Abstract
Aim:
Japanese encephalitis (JE) virus is the leading cause of viral neurologic disease and disability in Asia. In the present study JE virus-specific IgM in serum and CSF from acute encephalitis syndrome (AES) patients, attending Assam Medical College and Hospital (AMC and H), Dibrugarh, Assam from 2007 to 2009 were detected and different epidemiological parameters namely age, season and vaccination campaign were enumerated.
Materials and Methods:
A cross-sectional study on patients with AES admitted in AMC and H, Dibrugarh, Assam was done during 2007 to 2009. The different epidemiological features were characterized depending on a pretested structured questionnaire called the clinical information form (CIF). Serum and CSF obtained were tested by a Panbio JE-Dengue IgM Combo ELISA kit and JEV Chex kit (Xycton).
Statistical Analysis:
A z-test was used for the statistical analytic assessment.
Results:
Detection rate of JE was 39.4%, 51.1%, and 51.3% in the years 2007, 2008, and 2009 respectively. Cases of JE increased in the age group more than 15 years in the district where the vaccination program was undertaken. This increase of cases from pediatric to adults is also statistically significant by the z-test (P<0.05).
Conclusion:
There was an increase in AES cases and also JE cases from 2007 to 2009. JE also showed a seasonal variation with maximum cases in the months of July and August. Although vaccination campaigns with the live attenuated vaccine SA-14-14-2 have started and are protecting the under-15 children, there is a shift of disease pattern in the older population.
Keywords: Epidemiological parameters, Japanese encephalitis, Vaccination
INTRODUCTION
Japanese encephalitis (JE) virus is the leading cause of viral neurologic disease and disability in Asia.[1,2] JE virus is a member of the family Flaviviridae, genus Flavivirus and is transmitted to humans through a pig-culex mosquito-pig cycle. Humans are an incidental host. The clinical features are manifested as a febrile headache syndrome, aseptic meningitis or encephalitis.[3,4] Approximately 3 billion people live in JE-endemic areas where at least 50,000 cases of JE are reported every year. Of these about 10,000 cases result in death and high proportion of survivors develop serious neurological and psychiatric sequelae.[5]
JE virus cannot usually be isolated from clinical specimens because of low levels of viremia and rapid development of neutralizing antibodies.[6] The detection of JE virus specific IgM by IgM captive-enzyme linked-immunosorbent assay (IgM-Captive ELISA) has been accepted as the standard for serological diagnosis.[7] The presence of JE virus-specific IgM in cerebrospinal fluid (CSF) is considered to be a sign of JE virus infection of the central nervous system. Serum samples are also used for detection of specific IgM, because serum can be obtained from patients more easily than CSF.
The main pillar of JE control is the use of a live attenuated vaccine. The candidate is an attenuated SA-14-14-2 virus strain, adjuvanted with aluminum hydroxide and also grown in Vero cells.[8] India started JE vaccination in which children 1–15 years of age will be vaccinated. This immunization program started in Assam in the year 2006. The incidence of JE in India is still increasing and the case fatality rate of reported cases is high, i.e., 10–30%.[9] However overall trends for India are difficult to predict because JE endemicity is heterogenous and because socioeconomic conditions for control differ substantially from one state to another.[10]
The WHO National Network Laboratory for JE started functioning in Assam Medical College and Hospital (AMC and H), Dibrugarh from the year 2006. In the present study JE virus specific IgM in serum and CSF from Acute Encephalitis Syndrome (AES) patients, attending AMC and H, Dibrugarh from 2007 to 2009 were detected and different epidemiological parameters namely age, season, and vaccination campaign were enumerated.
MATERIALS AND METHODS
A cross-sectional study was done in the years 2007 to 2009. Patients with AES admitted in AMC and H, Dibrugarh, Assam were considered. AES consists of patients who present with fever, altered sensorium (including symptoms such as confusion, disorientation, coma or inability to talk), and new onset of seizures.[11] The different epidemiological features were characterized depending on a pretested structured questionnaire called the clinical information form (CIF). The project had been approved by suitably constituted Ethics Committee of the Institution within which the work was undertaken.
Serum and CSF were obtained from the AES cases. Clotted blood and CSF were collected from AES patients within 7 days of admission to AMC and H, Dibrugarh. Samples were collected in sterile vials and immediately transported to the laboratory of the Department of Microbiology, AMC and H, Dibrugarh. The serum was separated from the blood and both serum and CSF were stored at −30°C.
The serum samples were tested by the Panbio JE - Dengue IgM Combo ELISA kit. The CSF samples were tested by the JEV Chex kit (Xycton).
All collected data were later on statistically analyzed and presented. A-z-test was used for the analytic assessment using SPSS 14 software. The differences were considered to be statistically significant when the P value obtained was less than 0.05.
RESULTS
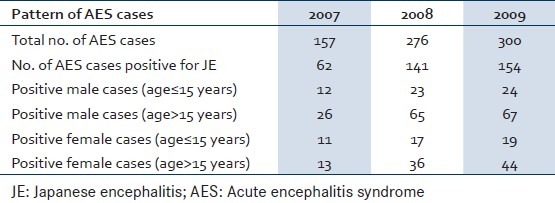
In 2007 157 AES cases were admitted in AMC and H of which 62 were positive for JE. The detection rate of JE was 39.4%. The total number of male cases (age≤15 years) was 12 and the number of males (age>15 years) was 23. Females (age≤15 years) were 11, and 13 cases belonged to the age group of more than 15 years. In 2008 total AES cases were 276 out of which 141 cases were positive for JE. A total of 23 cases were males aged≤15 years whereas 65 cases were males in the age group of more than 15 years. Females (age≤15 years) were 17, and 36 cases belonged to the age group of more than 15 years. The detection rate of JE in year 2008 was 51.1%. Total AES cases in 2009 were 300 of which 154 were positive for JE. So the JE detection rate was 51.3%. The total number of male cases (age≤15 years) was 24 and the number of males (age>15 years) was 67. Females (age≤15 years) were 19, and 44 cases belonged to the age group of more than 15 years [Table 1].
Table 1.
Age and sex distribution of JE cases in 3 years
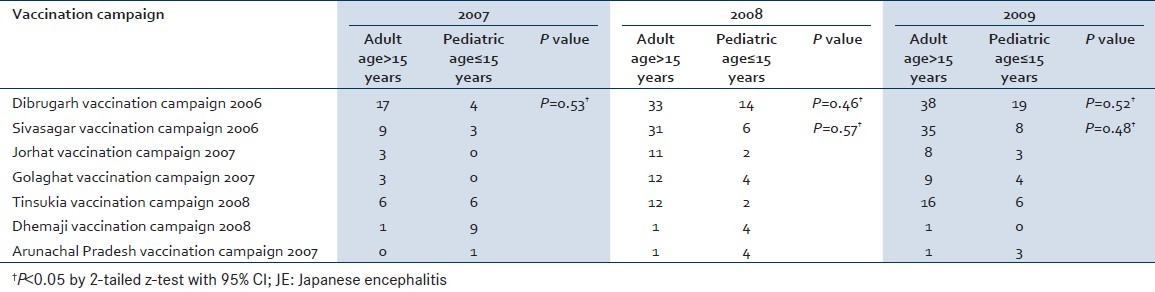
The seasonal distribution shows clustering of cases during July and August month. In 2007, the July month had 40 JE cases and the August month had 16 cases. A similar picture was seen in 2008 where July had 90 cases and August had 37 cases, and in 2009 July had 85 cases and August had 54 cases [Figure 1]. Vaccination for JE was started in a phased manner in Assam, a state in North East India in 2006 by the Government of India in collaboration with WHO. In the year 2006 the vaccination program was undertaken in Dibrugarh and Sivasagar, two districts in the state of Assam. In the subsequent year 2007 the vaccination program was initiated in two other districts Jorhat and Golaghat. Vaccination of two districts Tinsukia and Dhemaji was done in 2008. Vaccination was done with live-attenuated vaccine SA-14-14-2. A dose of vaccine was given to children aged 1–15 years. There is a definite reduction of cases of JE in the age group of less than 15 years of age. This reduction is found in the districts from the year vaccination initiative was done. Cases of JE increased in the age group of more than 15 years in the district where the vaccination program was undertaken. This increase of cases from pediatric to adults is also significant statistically by the z-test (P<0.05). Arunachal Pradesh a state adjoining Assam did not have this vaccination program. The number of cases of JE in Arunachal Pradesh is more in the age group of less than 15 years [Table 2].
Figure 1.
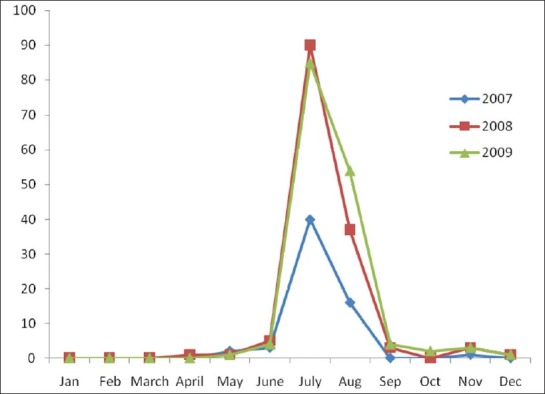
Seasonal variation of JE cases
Table 2.
Correlation of vaccination and JE cases in the adult and pediatric groups
DISCUSSION
There is an increase in AES cases and also JE cases from 2007 to 2009. This increase may be due to the establishment of sentinel surveillance which was started in 2006. The male adult population were most affected as they worked outdoors and were more exposed to the vector C. tritaeniorhynchus that breed abundantly in the rice fields. Assam is an endemic region for JE,[12] and here JE shows a seasonal variation. There is a clustering of cases in July and August. The rainfall starts from the month of June and there is an increase in the mosquito population. The results were also consistent with other studies. An epidemiological survey in Sri Lanka demonstrated an increase in mosquitoes following heavy rainfall.[13,14] This further led to the presence of seroconversion and JE in humans. Another study in Thailand indicated that nonimmune sentinel pigs in the dry hot season were not susceptible to infection until several weeks after the first rains of the weather season.[15] One study in China also found that reduced rainfall led to a decrease in the C. tritaeniorhynchus mosquito population in 1980 and 1981. This further resulted in a decrease in JE in Beijing in 1982 and 1983.[16] It should be noted that the above temporal relationship may vary by different geographic areas and other factors, such as pig density and vaccine coverage rate. The finding on time lag 1 of climate variables for the Southern area in Taiwan suggests that it takes 1 month for temperature and precipitation to effect occurrence of JE in humans.[17] This study in Taiwan also concluded that the seasonal pattern on occurrence of JE cases clusters between May and August during the period from 1991 to 2005.[17]
Coverage of immunization programs has further influenced JE transmission. The number of JE cases is more in the age group of more than 15 years in districts where vaccination was done. This shift of JE cases from children less than 15 years to adults is significant and can be explained by high coverage of vaccinated children. This finding is very similar to a study in Taiwan where the average age for onset of confirmed JE cases shifted from children <10 years to adulthood.[18]
In summary, Assam is still an endemic area and JE affects the adult male population here. JE also shows a seasonal variation with maximum cases in the month of July and August. Although vaccination campaigns with the live attenuated vaccine SA-14-14-2 has started and is protecting the under 15 children, there is a shift of disease pattern in the older population, so a concerted effort in surveillance and immunization is required to keep this vector borne disease problem in control.
ACKNOWLEDGEMENT
The authors acknowledge the contribution of WHO SEARO New Delhi office for providing the kits in time for testing of serum and CSF samples.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vaughn DW, Hake CH. The epidemiology of Japanese encephalitis: prospectus for prevention. Epidemiol Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- 2.Halstead SB, Tsai TF. Japanese encephalitis vaccine. In: Platkin SA, Orenstein WA, editors. Vaccines. Philadelphia: WB Sanders; 2004. [Google Scholar]
- 3.Monath TP, Heinz FX. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, USA: Lippincott-Raven Publishers; 1996. [Google Scholar]
- 4.Solemon T. Viral Encephalitis in South East Asia. Neurol Infect Epidemiol. 1997;2:191–9. [Google Scholar]
- 5.World Health Organization. Japanese Encephalitis vaccine. Wkly Epidemiol Rec. 1998;73:334–44. [Google Scholar]
- 6.Buescher EL, Seherer WF. Immunological studies of Japanese Encephalitis virus in man. I. Antibody responses following overt infection of man. J Immonol. 1959;83:582–3. [PubMed] [Google Scholar]
- 7.Cuzzubo AJ, Endy TP, Vaughn DW, Solemon T, Nisalak A, Kalayanarooj S, et al. Evaluation of a new commercially available immunoglobulin M capture enzyme- linked immunosarbent assay for diagnosis of Japanese Encephalitis infections. J Clin Microbiol. 1999;37:3738–41. doi: 10.1128/jcm.37.11.3738-3741.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tauber E, Dewasthaly S. Japanese Encephalitis vaccines-needs, flaws and achievements. Biol Chem. 2008;389:547–50. doi: 10.1515/bc.2008.062. [DOI] [PubMed] [Google Scholar]
- 9.Online Technical Appendix supplementary reference 41. Available from: www.cdc.gov./EID/content/15/1/1-Techapp.pdf .
- 10.Online Technical Appendix supplementary reference 42. Available from: www.cdc.gov./EID/content/15/1/1-Techapp.pdf .
- 11.WHO/VandB/03.01. Geneva, Switzerland: World Health Organization; 2006. WHO - Recommended Standards for surveillance of Selected Vaccine - Preventable Diseases. [Google Scholar]
- 12.WHO regional office for S.E. Asia, New Delhi. Health situation in SE Asia Region. 1998-2000:92–93. [Google Scholar]
- 13.Peiris JS. Japanese Encephalitis in Sri Lanka- the study of and epidemic: Vector incrimination, porcine infection and human disease. Trans R Soc Trop Med Hyg. 1992;86:307–13. doi: 10.1016/0035-9203(92)90325-7. [DOI] [PubMed] [Google Scholar]
- 14.Peiris JS. Japanese Encephalitis in Sri Lanka- Comparison of vectors and virus ecology in different agroclimatic areas. Trans R Soc Trop Med Hyg. 1993;87:541–8. doi: 10.1016/0035-9203(93)90080-a. [DOI] [PubMed] [Google Scholar]
- 15.Gingrich JB. Japanese Encephalitis virus in Bangkok: Factors influencing vector infection in 3 suburban communities. J Med Entomol. 1992;29:436–44. doi: 10.1093/jmedent/29.3.436. [DOI] [PubMed] [Google Scholar]
- 16.Wang YM. A survey on infection rate of Japanese Encephalitis virus in Culex. Tritaeniorhynchus in Beijing area Chung Hua Liu Hsing Ping Hsuh Tsa Chih. Chin J Epidemiol. 1983;4:273–6. [PubMed] [Google Scholar]
- 17.Hsu SM, Yen AM, Chen TH. Impact of climate on Japanese Encephalitis. Epidemiol Infect. 2008;136:980–7. doi: 10.1017/S0950268807009454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Online Technical Appendix, supplementary reference 43. Available from: www.cdc.gov./EID/content/15/1/1-Techapp.pdf .


