Abstract
Background:
Helicobacter pylori (H. pylori) infection is a risk factor for peptic ulcer. There have been no studies addressing environmental and dietary risk factors in western India. We conducted a case control study enrolling peptic ulcer patients in Pune, India.
Materials and Methods:
Risk factors for peptic ulcer and H. pylori infection were assessed in a participant interview. H. pylori status was assessed from stool by monoclonal antigen detection.
Results:
We enrolled 190 peptic ulcer, 35 stomach cancer patients, and 125 controls. Fifty-one percent (180/350) of the participants were infected with H. pylori. Lower socioeconomic status (SES) [odds ratio (OR): 1.10, 95% confidence interval (CI): 1.02–1.39], meat consumption (OR: 2.35, 95% CI: 1.30–4.23), smoking (OR: 2.23, 95% CI: 1.24–4.02), eating restaurant food (OR: 3.77, 95% CI: 1.39–10.23), and drinking nonfiltered or nonboiled water (OR: 1.05, 95% CI: 1.01–1.23) were risk factors for H. pylori infection. H. pylori infection (OR: 1.70, 95% CI: 1.03–2.89), meat (OR: 1.10, 95% CI: 1.02-1.75), fish (OR: 1.05, 95% CI: 1.02–1.89) consumption, and a family history of ulcer (OR: 1.20, 95% CI: 1.08–1.60) were risk factors for peptic ulcer. Consumption of chili peppers (OR: 0.20, 95% CI: 0.10–0.37) and parasite infestation (OR: 0.44, 95% CI: 0.24–0.80) were protective against H. pylori infection.
Conclusion:
H. pylori infection is associated with peptic ulcer. Lower SES, consumption of restaurant food, meat, nonfiltered water, and smoking are risk factors for H. pylori. Consumption of meat, fish, and a family history of peptic ulcer are risk factors for peptic ulcer. Consumption of chili peppers and concurrent parasite infestation appear to be protective against H. pylori.
Keywords: Case control study, Chili peppers, Helicobacter pylori, India, Parasites, Pune
INTRODUCTION
Helicobacter pylori (H. pylori) infect more than 50% of the world’s population.[1] There is significant variation in the prevalence of H. pylori across the world.[1] Previous studies from India have reported a high, up to 80%, prevalence of H. pylori.[2] H. pylori are known to be associated with peptic ulcer and gastric cancer.[3] Peptic ulcer is frequent in India but there is a paucity of data on its prevalence.[4,5] Prevalence of peptic ulcer is noted to be, on an average, around 8.0 per 100,000 in India.[5] The age-adjusted incidence rate of gastric cancer in urban registries in India is 3.0 per 100,000 which is lower compared to the average of 14 per 100,000 worldwide.[6]
The association between H. pylori and peptic ulcer is being investigated widely. Researchers have reported the role of H. pylori infection in the pathogenesis of peptic ulcer and stomach cancer in India, and some have reported no association between stomach cancer and H. pylori infection.[7–12] These studies are criticized to have methodological pitfalls such as small sample size and inadequate description of sample size calculations, and case definitions.[13,14] Several factors have been associated with the aggressiveness of H. pylori and hence implicated in epithelial damage, including the virulent East Asian CagA genotype, and environmental and dietary factors.[3] Recently, some researchers have also proposed that certain dietary elements such as chili peppers and garlic[15] play a protective role against H. pylori infection. Fox and colleagues have shown the protective role of infection of helminths and parasites against H. pylori infection.[16,17] However, the risk factors for H. pylori infection, especially the Indian context of environmental and dietary factors, are not comprehensively investigated. Specifically, we are not aware of any studies conducted in western India to assess the relationship between peptic ulcer and H. pylori and their risk factors.
Here we report findings from a case control study conducted in Pune, Maharashtra, India. The objectives of this study were a) to determine risk factors for peptic ulcer, stomach cancer, and H. pylori infection in Pune, India and b) to explore the association between peptic ulcer and H. pylori infection.
MATERIALS AND METHODS
Study design
Case control study: Our case control study had three groups: (1) patients diagnosed with peptic ulcer, (2) patients diagnosed with stomach cancer, and (3) controls.
Study location
The study was conducted at Deenanath Mangeshkar Hospital and Research Center (DMH) and private clinics affiliated with DMH in Pune, Maharashtra State, India during January 2008 and November 2010. The study was approved by the University of South Florida intuitional review board and research ethics committee at DMH.
Selection of cases
The peptic ulcer and stomach cancer cases were identified via the DMH database using the ICD10 coding. Any patient more than 18 years of age and listed in the DMH database as having a diagnosis of either peptic ulcer or stomach cancer was eligible for enrollment in the peptic ulcer or stomach cancer arm of the study, respectively. The participants who were enrolled in the peptic ulcer and stomach cancer group from the satellite clinics were diagnosed by their respective attending physicians. We used SAS software random number generation code to generate a list of potential eligible patients.
Selection of controls
The controls were identified via the DMH database using the ICD10 coding. Any patient more than 18 years of age and listed in the DMH database as having a diagnosis of other than peptic ulcer or stomach cancer was eligible for enrollment in the control arm of the study.
The trained study personnel contacted patients at DMH and private clinics, requesting them to participate in the study. The study was explained in detail by the study personnel to each potential participant in their native Marathi language.
Design of data collection instrument
We utilized the revised version of the semistructured questionnaire used by Sasaki et al. for data collection.[18] The questionnaire was pilot tested at the DMH on a small focus group of physicians (n = 3) and their patients (n = 12). Based on their feedback, the questionnaire was revised. We translated the questionnaire into the native Marathi language and translated it back into English to ensure consistency in terms of validity of content and criteria.
Data collection
Data regarding the risk factors for peptic ulcer and H. pylori infection was collected using a semistructured questionnaire by two trained physicians in a one-on-one participant interview. The physicians were bilingual (English-Marathi) natives. The principle investigator (RM) checked all the data for accuracy.
H. pylori antigen detection
We collected a single stool sample from each participant for the detection of H. pylori. H. pylori antigen was detected using ImmunoCard STAT HpSA antigen detection kit.[19,20] Briefly, a small portion (5–6 mm diameter) of stool specimen was transferred into the sample diluent vial using the applicator stick, vortexed for 15 seconds, and then four drops were dispensed into the round window at the lower end of the device. The results were read five minutes later. The tests were performed by trained pathology laboratory technicians.
Sample size calculation
We based our sample size calculations assuming the following parameters: Alpha error = 0.5, power = 90%, expected effect size: Odds ratio (OR) = 2.1, proportion of controls with the exposure (H. pylori) = 0.40, and proportion of cases with the exposure = 0.50.
Statistical analyses
The association between each potential risk factor and the outcome (H. pylori and peptic ulcer status) was measured using logistic regression with backward stepwise elimination (P < 05 to retain). Statistical analyses were conducted using SAS software version 9.1.3.[21]
RESULTS
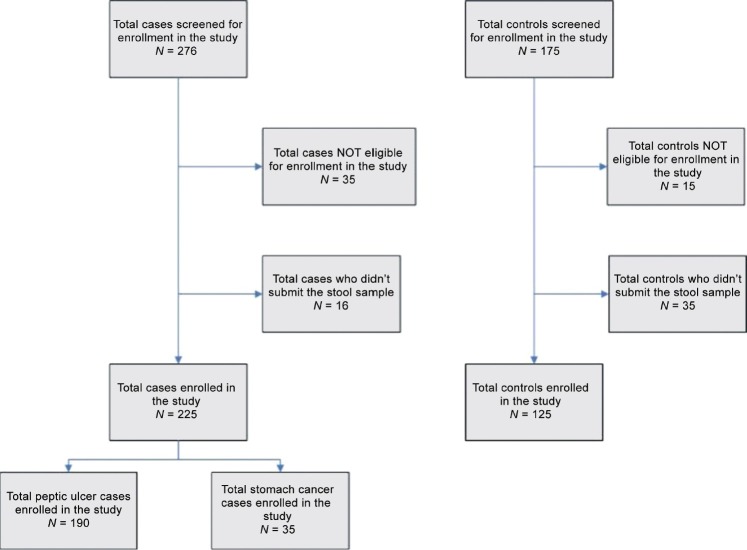
We contacted a total of 451 potential participants. We enrolled 190 patients diagnosed with peptic ulcer and 125 controls in our study [Figure 1]. We could enroll only 35 gastric cancer patients in this study. The probability of H. pylori thriving in the stomach lining with the metaplasia is known to be low,[21] and the inadequate sample size may lead to bias. Hence, we eliminated these 35 patients from our analysis investigating the association between disease status of the participants and various risk factors.
Figure 1.
Flow chart showing recruitment of study participants
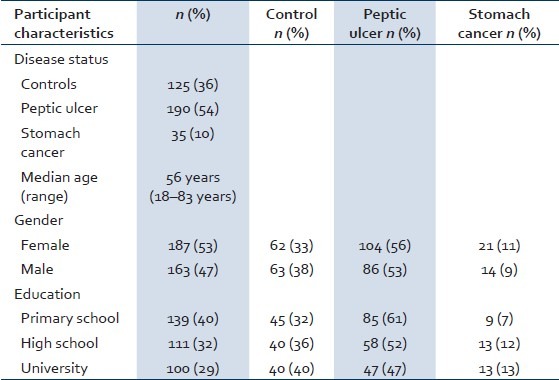
The median age of the enrolled participants was 56 years (range: 18–83 years). Fifty-three percent (187/350) of the participants were females and 47% (163/350) were males. Forty percent (139/350) of the participants had primary education, whereas 32% (111/350) had completed high school. Twenty-nine percent (100/350) of the participants had university-level education [Table 1]. Fifty-one percent (180/350) of the participants enrolled in the study were diagnosed with H. pylori infection.
Table 1.
Demographic characteristics of study participants (n=350)
Assessment of risk factors for H. pylori infection
In the univariate analysis, demographic characteristics such as gender (P value = 0.74), education (P value = 0.12), and age were not associated with H. pylori status. Current tobacco use of the participants was associated with H. pylori (P value = 0.01). Consumption of snacks by the participants while drinking alcohol was not associated with H. pylori status (P value = 0.10). Consumption of chili peppers (P value 0.01) and meat (consumption of mostly chicken and goat meat) (P value = 0.0003) had a statistically significant association with H. pylori status. However, fish consumption (P value = 0.31) was not associated with H. pylori status. Consuming the food items while they were hot (in terms of food temperature) (P value = 0.07) was not associated with H. pylori status. Frequency of food consumption from outside was associated with H. pylori status (P value < 0.01). Chronic gastric status (P value <0.01) of the participants and its frequency (P value = 0.02) were associated with their H. pylori status. Similarly, family history of peptic ulcer was associated with H. pylori status (P value = 0.008). Moreover, parasite (P value = 0.001) and protozoa infestations (P value = 0.02) were associated with H. pylori status. However, helminth infection was not associated with H. pylori status (P value = 0.55). Antiparasitic drug use was associated with H. pylori status (P value = 0.04). Prior treatment of H. pylori was not associated with current H. pylori status (P value = 0.80) [Table 2].
Table 2.
Risk factors for H. pylori infection
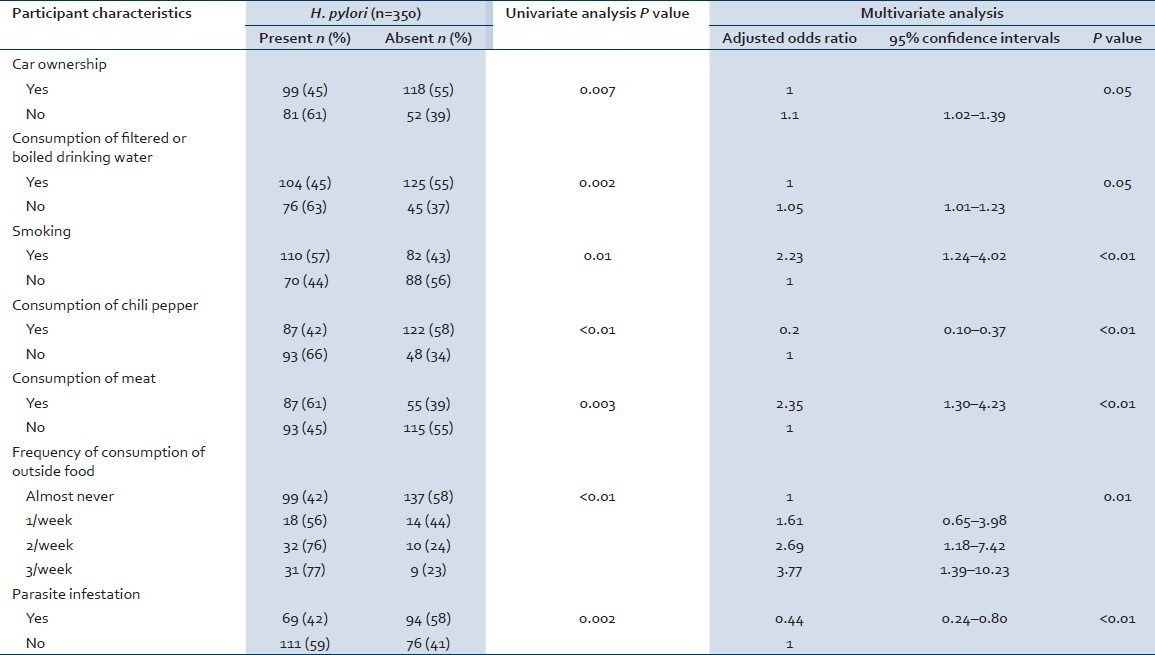
The key results of multivariate logistic regression analysis for the association between characteristics of participants and H. pylori status are shown in Table 2. Individuals with low socioeconomic status (SES) (not owning a car was used as a parameter for low SES) were more likely to be infected with H. pylori status compared with individuals with car ownership (OR: 1.10 95% CI: 1.02–1.39). Participants with chronic gastric ailments were more likely to suffer from H. pylori infection compared with individuals with no gastric ailments (OR: 3.73 95% CI: 1.69–8.26). Participants who reported tobacco use were more likely to be infected with H. pylori infection compared with participants who were not using tobacco (OR: 2.23 95% CI: 1.24–4.02). Meat consumption was a high risk factor for H. pylori infection (OR: 2.35 95% CI: 1.30–4.23). Consumption of chili peppers was a protective factor against H. pylori infection (OR: 0.20 95% CI: 0.10–0.37). Participants who did not drink filtered or boiled water were more likely to suffer from H. pylori infection compared with individuals who drank filtered or boiled water (OR: 1.05 95% CI: 1.01–1.23). Consumption of restaurant food was also a risk factor for H. pylori infection [Table 2].
Assessment of risk factors for peptic ulcer
In the univariate analysis, demographic characteristics such as gender (P value = 0.42), education (P value = 0.23), and age were not associated with disease status (having peptic ulcer vs. not having any gastric ailment). Sixty percent (115/190) of the patients suffering from peptic ulcer were diagnosed with H. pylori infection compared with 45% (57/125) of the participants enrolled in the control group. Disease status of the study participants was associated with H. pylori status (P value = 0.01).
Current tobacco use of the study participants was not associated with disease status (P value = 0.16). Consumption of snacks by the participants while drinking alcohol was associated with disease status (P value < 0.01). Consumption of chili peppers (P value = 0.06) did not have a statistically significant association with disease status. However, consuming the food items while they were hot (in terms of food temperature) (P value = 0.04), and consumption of meat (P value = 0.02) and fish (P value = 0.01) were associated with disease status. Frequency of the consumption of food from outside was not associated with disease status (P value = 0.30). Family history of peptic ulcer of the participants was associated with disease status (P value = 0.04). Parasite (P value = 0.56), protozoa (P value = 0.20), and helminth (P value = 0.25) infestations were not associated with disease status. Prior treatment of H. pylori was associated with current disease status (P value = 0.03) [Table 3].
Table 3.
Risk factors for peptic ulcer
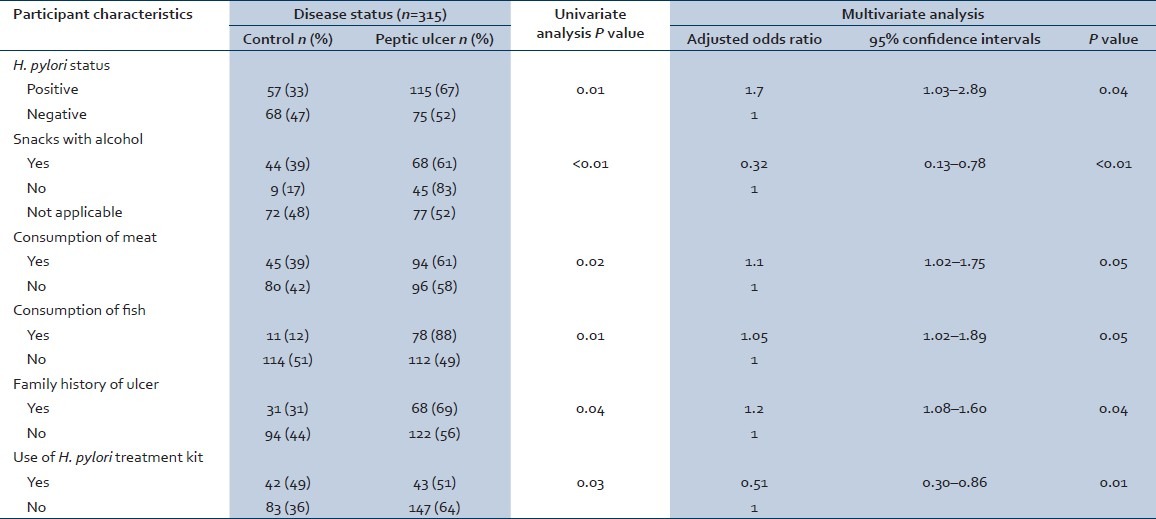
The key results of multivariate logistic regression analysis for the association between characteristics of participants and disease status are shown in Table 3. Participants with peptic ulcer were more likely to be diagnosed with H. pylori infection than controls (OR: 1.70 95% CI: 1.03–2.89). Participants with peptic ulcer were more likely consuming meat (OR: 1.10 95% CI: 1.02–1.75) and fish (OR: 1.05 95% CI: 1.02–1.89) compared with controls. Participants with a positive family history of ulcer were more likely to suffer from peptic ulcer compared with participants with no family history of peptic ulcer (OR: 1.20 95% CI: 1.08–1.60). Anti-H. pylori treatment kit use was a protective factor against peptic ulcer (OR: 0.51 95% CI: 0.30–0.86). Consumption of snacks with alcohol was a protective factor against peptic ulcer (OR: 0.32 95% CI: 0.13–0.78) [Table 3].
DISCUSSION
To our knowledge, this is the first study to document the prevalence of H. pylori among patients with peptic ulcer, stomach cancer, and individuals not diagnosed with peptic ulcer or stomach cancer in Pune, Maharashtra. This is also the largest study investigating the risk factors for H. pylori and peptic ulcer in Maharashtra, India. In this study, 51% (180/350) of the participants were infected with H. pylori. Fifty-five percent (123/225) of symptomatic participants and 45% (57/125) of asymptomatic individuals were diagnosed with H. pylori. This study shows that lower SES, consumption of restaurant food, meat, nonfiltered or nonboiled water, and smoking are risk factors for H. pylori infection. Consumption of chili peppers and concurrent parasite infestation are protective against H. pylori infection. Consumption of meat, fish, and a positive family history of peptic ulcer are risk factors for peptic ulcer. History of anti-H. pylori treatment protects against peptic ulcer.
We showed that individuals in the lower SES category were more likely to suffer from H. pylori infection compared with individuals who owned a car (an indicator of better SES). Our results highlighted the association between the source of drinking water and H. pylori infection. Prevalence of H. pylori was higher in participants consuming nonfiltered or nonboiled water compared with participants using water filters or boiling the water before consumption. Our findings are in line with previous research on this topic.[22] In our study, individuals who ate food from outside three times or more during the week were four times more likely to have H. pylori infection compared with individuals who almost never ate outside. Poor hygienic practices and the use of nonboiled water in the restaurants in the city may contribute to the transmission of H. pylori. Meat has been shown to be a risk factor for H. pylori infection.[23,24] Similarly, in our study, individuals consuming meat were twice as likely to be infected with H. pylori compared with participants not consuming meat.
The odds of H. pylori infection were lower in our study participants who consumed chili peppers compared with individuals who did not consume them. In Maharashtra, chili peppers are common ingredients of food recipes. The chili peppers are added with turmeric (curcuma longa) and cooking oil while preparing food items from produce and meat. Previous studies have shown the protective role of turmeric, chili peppers, and some spices against H. pylori infection.[15] Some of these studies have been validated in animals and have confirmed plants as the source of antimicrobial agents against H. pylori.[15] Specifically, capsaicin, the active ingredient in chili, has been shown to have a protective effect against H. pylori infection. It is shown that capsaicin exhibits bactericidal activity when incubated at pH values as low as 5.4. Ingestion of chili, therefore, could have a protective effect against H. pylori infection and associated gastroduodenal ailments.[25] Capsaicin has also been proposed as a potential anti-inflammatory drug by the inhibition of the production of interleukin (IL)-8 in H. pylori-infected gastric epithelium.[26] However, the exact mechanism behind the bactericidal action of chili peppers is being debated.
It is hypothesized that concurrent enteric helminth infection can attenuate gastric atrophy via immune modulation.[16,17] That is, helminthiasis and other parasitic infestation can change the T helper (Th) cell immune response from a predominantly Th1 type to a Th2 type protective response.[27,28] In our study, 27% of the participants were infected with helminth and 54% with protozoa, and 47% had parasite infection. Individuals who suffered from parasite infestations were less likely to have H. pylori infection. Recently, Fox et al.[17] showed that mice infected with Helicobacter felis alone showed a Th1 response, but in mice coinfected with H. felis and the helminth H. polygyrus, there was a shift in cytokine expression consistent with a Th2 immune response.[16] This hypothesis, If extrapolated to humans, may show that intestinal helminth infection may provide a protective effect against gastric cancer.[16]
In our study, H. pylori infection was seen as a significant risk factor for peptic ulcer disease. Meat and fish consumption were also noted to be risk factors for peptic ulcer. Our findings are in line with current research which showed that consumption of meat and salted fish are risk factors for stomach cancer.[29–32] This study reiterates the association between a positive family history of peptic ulcer and the prevalence of peptic ulcer.[33] Consumption of alcohol and tobacco did not emerge as risk factors for peptic ulcer in our study. However, consumption of snacks with alcohol was protective against peptic ulcer. The link between SES and the type and quantity of alcohol consumed can explain this partially. Usually, individuals with a higher SES consume snacks with alcohol; these individuals may drink in moderation and may opt for treatment for their ailments more frequently compared with individuals from a lower SES who do not consume snacks with their alcohol. These interlinked factors may play a role in protection of the gastric mucosal layer in people who drink but consume snacks while drinking. However, this hypothesis needs to be formally investigated.
Our study has some limitations. First, we could enroll only 35 stomach cancer patients. We believe this is due to the high mortality rate and low proportion of stomach cancer patients seeking active treatments. From most of the studies from developing countries, it is noted that within the first 10 years of life, the prevalence of H. pylori increases with age.[34–37] We enrolled only adults in this study and hence were unable to explore the association between age and prevalence of H. pylori from childhood. Education was not associated with the prevalence of H. pylori in this study. However, previous studies have shown the association between lower education and a high prevalence of H. pylori.[1,38] We recruited participants from the city of Pune but not from the nearby villages, and none of the participants in our study was uneducated. We came across a significant amount of missing data for the quantification of cigarettes and alcohol consumption. We believe that owing to the stigma attached to smoking and the consumption of alcohol, individuals in this study were reluctant to share this information. Hence, we were unable to investigate the dose-response of the consumption of tobacco and alcohol. We also made an attempt to elucidate the intrafamilial transmission pathways of H. pylori by enquiring about H. pylori status of spouses. However, 74% of our participants were unaware of the H. pylori status of their spouses. Previous research has shown the virulent nature of CagA-positive H. pylori.[39,40] Moreover, it is well documented that grades of inflammation, activity of gastritis, and atrophy are significantly higher in gastritis patients infected with the East Asian CagA-positive strain than in gastritis patients infected with the CagA-negative or western CagA-positive strains.[41,42] However, due to the lack of availability of biomolecular testing, we were unable to conduct the genotyping of H. pylori to detect the prevalence of CagA-positive and East Asian CagA H. pylori infection.
CONCLUSION
This largest study investigating the risk factors for H. pylori and peptic ulcer conducted in Maharashtra showed the association between H. pylori and peptic ulcer and highlighted the risk and protective factors for H. pylori infection. Specifically, lower SES, consumption of restaurant food, meat, and nonfiltered water, and smoking are the risk factors for H. pylori. Consumption of meat, fish, and a family history of peptic ulcer are risk factors for peptic ulcer. Consumption of chili peppers and concurrent parasite infestation appear to be protective against H. pylori. Further studies are needed to explore the protective mechanism of chili peppers against H. pylori infection and the association between helminths, parasite infestation, and H. pylori infection. Moreover, the role of East Asian CagA H. pylori infection in India should be investigated.
ACKNOWLEDGMENT
We thank Dr. Paranjape S. Y., Dr. Patwardhan S. A., and Dr. Kelkar D. S. from Deenanath Mangeshkar Hospital, Pune for their support of this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.The EUROGAST Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;34:1672–6. doi: 10.1136/gut.34.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunet N, Barros H. Helicobacter pylori infection and gastric cancer: Facing the enigmas. Int J Cancer. 2003;106:953–60. doi: 10.1002/ijc.11306. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins DJ. Helicobacter pylori and its interaction with risk factors for chronic disease. Bmj. 1997;315:1481–2. doi: 10.1136/bmj.315.7121.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tovey F. Peptic ulcer in India and Bangladesh. Gut. 1979;20:329–47. doi: 10.1136/gut.20.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khuroo MS, Zargar SA, Mahajan R, Banday MA. High incidence of oesophageal and gastric cancer in Kashmir in a population with special personal and dietary habits. Gut. 1992;33:11–5. doi: 10.1136/gut.33.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavithran K, Doval DC, Pandey KK. Gastric cancer in India. Gastric Cancer. 2002;5:240–3. doi: 10.1007/s101200200042. [DOI] [PubMed] [Google Scholar]
- 7.Gill HH, Desai HG. Helicobacter pylori and gastroduodenal disorders in India-lessons from epidemiology. J Clin Gastroenterol. 1993;16:6–9. [PubMed] [Google Scholar]
- 8.Jain A, Buddhiraja S, Khurana B, Singhal R, Nair D, Arora P, et al. Risk factors for duodenal ulcer in north India. Trop Gastroenterol. 1999;20:36–9. [PubMed] [Google Scholar]
- 9.Jain AK, Dayal VM. Helicobacter pylori recolonization and ulcer relapse after its eradication in India. Indian J Gastroenterol. 1997;16:S22–4. [PubMed] [Google Scholar]
- 10.Katelaris PH, Tippett GH, Norbu P, Lowe DG, Brennan R, Farthing MJ. Dyspepsia, Helicobacter pylori, and peptic ulcer in a randomly selected population in India. Gut. 1992;33:1462–6. doi: 10.1136/gut.33.11.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh V, Trikha B, Nain CK, Singh K, Vaiphei K. Epidemiology of Helicobacter pylori and peptic ulcer in India. J Gastroenterol Hepatol. 2002;17:659–65. doi: 10.1046/j.1440-1746.2002.02746.x. [DOI] [PubMed] [Google Scholar]
- 12.Tovey FI, Hobsley M, Kaushik SP, Pandey R, Kurian G, Singh K, et al. Duodenal gastric metaplasia and Helicobacter pylori infection in high and low duodenal ulcer-prevalent areas in India. J Gastroenterol Hepatol. 2004;19:497–505. doi: 10.1111/j.1440-1746.2003.03320.x. [DOI] [PubMed] [Google Scholar]
- 13.Abraham P. Helicobacter pylori: A review of practices and research in India. Indian J Gastroenterol. 1997;16(Suppl 1):S1–2. [PubMed] [Google Scholar]
- 14.Ahuja V. The case for Helicobacter pylori eradication in India: Sensationalism, skepticism and scientific salesmanship. Indian J Gastroenterol. 2006;25:20–4. [PubMed] [Google Scholar]
- 15.O’Mahony R, Al-Khtheeri H, Weerasekera D, Fernando N, Vaira D, Holton J, et al. Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol. 2005;11:7499–507. doi: 10.3748/wjg.v11.i47.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 17.Fox JG, Wang TC, Nagler-Anderson C. The African enigma: The parasite’s perspective. Gut. 2001;49:156–7. doi: 10.1136/gut.49.1.156a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki T, Hirai I, Izurieta R, Kwa BH, Estevez E, Saldana A, et al. Analysis of Helicobacter pylori genotype in stool specimens of asymptomatic people. Lab Med. 2009;40:412–4. [Google Scholar]
- 19.Nares-Cisneros J, Jaramillo-Rodríguez Y, Martínez-Ordaz VA, Velasco-Rodríguez VM, Madero A, Mena-Arias G, et al. Immunochromatographic monoclonal test for detection of Helicobacter pylori antigen in stool is useful in children from high-prevalence developing country. Helicobacter. 2007;12:354–8. doi: 10.1111/j.1523-5378.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- 20.Faruqui AN, Majid U, Ahmad L, Khalil M, Hassan MU. Helicobacter pylori stool antigen test (HpSA) for the diagnosis of gastric infection. J Coll Physicians Surg Pak. 2007;17:316–9. [PubMed] [Google Scholar]
- 21.SAS. 2012. [Last cited on 2012 Jan 20]. Available from: http://www.sas.com/
- 22.Rodrigues MN, Queiroz DM, Bezerra Filho JG, Pontes LK, Rodrigues RT, Braga LL. Prevalence of Helicobacter pylori infection in children from an urban community in north-east Brazil and risk factors for infection. Eur J Gastroenterol Hepatol. 2004;16:201–5. doi: 10.1097/00042737-200402000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Palli D, Caporaso NE, Shiao YH, Saieva C, Amorosi A, Masala G, et al. Diet, Helicobacter pylori, and p53 mutations in gastric cancer: A molecular epidemiology study in Italy. Cancer Epidemiol Biomarkers Prev. 1997;6:1065–9. [PubMed] [Google Scholar]
- 24.Phukan RK, Narain K, Zomawia E, Hazarika NC, Mahanta J. Dietary habits and stomach cancer in Mizoram, India. J Gastroenterol. 2006;41:418–24. doi: 10.1007/s00535-006-1761-x. [DOI] [PubMed] [Google Scholar]
- 25.Jones NL, Shabib S, Sherman PM. Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS Microbiol Lett. 1997;146:223–7. doi: 10.1111/j.1574-6968.1997.tb10197.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee IO, Lee KH, Pyo JH, Kim JH, Choi YJ, Lee YC. Anti-inflammatory effect of capsaicin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter. 2007;12:510–7. doi: 10.1111/j.1523-5378.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 27.Bamford KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 28.Gordon D. Solving the African enigma: Parasites may have their place. Gastroenterology. 2000;119:611. doi: 10.1016/s0016-5085(00)70104-1. [DOI] [PubMed] [Google Scholar]
- 29.Kato S, Tsukamoto T, Mizoshita T, Tanaka H, Kumagai T, Ota H, et al. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119:1558–66. doi: 10.1002/ijc.21810. [DOI] [PubMed] [Google Scholar]
- 30.Willis P, Lynch DA, Prescott R, Lamonby S. Cell proliferation in the post-surgical stomach, dietary salt, and the effect of H. pylori eradication. J Clin Pathol. 1999;52:665–9. doi: 10.1136/jcp.52.9.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:345–54. doi: 10.1093/jnci/djj071. [DOI] [PubMed] [Google Scholar]
- 32.Nomura A. Searching for the causes of gastric cancer. Hawaii Med J. 2002;61:33–4. [PubMed] [Google Scholar]
- 33.Tarpila S, Kekki M, Samloff IM, Sipponen P, Siurala M. Morphology and dynamics of the gastric mucosa in duodenal ulcer patients and their first-degree relatives. Hepatogastroenterology. 1983;30:198–201. [PubMed] [Google Scholar]
- 34.Bateson MC. Helicobacter pylori infection with age. Lancet. 1992;339:1121. doi: 10.1016/0140-6736(92)90721-e. [DOI] [PubMed] [Google Scholar]
- 35.Malaty HM, El-Kasabany A, Graham DY, Miller CC, Reddy SG, Srinivasan SR, et al. Age at acquisition of Helicobacter pylori infection: A follow-up study from infancy to adulthood. Lancet. 2002;359:931–5. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- 36.Sinha SK, Martin B, Sargent M, McConnell JP, Bernstein CN. Age at acquisition of Helicobacter pylori in a pediatric Canadian First Nations population. Helicobacter. 2002;7:76–85. doi: 10.1046/j.1083-4389.2002.00063.x. [DOI] [PubMed] [Google Scholar]
- 37.Tiwari SK, Manoj G, Kumar GV, Sivaram G, Hassan SI, Prabhakar B, et al. Prognostic significance of genotyping Helicobacter pylori infection in patients in younger age groups with gastric cancer. Postgrad Med J. 2008;84:193–7. doi: 10.1136/pgmj.2007.065060. [DOI] [PubMed] [Google Scholar]
- 38.Chang WK, Kim HY, Kim DJ, Lee J, Park CK, Yoo JY, et al. Association between Helicobacter pylori infection and the risk of gastric cancer in the Korean population: Prospective case-controlled study. J Gastroenterol. 2001;36:816–22. doi: 10.1007/s005350170003. [DOI] [PubMed] [Google Scholar]
- 39.Atherton JC. CagA: A role at last. Gut. 2000;47:330–1. doi: 10.1136/gut.47.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azuma T, Yamakawa A, Yamazaki S, Fukuta K, Ohtani M, Ito Y, et al. Correlation between variation of the 3’ region of the cagA gene in Helicobacter pylori and disease outcome in Japan. J Infect Dis. 2002;186:1621–30. doi: 10.1086/345374. [DOI] [PubMed] [Google Scholar]
- 41.Kanada R, Uchida T, Tsukamoto Y, Nguyen LT, Hijiya N, Matsuura K, et al. Genotyping of the cagA gene of Helicobacter pylori on immunohistochemistry with East Asian CagA-specific antibody. Pathol Int. 2008;58:218–25. doi: 10.1111/j.1440-1827.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim SY, Lee YC, Kim HK, Blaser MJ. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol. 2006;8:97–106. doi: 10.1111/j.1462-5822.2005.00603.x. [DOI] [PubMed] [Google Scholar]