification in PCR. An alternative approach is to use multiplex PCR (MPCR) in which several target genes are amplified simultaneously. Various targets have been used alone or in combination in diagnosis of Mycobacterium tuberculosis infection (MTB). We have chosen IS6110 primers because of multiple copy numbers (6–24) in the Mycobacterium genome.[11] MPB64 primer has shown good sensitivity for diagnosis of CNS TB.[13] To the best of our knowledge, this is one of the first few studies in which role of multiplex PCR using IS6110 and MPB64 for early diagnosis of GITB has been evaluated. Therefore, in the present study, we share our experience of multiplex PCR using IS6110 and MPB64 for rapid diagnosis of M. tuberculosis in endoscopic biopsy samples of patients of GI tuberculosis.
MATERIALS AND METHODS
A total of 70 endoscopic ileocecal biopsy received for acid fast staining and culture were tested from December 2008 to March 2010. The patients were in the age range of 19 to 68 years. Out of these 70 cases, 11 were confirmed GITB cases, 29 were clinically suspected GITB cases, 20 were Crohn’s disease cases and 10 were non TB cases, who also acted as control group. In the control group we have included patients of CD and non TB cases. The relevant history and other details of the patients were noted and they were divided into two groups on the basis of following criteria.[14]
Group I: GITB (n = 40)
Confirmed GITB cases (n = 11): Culture/smear positive/Histopathology Positive
Suspected GITB cases (n = 29): Smear/culture/Histopathology negative but suspected of having GITB on clinical grounds and endoscopic findings and response to ATT
Group II: Control Group (n = 30):
Crohn’s disease (n = 20)
Non TB cases (n = 10)
Specimen collection
Endoscopic ileocecal biopsy samples were collected by taking full aseptic precautions and sent to laboratory with in 1 hour in normal saline for AFB staining, culture and Multiplex PCR and part of endoscopic biopsy was kept in 10% formalin and sent for histopathology.
Sample processing
All samples were processed in class II biosafety cabinet. The endoscopic biopsy samples were decontaminated and concentrated by using N-acetyl-L-cystine sodium hydroxide (NALC-NaOH) method followed by mixing with equal amount of decontamination solution and kept for 15–20 minutes at room temperature. The samples were vortexed for 5–20 seconds. Equal volumes of phosphate buffer were added to the samples and were centrifuged at 3000 rpm for 20 minutes. The supernatant was discarded and the sediment was re-suspended in 1–3 ml of phosphate buffer. The re-suspended sample was processed for preparation of smear, culture and, MPCR and part of sample was stored at −20°C.
AFB staining and culture
Decontaminated samples were examined for AFB by Ziehl-Neelsen method and culture was done on two LJ slants using standard procedures and incubated for 6 weeks.
Histopathology
Paraffin-embedded tissue section were prepared and stained with Hematoxylin-eosin and examined for granulomatous reactions suggestive of Mycobacterial tuberculosis.
Multiplex PCR
DNA was extracted from tissue samples as previously described by Van Soolingen[15] using Chloroform: Isoamyl alcohol extraction method and was stored at −20°C. Multiplex PCR was standardized and was found to have quantitative sensitivity to detect the DNA equivalent to 2–3 organisms. It tested positive with standard strain of M. tuberculosis, H37RV. In each independent MPCR assay, test results were compared with the results for one positive and one negative control. The positive control included was the DNA of H37Rv and negative control included was the PCR grade water. Identification of M. tuberculosis was done using a specific pair of primers designed to amplify IS6110 and MPB64 in the M. tuberculosis complex and the expected band size was about 123bp for IS611O, and 240 bp for MPB64. The sequence of primers used for IS6110 was ISI: 5’-CCTGCGAGCGTAGGCGT 3, IS2: 5’-CTCGTCCAGCGCCGCTTCGG 3’. Primers used for MPB64 were MPB1:5’-TCC GCT GCC AGT CGT CTT CC-3’, MPB2:5’-GTC CTC GCG AGT CTA GGC CA-3’.
Following components were added to eppendrof (for 50 μl reaction). PCR buffer 10X, dNTPs (Mix) 10 mM, Primer IS1 (10 pm/μl), IS2 (10 pm/μl), MPB1 (10 pm/μl) and MPB2 (10 pm/μl), Taq polymerase 5 U/μl, DNA template and water. DNA amplification was performed for 40 cycles following an initial denaturation step at 95°C for 5 minutes in thermo cycler by using the following program: Denaturation at 94°C for 1 minutes, annealing at 65°C for 1.5 minutes, extension at 72°C for 1.5 minutes and final Extension: 72°C for 10 minutes.
The amplified product was stored at 4°C till the detection. For detection of amplified products, samples were run on 1.5% agarose gel stained with ethidium bromide. The stained gel was examined under UV light to look for bands 123 bp of IS611O, and 240 bp of MPB64 using molecular weight marker of 100bp ladder. The samples showing the presence of these bands under ultraviolet transilumination were considered positive [Figure 1].
Figure 1.
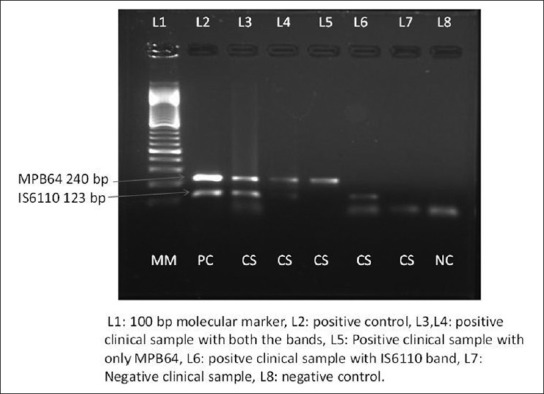
Gel photograph of multiplex PCR
Specificity and sensitivity of the MPCR assay
In agreement with Eisenach et al.,[16] MPCR was highly specific for M. tuberculosis in a preliminary study. No amplification product was produced with other mycobacterium species, such as M. avium, M. fortutium, or M. kansasii (data not shown). Sensitivity was estimated by serial dilutions of M. tuberculosis DNA. The MPCR detected 10 fg, which is equivalent to two mycobacterial genomes.
MPCR quality control
To avoid contamination during DNA extraction and amplification strict precautions were taken, including separate areas for DNA extraction, reagent preparation, amplification and product detection, and regular meticulous cleaning of surfaces with 10% hypochlorite. In addition all the reagents were aliquoted upon arrival in the laboratory. Positive and negative control was included with each set of reaction. The positive control was DNA extracted from H37RV whereas negative control was PCR grade water. To demonstrate the presence of inhibitors in MPCR all negative samples were spiked with positive control DNA and no inhibitors were detected on spiked samples as all were positive with spiked DNA.
Statistical methods
The sensitivity, specificity, positive predictive value and the negative.
Predictive value was calculated using the standard formulae. This study was part of study approved by the institute ethics committee.
RESULTS
The present study evaluated the role of Multiplex PCR, histopathology, culture and AFB smear for diagnosis of GITB in 40 patients. In the control group, we have included 20 patients of CD and 10 of non TB cases. Out of 40 cases of GITB, 11 were confirmed GITB cases, and 29 were clinically suspected GITB cases, who also responded to ATT. Figure 1 shows the gel photograph of Multiplex PCR. (MPCR is considered as positive if band is present for both MPB64 and IS6110 or band is there for any of two i.e., MPB64 or IS6110).
Out of 11 confirmed GITB cases, culture was positive one patient, 8 patients were diagnosed on histopathology [Table 1], AFB smear was positive in 2 cases, and MPCR was positive in 10 cases respectively. Culture and smear positive cases were also positive by MPCR. Out of 8 histopathology positive cases, 7 were positive by MPCR [Table 1].
Table 1.
Comparison of mPCR with smear culture and histopathology

In 29 clinically suspected cases of GITB, MPCR was positive in 21 (72.41%) cases, and out of these 21 cases, MPB64 bands were present in 20 (68.96%) cases and IS6110 bands were present in 18 (62.20%) cases respectively. MPCR was negative in control group thus giving specificity of 100%. A final diagnosis of GITB was made for 40 patients based on result of culture, microscopy, histopathology and response to ATT. MPCR was positive in 31 cases, histopathology in 8 and culture in 1 and AFB smear in 2 cases respectively. Thus sensitivity of MPCR, histopathology, culture and microscopy was 77.5%, 20%, 2% and 5% respectively. However, the sensitivity of MPCR in confirmed GITB cases and suspected GITB was 90.90% and 72.41% respectively. There were total 2 (5%) cases out of 40, which were missed by IS6110 but were diagnosed by MPB64. Similarly there was 1 of 70 cases which were only IS6110 positive [Table 1]. By using two primers together in MPCR there was an increase in the sensitivity to 77.5% for MPCR whereas sensitivity of MPB64 and IS6110 alone was 75% and 70% respectively. In the control group all the tests were negative thus giving specificity of 100% [Table 2].
Table 2.
Sensitivity and specificity of multiplex PCR compared to other tests for diagnosis of GITB
DISCUSSION
GITB is mostly based on clinical, radiological and endoscopic features as histological and microbiological results are often inconclusive due to paucibacilliary nature of the disease. In the recent years, there is increase in incidence of CD in Indian Population[4] moreover Crohn’s disease mimics GITB in clinical presentation, radiological, colonoscopy, and histological features.[16] Treatment modalities for two disease is totally different, therefore it is important to establish the diagnosis as accurately as possible as GITB is potentially curable with anti tubercular treatment.[17,18]
The most promising new approach to this problem is NAA technique such as PCR. In this study, we evaluated MPCR using two target together i.e., IS6110 and MPB64 for diagnosis of M. tuberculosis complex in patients of GITB and compared it with histopathology, culture and ZN smear examination. In our study, histopathology, microscopy and culture were positive in 20%, 5% and 2% cases of GITB confirming the paucibacilliary nature of the disease, as earlier studies[19,20] also reported sensitivity in the range of 22–30%, 7–10% and 0–20% for histopathology. AFB smear and culture respectively. The factors responsible for low sensitivity in this study and also stated by other studies[21,22] such as, the paucibacilliary nature of GITB, granulomas in deeper layer, inadequate biopsy material, unequal distribution of organism, limitation of microbiological techniques or early lesions that may not show caseation necrosis.
Most of the PCR based studies had reported use of single target like IS6110 for the diagnosis of MTB. However, IS6110 is missing in 10–15% of Indian population.[12] Use of two or more gene targets for amplification has been demonstrated to increase the diagnostic yield of MTB infection in other clinical setting.[20,22] We evaluated the role of MPCR using two different target specific for Mycobacterium tuberculosis complex for diagnosis of GITB in proven and clinically suspected cases of GITB. The overall sensitivity of this MPCR was 77.5% and specificity was 100%. Whereas sensitivity in proven cases which were histopathology, culture, and smear positive cases was 90.90%, and in clinically suspected cases who also responded to ATT was 72.41%. There was one histopathology positive case which was missed by MPCR; the reason could be inadequate biopsy material. The single target PCR target based studies had reported sensitivity varying between 21.6–64.1%.[4,5] The overall sensitivity of MPCR in our study is higher as compared to earlier studies. The other studies have also reported similar factors for low sensitivity of PCR in the diagnosis of MTB infection.[23]
In our study, IS6110 bands were present in 90.90% of confirmed cases and 62.20% of clinically suspected cases. The findings of our study are similar to that reported by Moatter et al.[24] MPB64 bands were positive in 90.90% of confirmed cases and 68.96% of suspected cases which is similar to the study reported by Jin XJ et al.[25] There were two cases which were missed by IS6110 and one case was missed by MPB64.
This again highlights the facts that there are strains of Mycobacterium tuberculosis strains in India which lack not only IS6110 but MPB64 also. Thus, MPCR plays an important role to increase the overall sensitivity of PCR over uniplex PCR test for diagnosing GITB infection. The other advantage of MPCR over doing Uniplex PCR using different primers is the low cost, less chances of contamination, less time consumption and better utilization of manpower in resource constraint settings.
CONCLUSION
This study found that MPCR assay is useful for rapid and accurate diagnosis of GITB cases which are likely to be missed due to absence of IS6110 sequence in our Indian population and can supplement the clinical diagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Patel N, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Gastrointestinal luminal tuberculosis: Establishing the diagnosis. J Gastroenterol Hepatol. 2004;19:1240–6. doi: 10.1111/j.1440-1746.2004.03485.x. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni S, Vyas S, Supe A, Kadival G. Use of polymerase chain reaction in the diagnosis of abdominal tuberculosis. J Gastroenterol Hepatol. 2006;21:819–23. doi: 10.1111/j.1440-1746.2006.04030.x. [DOI] [PubMed] [Google Scholar]
- 3.Das K, Ghoshal UC, Dhali GK, Benjamin J, Ahuja V, Makharia GK. Crohn’s disease in India: A multicenter study from a country where tuberculosis is endemic. Dig Dis Sci. 2009;54:1099–107. doi: 10.1007/s10620-008-0469-6. [DOI] [PubMed] [Google Scholar]
- 4.Amarapurkar DN, Patel ND, Amarapurkar AD, Agal S, Baigal R, Gupte P. Tissue polymerase chain reaction in diagnosis of intestinal tuberculosis and Crohn’s disease. J Assoc Physicians India. 2004;52:863–7. [PubMed] [Google Scholar]
- 5.Gan HT, Chen YQ, Ouyang Q, Bu H, Yang XY. Differentiation between intestinal tuberculosis and Crohn’s disease in endoscopic biopsy specimens by polymerase chain reaction. Am J Gastroenterol. 2002;97:1446–51. doi: 10.1111/j.1572-0241.2002.05686.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin DK, Roy P, Sharma M, Singh K, Malik AK, Panigrahi D. Acid-fast bacilli in colonoscopic brushings. Lancet. 1991;338:184–5. doi: 10.1016/0140-6736(91)90172-l. [DOI] [PubMed] [Google Scholar]
- 7.Malik AK, Bhasin DK, Roy P, Singh K, Mehta SK. Demonstration of Mycobacterium tuberculosis in colonoscopic biopsies. Histopathology. 1993;23:199–200. [PubMed] [Google Scholar]
- 8.Sharma K, Sharma A, Singh M, Ray P, Dandora R, Sharma SK, et al. Evaluation of polymerase chain reaction using protein b primers for rapid diagnosis of tuberculous meningitis. Neurol India. 2010;58:727–31. doi: 10.4103/0028-3886.72189. [DOI] [PubMed] [Google Scholar]
- 9.Sharma K, Sharma A, Sharma SK, Sen RK, Dhillon MS, Sharma M. Does multiplex polymerase chain reaction increase the diagnostic percentage in osteoarticular tuberculosis. A prospective evaluation of 80 cases? Int Orthop. 2012;36:255–9. doi: 10.1007/s00264-011-1241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusum S, Aman S, Pallab R, Kumar SS, Manish M, Sudesh P, et al. Multiplex PCR for rapid diagnosis of tuberculous meningitis. J Neurol. 2011;258:1781–7. doi: 10.1007/s00415-011-6010-4. [DOI] [PubMed] [Google Scholar]
- 11.Pai M, Flores LL, Pai N, Hubbard A, Riley LW, Colford JM., Jr Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect Dis. 2003;3:633–43. doi: 10.1016/s1473-3099(03)00772-2. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan DS, Sharma VD, Parashar D, Chauhan A, Singh D, Singh HB, et al. Molecular typing of Mycobacterium tuberculosis isolates from different parts of India based on IS6110 element polymorphism using RFLP analysis. Indian J Med Res. 2007;125:577–81. [PubMed] [Google Scholar]
- 13.Afrose D, Mir AW, Kirmani A, Rhehman S, Eachkoti R, Siddiqi MA. Improved diagnosis of central nervous system tuberculosis by MPB64-target PCR. Braz J Microbiol. 2008;39:209–13. doi: 10.1590/S1517-83822008000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai CG, Khandige GK. Is Crohn’s disease rare in India? Indian J Gastroenterol. 2000;19:17–20. [PubMed] [Google Scholar]
- 15.VanSoolingen D, Hermans PW, de Haas PE, Soll DR, van Embden JD. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: Evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–86. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tandon HD, Prakash A. Pathology of intestinal tuberculosis and its distinction from Crohn’s disease. Gut. 1972;13:260–9. doi: 10.1136/gut.13.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor VK. Abdominal tuberculosis. Postgrad Med J. 1998;74:459–67. doi: 10.1136/pgmj.74.874.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989–99. [PubMed] [Google Scholar]
- 19.Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005;37:351–6. doi: 10.1055/s-2005-861116. [DOI] [PubMed] [Google Scholar]
- 20.Shah S, Thomas V, Mathan M, Chacko A, Chandy G, Ramakrishna BS, et al. Colonoscopic study of 50 patients with colonic tuberculosis. Gut. 1992;33:347–51. doi: 10.1136/gut.33.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhigjee AI, Padayachee R, Paruk H, Hallwirth-Pillay KD, Marais S, Connoly C. Diagnosis of tuberculous meningitis: Clinical and laboratory parameters. Int J Infect Dis. 2007;11:348–54. doi: 10.1016/j.ijid.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Rafi W, Venkataswamy MM, Ravi V, Chandramuki A. Rapid diagnosis of tuberculous meningitis: A comparative evaluation of in-house PCR assays involving three mycobacterial DNA sequences, IS6110, MPB-64 and 65 kDa antigen. J Neurol Sci. 2007;252:163–8. doi: 10.1016/j.jns.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Noordhoek GT, Kolk AH, Bjune G, Catty D, Dale JW, Fine PE, et al. Sensitivity and specificity of PCR for detection of Mycobacterium tuberculosis: A blind comparison study among seven laboratories. J Clin Microbiol. 1994;32:277–84. doi: 10.1128/jcm.32.2.277-284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moatter T, Mirza S, Siddiqui MS, Soomro IN. Detection of Mycobacterium tuberculosis in paraffin embedded intestinal tissue specimens by polymerase chain reaction: Characterization of IS6110 element negative strains. J Pak Med Assoc. 1998;48:174–8. [PubMed] [Google Scholar]
- 25.Jin XJ, Kim JM, Kim HK, Kim L, Choi SJ, Park IS, et al. Histopathology and TB-PCR kit analysis in differentiating the diagnosis of intestinal tuberculosis and Crohn’s disease. World J Gastroenterol. 2010;16:2496–503. doi: 10.3748/wjg.v16.i20.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]


