Abstract
Aims
The purpose of this study was to establish safety and tolerability of a single intravenous (IV) infusion of a p38 mitogen-activated protein kinase inhibitor, losmapimod, to obtain therapeutic levels rapidly for a potential acute coronary syndrome indication. Pharmacokinetics (PK) following IV dosing were characterized, and pharmacokinetic/pharmacodynamic (PK/PD) relationships between losmapimod and phosphorylated heat shock protein 27 (pHSP27) and high-sensitivity C-reactive protein were explored.
Methods
Healthy volunteers received 1 mg losmapimod IV over 15 min (n = 4) or 3 mg IV over 15 min followed by a washout period and then 15 mg orally (PO; n = 12). Pharmacokinetic parameters were calculated by noncompartmental methods. The PK/PD relationships were explored using modelling and simulation.
Results
There were no deaths, nonfatal serious adverse events or adverse events leading to withdrawal. Headache was the only adverse event reported more than once (n = 3 following oral dosing). Following 3 mg IV and 15 mg PO, Cmax was 59.4 and 45.9 μg l−1 and AUC0–∞ was 171.1 and 528.0 μg h l−1, respectively. Absolute oral bioavailability was 0.62 [90% confidence interval (CI) 0.56, 0.68]. Following 3 mg IV and 15 mg PO, maximal reductions in pHSP27 were 44% (95% CI 38%, 50%) and 55% (95% CI 50%, 59%) occurring at 30 min and 4 h, respectively. There was a 17% decrease (95% CI 9%, 24%) in high-sensitivity C-reactive protein 24 h following oral dosing. A direct-link maximal inhibitory effect model related plasma concentrations to pHSP27 concentrations.
Conclusions
A single IV infusion of losmapimod in healthy volunteers was safe and well tolerated, and may potentially serve as an initial loading dose in acute coronary syndrome as rapid exposure is achieved.
Keywords: losmapimod, pharmacodynamics, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Over the last 20 years, inhibition of p38 mitogen-activated protein kinase in numerous preclinical studies of inflammatory conditions (e.g. rheumatoid arthritis, as well as pulmonary and vascular disease) has demonstrated compelling evidence for benefit. Safety/toxicity concerns with translation to humans, in addition to unclear pharmacology, have contributed to limited success in clinical programmes. Losmapimod (a potent p38 mitogen-activated protein kinase inhibitor), provided orally and dosed submaximally, has been safe and well tolerated, but in addition has offered improved vascular benefits in several small clinical studies. These data have permitted consideration for treatment in acute coronary syndrome, which has multiple inflammatory components.
WHAT THIS STUDY ADDS
The pharmacokinetic information available in the literature for losmapimod is sparse, and currently non-existent for intravenous dosing. Elucidation of bioavailability of the oral dose, preliminary safety and tolerability of a single intravenous infusion, along with the comparative pharmacokinetics (PK) and PK/pharmacodynamics (PD) of intravenous and oral dosing are provided herein. Exploration of PK/PD relationships, particularly PK/PD modelling of the relationship between pHSP27 (a monocyte tissue marker) and losmapimod concentrations, is presented with a specific temporal examination. This study permits consideration of an intravenous formulation to be applied during the life-cycle of an acute coronary syndrome development plan.
Introduction
Losmapimod (GW856553) acts as a selective p38α/β mitogen-activated protein kinase (p38 MAPK) inhibitor by competitively binding to the ATP binding site of p38MAPK 1. In preclinical studies of myocardial ischaemia–reperfusion injury, inhibition of p38MAPK has been shown to be cardioprotective. Increased cell viability was noted, with reductions in infarct size, neutrophil accumulation and circulating markers of cell injury, such as creatine kinase and lactate dehydrogenase 2–4. In angiotensin II-induced atherosclerotic rats, inhibition of p38 MAPK reduced superoxide anion generation with subsequent improvements in endothelial function, mean blood pressure and cardiac hypertrophy 5. In untreated hypercholesterolaemic patients, losmapimod improved nitric oxide-mediated vasoregulation, a marker of endothelial function, and reduced levels of the inflammatory biomarker high-sensitivity C-reactive protein (hsCRP) 6. More recently, our group has also demonstrated that losmapimod reduces vascular inflammation as evidenced by fluorodeoxyglucose positron emission tomography-computed tomography in patients with pre-existing atherosclerosis on standard medical therapies, including statins 7. This body of evidence supports ongoing clinical trials of losmapimod for the indication of acute coronary syndrome (ACS; http://clinicaltrials.gov/ct2/show/NCT00910962).
To date, only oral (PO) doses of losmapimod have been studied. This may not be an ideal formulation in ACS as it takes approximately 1–4 h for losmapimod to reach maximal concentrations. The correlation between a delayed percutaneous coronary intervention and increased risk of various negative health outcomes, including mortality, is generally accepted 8–10. In particular, preclinical work highlighted that the cardioprotective effects of inhibiting the p38 MAPK pathway are rapidly attenuated after reperfusion 3. To achieve a rapid onset of action and maximize potential benefits of treatment, an intravenous (IV) formulation of losmapimod has been developed.
The primary purpose of this study was to assess the feasibility of providing a single IV infusion to establish rapid drug exposure. To this end, we evaluated preliminary safety and tolerability, determined the pharmacokinetics (PK) and explored the pharmacodynamics (PD) of an IV formulation of losmapimod. The absolute bioavailability for oral dosing was also determined. Concentrations of phosphorylated heat shock protein 27 (pHSP27) were determined following losmapimod dosing using standard statistical methods and modelling and simulation techniques. Phosphorylated HSP27 was measured because it is a fairly direct downstream phosphorylation target of p38 MAPK. High-sensitivity C-reactive protein was measured because it represents a commonly measured marker of inflammation and plaque vulnerability, and p38 MAPK inhibition has previously been shown to result in hsCRP reductions over 3 days 11. In future studies, hsCRP may serve some prognostic value to provide better assessment of the risk of experiencing a cardiovascular event 12.
Methods
Study design
This was a Phase 1, open-label, single-centre, multiple-cohort study in healthy adult volunteers (male or female subjects, aged 18–75 years, weighing > 50 kg, body mass index 19–30 kg m−2) that consisted of the following four study periods: screening, treatment, washout (for cohort 3 only) and follow-up. The protocol was approved by an independent ethics committee (Welwyn Clinical Pharmacology Ethics Committee) and was registered at clinicaltrials.gov (NCT01039961). The study complied with the Declaration of Helsinki, and full written informed consent was obtained from all participants before the performance of any study-specific procedures.
Cohort 1 (n = 4) received a single 1 mg IV dose of losmapimod as a constant IV infusion over 15 min. Pharmacokinetics results from cohort 1 were sufficient to obviate the need for an optional dose-finding cohort, cohort 2, and were used to determine the dose for cohort 3 predicted to achieve target concentrations. The primary PK parameter for dose selection for cohort 3 was the maximum concentration (Cmax). Total exposure area under the concentration-time curve (AUC) was also evaluated to ensure that exposure of the IV dose did not exceed exposures previously tested and known to be safe. The Cmax for cohort 3 was to approach but not exceed the maximal concentration after administration of a single 20 mg oral dose in healthy volunteers, i.e. 73.7 ng ml−1 [geometric mean, range 57.8–94.5 ng ml−1, n = 9 (data in house)]. A 20 mg single oral dose had previously been shown to be safe and well tolerated and within the dose-linear PK range. Cohort 3 (n = 12) received a single 3 mg IV dose of losmapimod infusion over 15 min and, following a 1 week washout period, a 15 mg oral dose of losmapimod given as two 7.5 mg tablets. Subjects fasted for approximately 10 h prior to receiving study drug and received a meal approximately 4 and 10 h postdose. Follow-up occurred 14 (±3) days following the last dose of study drug.
Safety assessments and safety analysis
All adverse events (AEs)/serious AEs were collected from the time of administration of the investigational product until follow-up. Additionally, serious AEs assessed as related to study participation were recorded from the time the subject gave consent. A complete set of safety observations was performed, including vital signs, physical examination, clinical laboratory evaluations (clinical chemistry, haematology and urinalysis) and 12-lead electrocardiograms (ECGs). The change from baseline was calculated by subtracting the baseline values from the individual postrandomization values. Laboratory, ECG or vital sign values of potential clinical importance based on predefined criteria were listed for each evaluation.
Pharmacokinetic assessments
Pharmacokinetic samples for determination of losmapimod and GSK198602 (the primary but inactive metabolite) were collected predose and at 5, 10, 15, 30, 45, 60 and 90 min, and 2, 3, 4, 6, 8, 10, 12, 16 and 24 h. Samples were chilled on wet ice immediately after collection into EDTA-containing tubes. Plasma was separated by centrifugation at 3000g, promptly transferred to an appropriately labelled polypropylene tube and frozen at approximately −20°C. The samples were analysed using a validated analytical method based on protein precipitation, followed by HPLC-MS/MS analysis. The lower limit of quantification for losmapimod was 0.2 μg l−1 and for GSK198602 it was 1 μg l−1 using a 50 μl aliquot of human plasma with a higher limit of quantification of 200 and 1000 μg l−1, respectively. The relative error (calculated using the average experimental concentration for each quality control level) and coefficient of variation (CV) for the quality controls for analysis of losmapimod were ≤10.1 and ≤10.7%, respectively, representing accuracy and precision for the analysis. For GSK198602, the precision and accuracy at a concentration of 3 μg l−1 (the low quality control) were ≤53.5 and ≤80.0%. At concentrations of 50 and 800 μg l−1 (the middle and high quality controls), the relative error and CV were ≤11.6 and ≤5.3%. Given that the quality control samples were run in duplicate, the run was accepted if no more than one-third of the quality control results exceeded acceptable limits and at least half of the results at each concentration were within the acceptable limit.
Plasma losmapimod and metabolite GSK198602 concentration–time data were analyzed by noncompartmental methods using WinNonlin 5.2 (Pharsight Corporation, Mountain View, CA, USA). From the plasma concentration–time data, the following PK parameters were determined: Cmax, time to maximum concentration (Tmax), area under the concentration time curve to last measured concentration (AUC0-t), area under the concentration time curve to infinity (AUC0–∞), half-life (t1/2), volume of distribution calculated by the steady-state method (VDss; losmapimod IV dosing only) and clearance (CL) for IV dosing or apparent clearance (CL/F) for oral dosing (losmapimod only). Absolute oral bioavailability was also calculated, and the calculation method is described in the ‘Statistical methods’ section.
Pharmacodynamic assessments
Phosphorylated HSP27 and hsCRP were collected from subjects in cohort 3 only. Blood samples for measurement of pHSP27 were collected predose and at 30 and 60 min, and 2, 4, 6 and 8 h. One whole blood sample (1 ml) was collected from each subject into a sterile polystyrene tube containing sodium heparin at each time point. Human whole blood was stimulated with sorbitol for 1 h at 37°C in air enriched with 5% CO2 or incubated with medium as a control. Sorbitol stimulation induces phosphorylation of HSP27 and serves as a measure of p38 activity. The whole blood samples were lysed on ice after sorbitol stimulation and the lysates frozen. The samples were analysed with a sandwich immunoassay using the Meso Scale Discovery Multi-Spot Biomarker Detection Whole Cell Lysate Kit Phospho-(Ser15)/Total-HSP27 and Meso Scale Discovery SECTOR Instrument (Meso Scale Discovery, Rockville, MD, USA). Details of a similar method are provided elsewhere 13. The quantifiable range for the assay was 0.78–50 ng ml−1. The absolute relative error (calculated using the average experimental concentration for each quality control level) and CV for the quality controls were ≤8.4 and ≤8.7%, respectively.
To determine hsCRP concentrations, serum samples were collected predose and at 6, 12 and 24 h. Immunonephelometry was used to measure hsCRP on the BN II system (Siemens, Tarrytown, NY, USA) using the CardioPhase hsCRP reagent (Siemens). The average accuracy of this method compared with the International Reference Standard CRM470 was ∼98%. The CVs at concentrations of 1.73 and 5.1 mg l−1 were 6.9 and 7.2%, respectively. The sensitivity of the assay was 0.175 mg l−1, with an upper reporting range of 220 mg l−1.
Statistical methods
Pharmacokinetic analysis
For the absolute bioavailability of oral losmapimod, following loge transformation, dose-normalized AUC0–∞ values of losmapimod in cohort 3 were analysed using a mixed effects model with a fixed effect term for treatment (IV treatment D or PO treatment E). Subjects were treated as a random effect. A point estimate and associated 90% confidence interval (CI) was constructed for the difference between oral vs. IV dosing (i.e. E minus D). The point estimate and associated 90% CIs were then back transformed to provide a point estimate and 90% CI for the ratio E:D (i.e. the absolute bioavailability).
Pharmacodynamic analysis
Effects of losmapimod on pHSP27 and hsCRP levels, following loge transformation, from cohort 3 were analysed separately using a repeated measure mixed effects model with fixed effect terms for time (baseline and other postdose time points), treatment (IV dosing and oral dosing) and time by treatment interaction. Subject was treated as a random effect in the model. Point estimates at each planned time point and their associated 95% CIs were constructed for the differences of postdose vs. baseline for each treatment. They were then back transformed to provide point estimates and 95% CIs for the ratios of postdose to baseline for each treatment at every planned postdose time point.
Pharmacokinetic/pharmacodynamic analysis
The relationship between losmapimod concentration and PD end-points (pHSP27 and hsCRP) were explored through modelling and simulation techniques. Modelling was performed using NONMEM VII (Icon Development Solutions, Ellicott, MD, USA) with the iterative two-stage (ITS) followed by the stochastic approximation expectation maximization (SAEM) estimation methods. The objective function was obtained using the Monte-Carlo importance sampling method assisted by mode a posteriori estimation (IMPMAP). The PK/PD models were compared based on visual inspection of the goodness-of-fit plots, a successful covariance step, and a significant change of the model selection criteria (i.e. the Akaike information criteria). Two PK/PD models were evaluated, the direct-link and indirect maximal inhibitory effect (Imax) models. The PD data were normalized to baseline and then converted to a symmetrized percentage change to create a normal distribution of the data for modelling 14. For plots, data were back transformed to the robust change from baseline for easier interpretation. The PK/PD model was evaluated by visual predictive check (n = 1000 per dose).
Results
Study population
A total of 16 healthy subjects were enrolled in the study (four in cohort 1 and 12 in cohort 3). Demographic data are provided in Table 1.
Table 1.
Patient demographics
| Demographics | 1 mg IV (n = 4) | 3 mg IV/15 mg PO (n = 12) |
|---|---|---|
| Age [years; mean (SD)] | 32.3 (8.54) | 43.3 (9.25) |
| Body mass index [kg m−2; mean (SD)] | 24.67 (2.01) | 25.00 (2.55) |
| Height [cm; mean (SD)] | 180.3 (5.56) | 175.4 (6.23) |
| Weight [kg; mean (SD)] | 79.96 (3.68) | 76.77 (7.13) |
| Male sex [n (%)] | 4 (100) | 12 (100) |
| Race [n (%)] | ||
| African American/African heritage | 0 | 1 (8) |
| White, Arabic/North African heritage | 1 (25) | 0 |
| White, White/Caucasian/European heritage | 3 (75) | 11 (92) |
Adverse events
There were few adverse events reported during the study. The only AE reported in more than one subject was headache, which was reported in three subjects receiving a 15 mg oral dose. In the 15 mg oral dosing arm, eye pain, nasopharyngitis and contusion were also reported as AEs. In the 1 mg IV arm, headache and thirst were reported once each. In the 3 mg IV arm, headache, neuralgia, catheter site haematoma, fatigue, dry mouth, nausea (the only drug-related AE determined by the investigator) and nasopharyngitis were reported once each. No subject died, experienced a serious AE or withdrew due to an AE during the study. There were no clinically significant alterations in vital signs, ECG, haematology, biochemistry and urinalysis.
Pharmacokinetic results
Mean losmapimod concentration–time profiles for the three doses are presented in Figure 1. The PK parameters obtained from the noncompartmental analysis for losmapimod and GSK198602 are presented in Table 2. Plasma concentrations for the 3 mg IV dose reached the target, i.e. they approached but did not exceed the Cmax of a 20 mg oral dose. Comparatively, the 1 mg IV dose did not reach this concentration. Absolute oral bioavailability was 0.62 (90% CI 0.56, 0.68).
Figure 1.
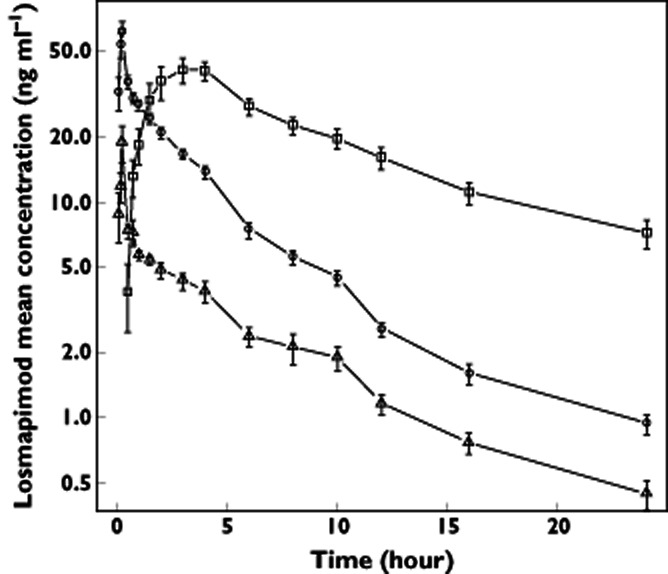
Mean concentration at each time point with standard error bars.  , 15 mg orally (PO);
, 15 mg orally (PO);  , 3 mg intravenously (IV); and
, 3 mg intravenously (IV); and  , 1 mg IV
, 1 mg IV
Table 2.
Results of noncompartmental analysis for losmapimod and the primary metabolite GSK198602
| Parameter | Compound | Dose | Geometric mean | 95% CI | CV% |
|---|---|---|---|---|---|
| AUC0–∞ (μg h l−1) | Losmapimod | 1 mg IV | 54.09 | 38.4, 76.2 | 21.8 |
| 3 mg IV | 171.1 | 148, 198 | 23.3 | ||
| 15 mg PO | 528.0 | 428, 652 | 34.1 | ||
| GSK198602 | 1 mg IV | 30.67 | 23.5, 40.0 | 16.8 | |
| 3 mg IV | 216.5 | 178, 263 | 31.2 | ||
| 15 mg PO | 1096 | 875, 1372 | 36.5 | ||
| AUC0–t (μg h l−1) | Losmapimod | 1 mg IV | 48.56 | 36.2, 65.1 | 18.6 |
| 3 mg IV | 161.7 | 141, 186 | 22.1 | ||
| 15 mg PO | 421.0 | 351, 506 | 29.5 | ||
| GSK198602 | 1 mg IV | 22.42 | 14.6, 34.5 | 27.5 | |
| 3 mg IV | 184.8 | 155, 221 | 28.4 | ||
| 15 mg PO | 717.9 | 616, 837 | 24.4 | ||
| Cmax (μg l−1) | Losmapimod | 1 mg IV | 18.00 | 8.91, 36.3 | 46.4 |
| 3 mg IV | 59.38 | 46.1, 76.5 | 41.5 | ||
| 15 mg PO | 45.90 | 36.7, 57.4 | 36.3 | ||
| GSK198602 | 1 mg IV | 5.228 | 4.01, 6.81 | 16.7 | |
| 3 mg IV | 22.24 | 20.1, 24.6 | 16.1 | ||
| 15 mg PO | 67.31 | 55.5, 81.6 | 30.9 | ||
| Tmax (h)* | Losmapimod | 1 mg IV | 0.250 | 0.17, 0.27 | — |
| 3 mg IV | 0.250 | 0.18, 0.75 | — | ||
| 15 mg PO | 3.500 | 2.00, 6.00 | — | ||
| GSK198602 | 1 mg IV | 1.000 | 0.75, 1.50 | — | |
| 3 mg IV | 1.515 | 1.00, 6.00 | — | ||
| 15 mg PO | 4.000 | 2.00, 10.00 | — | ||
| t1/2 (h) | Losmapimod | 1 mg IV | 8.328 | 4.84, 14.3 | 35.2 |
| 3 mg IV | 6.488 | 5.14, 8.20 | 38.1 | ||
| 15 mg PO | 9.439 | 7.37, 12.1 | 40.6 | ||
| GSK198602 | 1 mg IV | 4.014 | 2.96, 5.44 | 19.2 | |
| 3 mg IV | 7.312 | 5.83, 9.18 | 36.9 | ||
| 15 mg PO | 12.17 | 16.4, 0.474 | 50.2 | ||
| VDss (l) | Losmapimod | 1 mg IV | 171.8 | 139, 212 | 13.3 |
| 3 mg IV | 110.8 | 96.1, 128 | 22.7 | ||
| CL (l h−1CL/F (l h−1) | Losmapimod | 1 mg IV | 18.49 | 13.1, 26.0 | 21.8 |
| 3 mg IV | 17.54 | 15.2, 20.3 | 23.3 | ||
| 15 mg PO | 28.41 | 23.0, 35.1 | 34.1 |
Tmax is presented as the median and range.
Pharmacodynamic results
The postdose/baseline ratio at each time point, i.e. the change from baseline, for both biomarkers are presented in Table 3. Following IV dosing, the concentration of pHSP27 was reduced from baseline by 44% within 30 min of administration, was maintained at approximately the same level for 1 h, and then increased almost completely back to baseline by 8 h (Figure 2). Following oral dosing, the level of pHSP27 was decreased by 33% at 1 h, 55% at 4 h (Tmax for losmapimod), and diminished thereafter (Figure 2).
Table 3.
Parametric analysis for the comparison of phosphorylated heat shock protein 27 (pHSP27) and high-sensitivity C-reactive protein (hsCRP) levels between postdose and baseline (i.e. ratio of postdose/baseline)
| Parameter | Formulation | Time | Point estimate (95% confidence interval) | P Value |
|---|---|---|---|---|
| pHSP27 | IV | 30 min | 0.56 (0.50, 0.62) | <0.0001 |
| IV | 60 min | 0.56 (0.51, 0.63) | <0.0001 | |
| IV | 2 h | 0.65 (0.59, 0.73) | <0.0001 | |
| IV | 4 h | 0.70 (0.63, 0.78) | <0.0001 | |
| IV | 6 h | 0.84 (0.76, 0.94) | 0.0022 | |
| IV | 8 h | 0.89 (0.80, 0.99) | 0.0366 | |
| PO | 30 min | 0.96 (0.87, 1.07) | 0.5081 | |
| PO | 60 min | 0.67 (0.69, 0.74) | <0.0001 | |
| PO | 2 h | 0.55 (0.49, 0.61) | <0.0001 | |
| PO | 4 h | 0.45 (0.41, 0.50) | <0.0001 | |
| PO | 6 h | 0.51 (0.46, 0.57) | <0.0001 | |
| PO | 8 h | 0.54 (0.48, 0.60) | <0.0001 | |
| hsCRP | IV | 6 h | 1.00 (0.91, 1,11) | 0.9257 |
| IV | 12 h | 1.01 (0.92, 1.12) | 0.7888 | |
| IV | 24 h | 0.95 (0.87,1.05) | 0.3393 | |
| PO | 6 h | 0.98 (0.89, 1.08) | 0.7396 | |
| PO | 12 h | 0.95 (0.86, 1.05) | 0.3072 | |
| PO | 24 h | 0.83 (0.76, 0.91) | 0.0004 |
Figure 2.
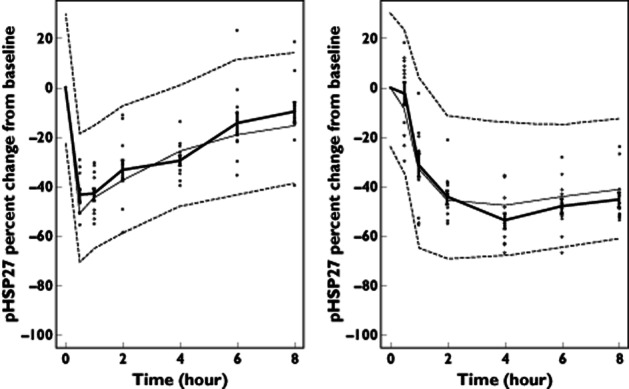
Visual predictive check forphosphorylated heat shock protein 27 (pHSP27) direct inhibitory model with observed data. Mean of observed data at each time point with standard error bars and thick continuous line. The median of simulated data at each time point is shown by the thin continuous line. The 90% prediction interval is indicated by the thin dashed lines.  , observed data. Left panel shows data for 3 mg IV and right panel 15 mg PO. The model appears to describe the central tendency and variability of the data well, supporting the suitability of the model
, observed data. Left panel shows data for 3 mg IV and right panel 15 mg PO. The model appears to describe the central tendency and variability of the data well, supporting the suitability of the model
There was a 17% reduction for hsCRP at 24 h in the oral treatment arm; however, a significant reduction in hsCRP was not seen at any other time point following 15 mg PO or at any time point following 3 mg IV dosing.
Pharmacokinetic/pharmacodynamic analysis
The direct-link Imax model was selected to describe the effect between pHSP27 concentrations and losmapimod plasma concentrations as follows:
where E0 is the effect at baseline (which was fixed to 1 because the data were normalized to baseline), E(t) is the effect observed at time t (i.e. the fraction of baseline pHSP27 at time t), Imax is the maximal inhibitory effect achievable, which was fixed to 0.667 (equivalent to a robust maximal change from baseline of 0.8 or 80% and based on previous data), C(t) is the plasma concentration of losmapimod at time t, and IC50 is the losmapimod concentration which results in half-maximal inhibition of the phosphorylation of HSP27. The only parameters estimated in this model were the IC50, the interindividual variability of the IC50 (CV%) and the residual error (additive), which were 37.4 ng ml−1 (9.83% relative standard error), 31.8% (32.1% relative standard error) and 0.0044 (26.8% relative standard error), respectively. The visual predictive check for this model, depicted as the percentage change from baseline to display the inhibitory effect more clearly, is presented in Figure 2. Essentially, this model demonstrates that the inhibition of phosphorylation of HSP27 is directly related to the plasma concentration of losmapimod and as the losmapimod concentration changes with time the resulting change in pHSP27 occurs almost instantaneously.
Discussion
Losmapimod, as a single IV dose (given as a 15 min infusion) administered to a small cohort of healthy volunteers, was well tolerated with an adverse event profile similar to that shown previously 6. This outcome was expected because exposures following IV dosing aligned with plasma concentrations previously shown to be generally safe and well tolerated in a larger number of subjects.
Losmapimod delivered as a 15 min IV infusion displayed approximate dose proportionality between the 1 and 3 mg doses. The volume of distribution at steady state of 171.8 l for the 1 mg IV dose and 110.8 l for the 3 mg IV dose indicates that this drug distributes extensively into tissues. There was a statistically significant difference in the VDss between these two doses. Elucidating the basis for this difference is difficult because there was a limited number of subjects, particularly in the 1 mg dose group (n = 4), and the ranges for VDss of the two doses overlapped; 140.9–186.0 l for the 1 mg dose and 76.8–153.2 l for 3 mg dose. However, one possible explanation may be due to covariates, particularly body weight, because bodyweight was found to be a significant covariate on clearance and volume of distribution terms in a population PK analysis in ACS patients (unpublished data on file; GlaxoSmithKline, King of Prussia, PA, USA). With regard to this study, the two patients who had the lowest bodyweights, i.e. 66.4 and 66.8 kg, received the 3 mg IV dose and had the lowest VDss, i.e. 76.8 and 86.1 l.
This is the only study involving the IV formulation of losmapimod thus far. However, numerous other phase I studies in healthy volunteers have been conducted utilizing a tablet formulation. The PK from those studies closely parallels our present findings. The half-life in this study ranged between 6.5 and 9.4 h (based on the geometric mean for each dose group), while the half-life in the first time in human (FTIH) escalating dose study ranged between 4.4 and 10.1 h for doses in the range of 7–60 mg (unpublished data on file; GlaxoSmithKline, King of Prussia, PA, USA). The Tmax for the oral dose in this study was also in good agreement with the Tmax of the 20 mg single dose in the previous FTIH study; 3.5 h (median), range 2–6 h compared with 3.0 h (median), range 1.5–4 h, respectively. Finally, the apparent clearance in the present study for the oral dose, 28.4 l h−1, also appeared to be similar to that for the 20 mg single dose in the FTIH study, 33.3 l h−1.
The PK/PD relationship between losmapimod and pHSP27, a fairly direct monocyte marker 15 within the p38 phosphorylation cascade, was characterized using a direct-link standard Imax model. This model demonstrated that the pharmacodynamic effect of losmapimod on this biomarker occurs instantaneously and correlates directly with losmapimod plasma concentrations, demonstrating that an IV infusion over a short duration of time achieves a more rapid inhibition of p38 MAPK compared with the oral dose. As noted, this may be a desirable effect in ACS. In contrast, hsCRP did not reveal such a PK/PD relationship (Figure 3). The most likely explanation as to why an exposure–response relationship for hsCRP could not be developed with these data is the long half-life of CRP (19–20 h 16, 17). Several consecutive days of dosing would be needed in order to observe the maximal effect of losmapimod on hsCRP. Another major contributory factor is the fact that healthy volunteers were used in this study, with low baseline hsCRP levels (geometric mean baseline hsCRP 0.6 and 0.55 mg l−1 for the 3 mg IV and 15 mg oral dose, respectively) compared with the higher hsCRP levels typically observed in a population with coronary disease. Other possible explanations include high variability of hsCRP and small sample size of this study. However, despite this rationalization as to why an exposure–response relationship was not observed, a trend of reduced hsCRP was noted for both formulations at 24 h (which was statistically significant for the PO formulation) and for only the PO formulation at 12 h.
Figure 3.
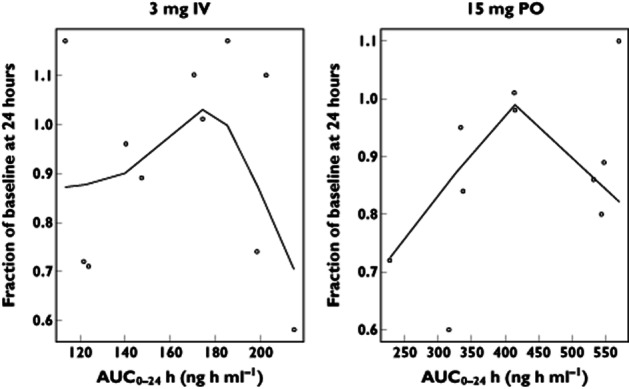
AUC0–24 vs. change from baseline high-sensitivity C-reactive protein (hsCRP) with smooth (i.e. a lowess fit curve). No exposure–response relationship was demonstrated. The hsCRP values were below the limit of quantification for one subject following 3 mg IV dosing and two subjects following 15 mg PO dosing
Conclusion
A single IV infusion of losmapimod in healthy volunteers is safe and well tolerated and, based on its effect on pHSP27, demonstrates rapid blockade of the p38 MAPK receptor. These data suggest that an initial IV loading dose of losmapimod may be of potential benefit in acute coronary syndrome.
Acknowledgments
We would like to thank participating volunteers and to acknowledge all clinical study site personnel who contributed to the conduct of this trial. In particular, the authors acknowledge the support of Dr Joao Oliveira who helped with the practical aspects of the trial. The authors also acknowledge the support of the Department of Clinical Immunology, Cambridge University Hospital for conducting the hsCRP assay. All authors contributed to data interpretation and manuscript development. All authors approved the submitted draft and assume responsibility for manuscript content. Dr Cheriyan and the Cambridge University Hospitals Core Biochemistry Assay Laboratory (Biochemistry, Haematology and Immunology) acknowledge the funding support received from the National Institute for Health Research Cambridge Comprehensive Biomedical Research Centre.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). A.T.M. and J.G. declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. A.M.B., L.S.-B., G.C., M.J.F., J.M. and D.L.S. had support from GlaxoSmithKline in the form of employment as A.M.B., L.S.-B., G.C., M.J.F., J.M. and D.L.S. are full-time employees of GlaxoSmithKline. A.M.B., L.S.-B., G.C., M.J.F., J.M. and D.L.S. also own stock in GlaxoSmithKline. D.L.S. declares that he led the full clinical discovery for losmapimod, which could appear to have influenced the submitted work. J.C. declares that he is a full-time employee of Cambridge University Hospitals NHS Foundation Trust but is obligated to spend 50% of his time on GlaxoSmithKline clinical trial research, representing a significant relationship which could appear to have influenced the submitted work; however, he receives no other benefits or compensation from GlaxoSmithKline.
References
- 1.Willette RN, Eybye ME, Olzinski AR, Behm DJ, Aiyar N, Maniscalco K, Bentley RG, Coatney RW, Zhao S, Westfall TD, Doe CP. Differential effects of p38 mitogen-activated protein kinase and cyclooxygenase 2 inhibitors in a model of cardiovascular disease. J Pharmacol Exp Ther. 2009;330:964–970. doi: 10.1124/jpet.109.154443. [DOI] [PubMed] [Google Scholar]
- 2.Saurin AT, Martin JL, Heads RJ, Foley C, Mockridge JW, Wright MJ, Wang Y, Marber MS. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 2000;14:2237–2246. doi: 10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- 3.Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 4.Schwertz H, Carter JM, Abdudureheman M, Russ M, Buerke U, Schlitt A, Muller-Werdan U, Prondzinsky R, Werdan K, Buerke M. Myocardial ischemia/reperfusion causes VDAC phosphorylation which is reduced by cardioprotection with a p38 MAP kinase inhibitor. Proteomics. 2007;7:4579–4588. doi: 10.1002/pmic.200700734. [DOI] [PubMed] [Google Scholar]
- 5.Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC, Johns DG, Woods TN, Doe CP, Coatney RW, Ohlstein JF, Douglas SA, Willette RN, Yue TL. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol. 2007;49:362–368. doi: 10.1097/FJC.0b013e318046f34a. [DOI] [PubMed] [Google Scholar]
- 6.Cheriyan J, Webb AJ, Sarov-Blat L, Elkhawad M, Wallace SM, Maki-Petaja KM, Collier DJ, Morgan J, Fang Z, Willette RN, Lepore JJ, Cockcroft JR, Sprecher DL, Wilkinson IB. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation. 2011;123:515–523. doi: 10.1161/CIRCULATIONAHA.110.971986. [DOI] [PubMed] [Google Scholar]
- 7.Elkhawad M, Rudd JH, Sarov-Blat L, Cai G, Wells R, Davies LC, Collier DJ, Marber MS, Choudhury RP, Fayad ZA, Tawakol A, Gleeson FV, Lepore JJ, Davis B, Willette RN, Wilkinson IB, Sprecher DL, Cheriyan J. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. JACC Cardiovasc Imaging. 2012;5:911–922. doi: 10.1016/j.jcmg.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, Krumholz HM. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ. 2009;338:b1807. doi: 10.1136/bmj.b1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan MY, Sun JL, Newby LK, Shaw LK, Lin M, Peterson ED, Califf RM, Kong DF, Roe MT. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation. 2009;119:3110–3117. doi: 10.1161/CIRCULATIONAHA.108.799981. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, Afzal R, Chrolavicius S, Jolly SS, Widimsky P, Avezum A, Rupprecht HJ, Zhu J, Col J, Natarajan MK, Horsman C, Fox KA, Yusuf S. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360:2165–2175. doi: 10.1056/NEJMoa0807986. [DOI] [PubMed] [Google Scholar]
- 11.Sarov-Blat L, Morgan JM, Fernandez P, James R, Fang Z, Hurle MR, Baidoo C, Willette RN, Lepore JJ, Jensen SE, Sprecher DL. Inhibition of p38 mitogen-activated protein kinase reduces inflammation after coronary vascular injury in humans. Arterioscler Thromb Vasc Biol. 2010;30:2256–2263. doi: 10.1161/ATVBAHA.110.209205. [DOI] [PubMed] [Google Scholar]
- 12.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 13.Singh D, Smyth L, Borrill Z, Sweeney L, Tal-Singer R. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 2010;50:94–100. doi: 10.1177/0091270009347873. [DOI] [PubMed] [Google Scholar]
- 14.Berry DA, Ayers GD. Symmetrized percent change for treatment comparisons. Am Stat. 2006;60:27–31. [Google Scholar]
- 15.Voss OH, Batra S, Kolattukudy SJ, Gonzalez-Mejia ME, Smith JB, Doseff AI. Binding of caspase-3 prodomain to heat shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activation. J Biol Chem. 2007;282:25088–25099. doi: 10.1074/jbc.M701740200. [DOI] [PubMed] [Google Scholar]
- 16.Kao PC, Shiesh SC, Wu TJ. Serum C-reactive protein as a marker for wellness assessment. Ann Clin Lab Sci. 2006;36:163–169. [PubMed] [Google Scholar]
- 17.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]


