Abstract
Factor V must be converted to Factor Va in order to bind to a high affinity platelet surface site and participate in prothrombin activation. Osterud et al. (10) presented data that suggested that human platelets contain an activated form of Factor V and a Factor V activator. We find that the Factor V released when platelets are disrupted by freezing and thawing or sonication is activated 3- to 10-fold by thrombin as determined by coagulation assay and is therefore stored as the relatively inactive procofactor rather than in the active form Factor Va.
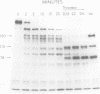
We incubated purified Factor V, which had a specific activity of 140±30 U/mg, with Factor V-deficient frozen and thawed platelets (109 platelets/ml) obtained from a patient with Factor V deficiency. The specific activity of the Factor V increased to a maximum of 740±240 U/mg (mean±SD of three experiments). When this partially activated Factor V was incubated with thrombin its specific activity increased further to 1,440±280 U/mg, which is similar to the activity of Factor V activated with thrombin alone (1,540±60 U/mg).
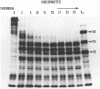
The platelet Factor V activator is not inhibited by dansyl arginine-4-ethylpiperidine amide, 93 μM, indicating that it is not thrombin. When thrombin-stimulated platelets, to which dansyl arginine-4-ethylpiperidine amide had been added to inhibit the further action of thrombin, were incubated with 125-labeled Factor V, there was no detectable proteolysis of the Factor V molecule. Our failure to detect activation of Factor V under these conditions suggests that <4% of the platelet protease is released by thrombin. Subcellular fractionation of platelets indicates that the platelet protease that activates Factor V is in the soluble fraction.
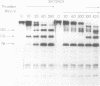
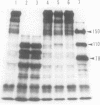
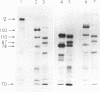
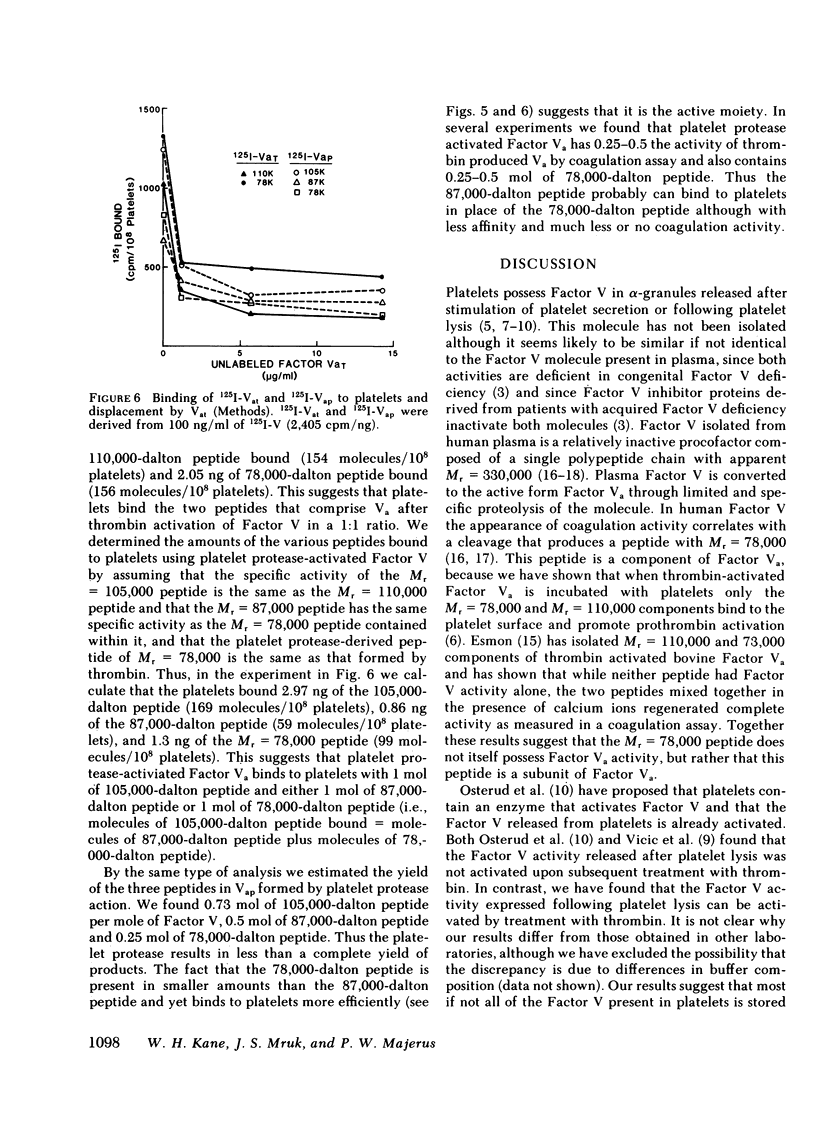
When Factor Va formed by the action of platelet protease is incubated with platelets, peptides with Mr = 105,000, 87,000, and 78,000 bind to the platelet surface. All three radiolabeled peptides are displaced from platelets by unlabeled Factor Va formed by the action of thrombin. The stoichiometry of binding suggests that the 105,000-dalton peptide is associated with either an 87,000- or a 78,000-dalton peptide. The 78,000-dalton peptide binds with greater affinity and probably accounts for the bulk of the activity of Factor Va in coagulation assays. Whether or not the platelet protease serves to activate Factor V before thrombin formation during normal hemostasis remains to be determined.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breederveld K., Giddings J. C., ten Cate J. W., Bloom A. L. The localization of factor V within normal human platelets and the demonstration of a platelet-factor V antigen in congenital factor V deficiency. Br J Haematol. 1975 Mar;29(3):405–412. doi: 10.1111/j.1365-2141.1975.tb01838.x. [DOI] [PubMed] [Google Scholar]
- Broekman M. J., Westmoreland N. P., Cohen P. An improved method for isolating alpha granules and mitochondria from human platelets. J Cell Biol. 1974 Feb;60(2):507–519. doi: 10.1083/jcb.60.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney C. M., Pifer D., Colman R. W. Subcellular localization and secretion of factor V from human platelets. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5180–5184. doi: 10.1073/pnas.78.8.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B. Human coagluation factor V purification and thrombin-catalyzed activation. J Clin Invest. 1980 Sep;66(3):583–591. doi: 10.1172/JCI109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino G. N., Goldberg A. L. Identification and partial purification of an ATP-stimulated alkaline protease in rat liver. J Biol Chem. 1979 May 25;254(10):3712–3715. [PubMed] [Google Scholar]
- Esmon C. T. The subunit structure of thrombin-activated factor V. Isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J Biol Chem. 1979 Feb 10;254(3):964–973. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Rose I. A. Resolution of the ATP-dependent proteolytic system from reticulocytes: a component that interacts with ATP. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3107–3110. doi: 10.1073/pnas.76.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane W. H., Lindhout M. J., Jackson C. M., Majerus P. W. Factor Va-dependent binding of factor Xa to human platelets. J Biol Chem. 1980 Feb 10;255(3):1170–1174. [PubMed] [Google Scholar]
- Kane W. H., Majerus P. W. Purification and characterization of human coagulation factor V. J Biol Chem. 1981 Jan 25;256(2):1002–1007. [PubMed] [Google Scholar]
- Kane W. H., Majerus P. W. The interaction of human coagulation factor Va with platelets. J Biol Chem. 1982 Apr 10;257(7):3963–3969. [PubMed] [Google Scholar]
- Katzmann J. A., Nesheim M. E., Hibbard L. S., Mann K. G. Isolation of functional human coagulation factor V by using a hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Jan;78(1):162–166. doi: 10.1073/pnas.78.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meisler M., Paigen K. Coordinated development of -glucuronidase and -galactosidase in mouse organs. Science. 1972 Sep 8;177(4052):894–896. doi: 10.1126/science.177.4052.894. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Jackson C. M., Majerus P. W. Interaction of coagulation factor Xa with human platelets. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4033–4036. doi: 10.1073/pnas.74.9.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich J. P., Jackson C. M., Majerus P. W. Properties of the factor Xa binding site on human platelets. J Biol Chem. 1978 Oct 10;253(19):6908–6916. [PubMed] [Google Scholar]
- Miletich J. P., Kane W. H., Hofmann S. L., Stanford N., Majerus P. W. Deficiency of factor Xa-factor Va binding sites on the platelets of a patient with a bleeding disorder. Blood. 1979 Nov;54(5):1015–1022. [PubMed] [Google Scholar]
- Miletich J. P., Majerus D. W., Majerus P. W. Patients with congenital factor V deficiency have decreased factor Xa binding sites on their platelets. J Clin Invest. 1978 Oct;62(4):824–831. doi: 10.1172/JCI109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich J. P., Majerus D. W., Majerus P. W. Patients with congenital factor V deficiency have decreased factor Xa binding sites on their platelets. J Clin Invest. 1978 Oct;62(4):824–831. doi: 10.1172/JCI109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E., Canfield W. M., Kisiel W., Mann K. G. Studies of the capacity of factor Xa to protect factor Va from inactivation by activated protein C. J Biol Chem. 1982 Feb 10;257(3):1443–1447. [PubMed] [Google Scholar]
- Nesheim M. E., Myrmel K. H., Hibbard L., Mann K. G. Isolation and characterization of single chain bovine factor V. J Biol Chem. 1979 Jan 25;254(2):508–517. [PubMed] [Google Scholar]
- Nesheim M. E., Prendergast F. G., Mann K. G. Interactions of a fluorescent active-site-directed inhibitor of thrombin: dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Biochemistry. 1979 Mar 20;18(6):996–1003. doi: 10.1021/bi00573a010. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I., Lavine K. K. Factor V activity of platelets: evidence for an activated factor V molecule and for a platelet activator. Blood. 1977 May;49(5):819–834. [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Reitman M. L., Varki A., Kornfeld S. Fibroblasts from patients with I-cell disease and pseudo-Hurler polydystrophy are deficient in uridine 5'-diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminylphosphotransferase activity. J Clin Invest. 1981 May;67(5):1574–1579. doi: 10.1172/JCI110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman M. A., Levine S. P. Thrombin generation and secretion of platelet Factor 4 during blood clotting. J Clin Invest. 1978 Apr;61(4):1102–1106. doi: 10.1172/JCI109010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman M. A., Majerus P. W. The measurement of thrombin in clotting blood by radioimmunoassay. J Clin Invest. 1976 Nov;58(5):1249–1258. doi: 10.1172/JCI108579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]
- Tracy P. B., Eide L. L., Bowie E. J., Mann K. G. Radioimmunoassay of factor V in human plasma and platelets. Blood. 1982 Jul;60(1):59–63. [PubMed] [Google Scholar]
- Vicic W. J., Lages B., Weiss H. J. Release of human platelet factor V activity is induced by both collagen and ADP and is inhibited by aspirin. Blood. 1980 Sep;56(3):448–455. [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]