Abstract
Aims
Nevirapine (NVP) is a non-nucleoside reverse transcriptase inhibitor used for chronic human immunodeficiency virus infections in adults and children. The aims of this study were to investigate the population pharmacokinetics of NVP in children, establish factors that influence NVP pharmacokinetics and evaluate the current dosing recommendations.
Methods
Concentrations were measured on a routine basis in 94 children aged from 2 months to 17 years. A total of 390 NVP plasma concentrations were retrospectively collected, and a population pharmacokinetic model was developed with Monolix 4.0.
Results
Nevirapine pharmacokinetics was best described by a one-compartment model with first-order absorption and elimination. After standardization to a 70 kg adult using allometry, postmenstrual age had a significant effect on the bioavailability. Estimates of apparent clearance and volume of distribution were 3.9 l h−1 (70 kg)−1 and 140 l (70 kg)−1, respectively. Based on simulations of European Medicines Agency (EMA) and World Health Organization (WHO) dosing recommendations, the probability of observing minimal concentrations below the efficacy target of 3 mg l−1 is higher following the EMA recommendations than the WHO recommendations. However, NVP underdosing persists for the 3–6 and 6–10 kg weight ranges following the WHO recommendations.
Conclusions
It is suggested to increase doses to 75 and 100 mg twice daily for the 3–6 and 6–10 kg weight ranges, respectively, in order to obtain more than 95% of children with concentrations above 3 mg l−1.
Keywords: children, nevirapine, population pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Nevirapine pharmacokinetics has been studied in adults and children. Subtherapeutic concentrations have been reported by several studies with current recommendations, especially in young children.
WHAT THIS STUDY ADDS
This population pharmacokinetics analysis of nevirapine in human immunodeficiency virus-infected children provides new insights into the fate of this drug. The apparent clearance and apparent volume of distribution increased allometrically with bodyweight, whereas the relative bioavailability increased with postmenstrual age. Underexposure to nevirapine was found for the 3–10 kg bodyweight range, and dosing suggestions are provided.
Introduction
Nevirapine (NVP) is a non-nucleoside reverse transcriptase inhibitor used for chronic human immunodeficiency virus (HIV) infections in adults and children and in pregnant women for the prevention of mother-to-child transmission. Nevirapine has demonstrated a long-term suppression of viral replication in HIV-experienced and -naïve infected adults and children 1–4. Several studies have also been conducted in newborns aged less than 6 months 5–8.
The current NVP dosing recommendations of the European Medicines Agency (EMA) at steady state are 7 mg kg−1 twice daily (BID) in children from 2 months to 8 years, 4 mg kg−1 BID for children older than 8 years and 200 mg BID for children weighing more than 50 kg (equivalent to the dosing recommendation based on the body surface area of 150 mg m−2 BID for children older than 15 days) 1, 2, 9, 10. The total daily dose should not exceed 400 mg for any patient 1.
In adults, NVP is readily absorbed (>90%) after oral administration. The drug is highly lipophilic and is about 60% bound to plasma protein in the concentration range of 1–10 mg l−1. This drug is extensively and primarily metabolized by the cytochrome P450 (CYP) isoenzymes 3A4 and 2B6 to several hydroxylated metabolites. The half-life at steady state (following auto-induction) is 25–30 h following a 200 or 400 mg daily regimen 1.
Patients taking NVP must be closely monitored for adverse events during the first 18 weeks of treatment and more specifically during the first 6 weeks; indeed, during this period the risk of severe hepatic failure or skin reactions is higher. However, skin rashes, Stevens–Johnson syndrome or hepatic events may occur at any time during treatment 1.
Nevirapine has a high interindividual variability in its pharmacokinetics and a low genetic barrier, i.e. development of resistance to the treatment appears with very few mutations of the virus. It is therefore important to ensure that sufficient minimal concentrations (Cmin) are reached. It has been shown that minimal concentrations below 3 mg l−1 are associated with a higher risk of virological failure 11, 12. Some studies have considered a minimal concentration above 8 mg l−1 as a target for toxicity 13, 14; however, no relationships between toxicity and drug concentrations have been precisely defined. Since 2001, many clinical studies in children have reported subtherapeutic minimal concentrations of NVP < 3 mg l−1 following current EMA recommendations 11, 15. Thus, more reliable fixed-dose combinations, according to bodyweight (BW), were recommended by the World Health Organization (WHO). These fixed-dose combinations including nevirapine, stavudine (d4T) and lamivudine (3TC), and are administrated twice daily with a higher NVP dose: Triomune Baby® (Cipla Ltd., Mumbai, India) for children weighing from 3 to 10 kg, Triomune Junior® for children from 10 to 30 kg and Triomune 30® for children and adolescents weighing more than 30 kg 16.
However, recent studies have shown that these increased NVP doses per weight could be insufficient to correct subtherapeutic Cmin values in the bodyweight range 3–14 kg 13, 14, 17–20.
The aims of this study were as follows: (i) to investigate the population pharmacokinetics of NVP in children; (ii) to investigate the factors that influence NVP pharmacokinetics in this population; (iii) to compare NVP pharmacokinetic parameters with previous studies in children; and (iv) to evaluate the dosing recommendations in children.
Methods
Patients and treatment
Clinical data were collected retrospectively from the medical files of HIV-infected children and adolescents in five clinical centres. Blood samples were drawn at random time points using a therapeutic drug-monitoring methodology. Ethics committee approval and patient consent are not required in France in order to use therapeutic drug-monitoring data retrospectively. At each patient visit, time after the last dosing, BW, age and combined treatments were recorded. Nevirapine was administered as 7 mg kg−1 BID in children from 2 months to 8 years and 4 mg kg−1 BID for children older than 8 years. The pharmacokinetic study was performed after 15 days of treatment. All plasma samples were collected at steady state. The samples were not collected on the same occasion, the median (range) number of samples per patient was 3 (1–17) and the median (range) blood sampling time was 3.4 h (0.7–11.8).
Analytical method
Nevirapine was measured in a 100 μl plasma sample using a high-pressure liquid chromatography method (Beckman system GOLD®) with ultraviolet detection. Samples were extracted using tert-butyl methyl ether in the presence of 2-acetamidophenol as the internal standard. The separation was carried out on high-pressure liquid chromatography system with CLUZEAU column (250 mm × 3 mm, 5 μm) with a gradient of solvent A (0.87 g of pentane-1-sulfonic acid in 1 l of phosphate buffer) and solvent B (acetonitrile) as follows: 75% solvent A and 25% solvent B for 30 min.
The characteristics of the method were as follows: the calibration range was linear from 0.25 to 10 mg l–1; the lower limit of quantification was 0.25 mg l–1; the extraction yield was 88%; the reproducibility evaluated by the coefficient of variation was >93.6%; and the accuracy evaluated by the coefficient of variation was >96%. No analytical interference between the different antiretrovirals was noticed.
Modelling strategy and data analysis
Different structural models for NVP pharmacokinetics were investigated, i.e. one or two compartments with linear elimination and first-order or zero-order absorption, with or without a lag time or a transit compartment for absorption. As nevirapine was given exclusively by oral route, clearance (CL) and volume of distribution (V) are apparent parameters, V/F and CL/F, where F is the unknown bioavailability fraction.
Data were analysed using the nonlinear mixed-effect modelling software program Monolix version 4.0 ‘http://www.lixoft.eu/’ 21. Parameters were estimated by computing the maximum likelihood estimator of the parameters without any approximation of the model (no linearization) using the stochastic approximation expectation maximization (SAEM) algorithm combined to a Markov Chain Monte Carlo procedure. The number of Markov Chain Monte Carlo chains was fixed to five for all estimations. Several error models (proportional, additive or mixed) were investigated to describe the residual variability (ε). The between-subject variabilities (η or BSVs) were assumed to be exponential. The objective function value (OFV) was used to test different hypotheses regarding the final model, covariate effect(s) on pharmacokinetic parameter(s), residual variability model (proportional vs. proportional plus additive error model), and structure of the variance–covariance matrix for the BSV parameters.
Continuous covariates (CO) were tested according to the following equation, using CL for example, 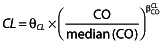 , where θCL is the typical value of clearance for a patient with the median covariate value and the power exponent
, where θCL is the typical value of clearance for a patient with the median covariate value and the power exponent 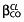 is the estimated influential factor. The main continuous covariates of interest in the population were age, postmenstrual age (PMA) and bodyweight. Parameter estimates were standardized for a mean standard BW using an allometric model. From allometric scaling theory the power exponents are typically 0.75 for clearance and one for volume of distribution 22.
is the estimated influential factor. The main continuous covariates of interest in the population were age, postmenstrual age (PMA) and bodyweight. Parameter estimates were standardized for a mean standard BW using an allometric model. From allometric scaling theory the power exponents are typically 0.75 for clearance and one for volume of distribution 22.
Age-related change functions for pharmacokinetic parameters have been described previously 23. The effect of PMA on the relative bioavailability was investigated following the Hill equation, 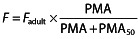 , where Fadult is the reference bioavailability in adults, fixed to one and PMA50 is the PMA corresponding to F = 0.5.
, where Fadult is the reference bioavailability in adults, fixed to one and PMA50 is the PMA corresponding to F = 0.5.
Binary covariates were tested according to the equation, 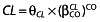 , where CO = 0 is for the reference θCL value and CO = 1 for the CL value in the presence of the covariate. The main binary covariates of interest in the population were sex and antiretroviral treatment. A covariate was finally retained if it met the following conditions: (i) its effect was biologically plausible; (ii) a reduction in OFV value was observed; and (iii) it produced a reduction in the variability of the pharmacokinetic parameter, assessed by the associated intersubject variability. Graphical evaluation of the goodness of fit was mainly assessed by observed vs. predicted concentrations (PRED-DV) and weighted residuals vs. time and/or weighted residuals vs. PRED.
, where CO = 0 is for the reference θCL value and CO = 1 for the CL value in the presence of the covariate. The main binary covariates of interest in the population were sex and antiretroviral treatment. A covariate was finally retained if it met the following conditions: (i) its effect was biologically plausible; (ii) a reduction in OFV value was observed; and (iii) it produced a reduction in the variability of the pharmacokinetic parameter, assessed by the associated intersubject variability. Graphical evaluation of the goodness of fit was mainly assessed by observed vs. predicted concentrations (PRED-DV) and weighted residuals vs. time and/or weighted residuals vs. PRED.
The final population model was mainly evaluated by the normalized prediction distribution errors (npde) metrics 24 and the prediction-corrected visual predictive check 25. Diagnostic graphics and distribution statistics were obtained using RfN (link on http://wfn.sourceforge.net) via the R program 26.
Individual Bayesian estimates of the pharmacokinetic parameters were used to calculate the individual area under the concentration–time curve from time zero to 12 h (AUC0–12) and the Cmin of NVP.
Dose simulations
Using the final population model, the probability of obtaining the target Cmin between 3 and 8 mg l−1 in 94 000 children (1000 simulations of our 94 children) was calculated for different twice-daily doses (50, 75, 100, 125, 150, 175 and 200 mg) in 13 bodyweight groups (3–6, 6–10, 10–15, 15–20, 20–25, 25–30, 30–35, 35–40, 40–45, 45–50, 50–55, 55–60 and >60 kg). Simulations were also performed in order to determine the percentage of patients below the efficacy target following the EMA and WHO dosing recommendations.
The EMA dosing recommendations are currently 7 mg kg−1 BID for children more than 2 months to 8 years and 4 mg kg−1 BID for children older than 8 years 2.
the WHO dosing recommendations are 50 mg BID (3–6 kg), 75 mg BID (6–10 kg), 100 mg BID (10–14 kg), 125 mg BID (14–20 kg), 150 mg BID (20–25 kg) and 200 mg BID above 25 kg 16.
Results
Demographic data
The median [95% confidence interval (CI)] follow-up duration of therapeutic drug monitoring for the 94 children (50 girls and 44 boys) was 12.5 months (range 0–93 months), and 390 NVP concentrations were available for pharmacokinetic evaluation. The pharmacokinetic study was performed after 15 days of treatment, in children older than 2 months. At baseline, the median (95% CI) age was 6.3 years (10 months to 14 years), the median (95% CI) bodyweight was 20 kg (3–64 kg) and the median (95% CI) NVP dose was 100 mg (21–200 mg) twice daily.
Population pharmacokinetics
Three concentrations were below the linit of quantification and were treated as left-censored data by the program. A one-compartment model with first-order absorption and elimination described the data adequately. The absorption constant could not be estimated adequately (no sample between 0 and 1 h after drug intake) and was finally fixed to 0.4 h−1 27. Between-subject variability could be estimated only for apparent clearance. The proportional model for the residual variability ensured a good agreement between observed and predicted values. Bodyweight was included in the model, using a one and a three-quarters allometric scaling for V/F and CL/F, respectively. These parameters were normalized for a 70 kg BW adult and decreased the objective function value by 66 units and the CL/F BSV from 0.47 to 0.34. The effect of PMA on the bioavailability was statistically significant, decreasing the objective function value by 8.2 units and the BSV on CL/F to 0.33. The effects of sex and other co-medications were also evaluated on the CL/F parameter, but no significant relationship appeared. The final model was then:
Table 1 summarizes the final population pharmacokinetic estimates, including the relative standard errors. All parameters were well estimated regarding the relative standard error values of the estimates. The empirical Bayesian estimate of η-shrinkage on CL/F was 0.02. Final model performance was appreciated by comparing population predicted and individual predicted with observed plasma concentrations and population weighted residuals vs. predicted concentrations for nevirapine (Figure 1).
Table 1.
Population pharmacokinetic parameters of nevirapine in 94 children (aged 2 months to 17 years)
| Parameter | RSE (%) | |
|---|---|---|
| Structural model | ||
| Ka (h−1) | 0.4 | — |
| V/F (l (70 kg)−1)* | 140 | 39 |
| CL/F (l h−1 (70 kg)−1)* | 3.93 | 5 |
| θPMA50 (years) | 0.55 | 36 |
| Statistical model | ||
| BSV CL/F | 0.33 | 10 |
| σproportional | 0.30 | 4 |
Abbreviations are as follows: F, bioavailability; BSV, between-subject variability estimates; CL/F, apparent elimination clearance; Ka, absorption rate constant; RSE, relative standard error; σ, residual variability estimates; θPMA50, PMA corresponding to F = 0.5; and V/F, apparent central volume of distribution.
The typical parameters refer to a patient weighing 70 kg according to the following allometric scaling model: [typical value] = [typical parameter] × (bodyweight/70)PWR, where PWR = 0.75 for the CL term and PWR = 1 for the V term. CL/F = 3.93 × (bodyweight/70)0.75/[PMA/(PMA50 + PMA)]. V/F = 140 × (bodyweight/70)1/[PMA/(PMA50 + PMA)].
Figure 1.
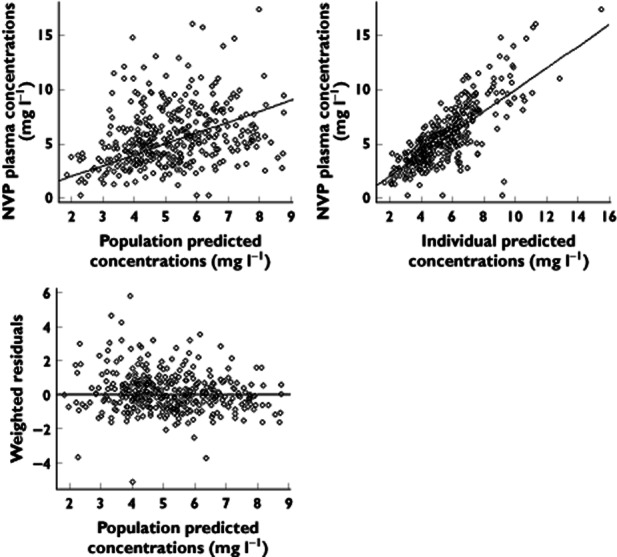
Observed nevirapine (NVP) concentrations vs. population predicted NVP concentrations (top left), observed NVP concentrations vs. individual predicted NVP concentrations (top right), and population weighted residuals vs. population predicted NVP concentrations (bottom left)
The prediction-corrected visual predictive check shows that the 5th, 50th and 95th percentiles of observed data are well included within the 90% CI of the 5th, 50th and 95th simulated percentiles (Figure 2). The mean and variance of the npde metrics were not significantly different from 0 (P = 0.13) and 1 (P = 0.17) and their distribution was not different from a normal one (P = 0.23; global adjusted P-value, P = 0.39). Table 2 summarizes values of AUC0–12 and Cmin obtained from the present study and from previous studies in children.
Figure 2.
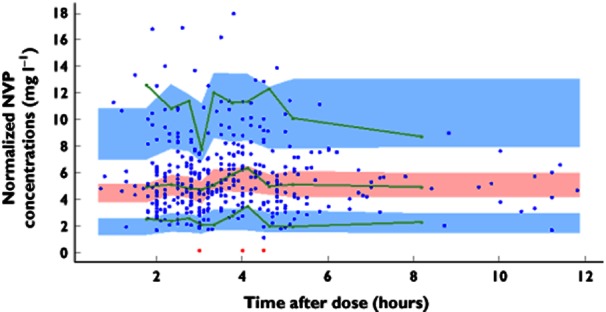
Prediction-corrected visual predictive check for nevirapine (NVP) concentrations vs. time (in hours). The green lines show the 5th, 50th and 95th percentiles of observed data; the areas represent the 90% confidence interval around the simulated percentiles; and the red dots correspond to values below the limit of quantification
Table 2.
Comparison of nevirapine-derived pharmacokinetic parameters between our study and previous studies of children
| Parameter [median (range)] | Present study | Previous paediatric studies | |
|---|---|---|---|
| Chokephaibulkit et al. 34 | Pollock et al. 14 | ||
| Number of children | 94 | 34 | 25 |
| Age (years) | 7.2 (0.17–17.5) | 8.4 (3–15) | 6.3 (0.8–16) |
| Bodyweight (kg) | 21 (2.9–67) | — (4–40) | 15.9 (6.3–38.9) |
| Daily dose | 200 (40–400) mg | 328 (244–435) mg m−2 | 250 (100–400) mg |
| CL (l h−1 kg−1) | 0.08 (0.03–0.14) | 0.08 (0.02–0.16) | 0.1 (0.03–0.18) |
| AUC0–12 (mg h−1 l−1) | 65.3 (25–191) | 78.4 (50–307) | 79.9 (44–146) |
| Cmin (mg−1 l−1) | 4.3 (1.3–14.2) | 5.98 (2.57–24.4) | 5.9 (2.3–9.1) |
Abbreviations are as follows: CL, clearance; AUC0-12, area under the concentration-time curve from 0 to 12 h; Cmin, minimal concentration.
Figure 3 displays the median NVP Cmin values as a function of bodyweight derived from 1000 simulations of the final model following the EMA or WHO dosing recommendations. Figure 3A shows that the EMA dosing recommendations lead to subtherapeutic Cmin values for children weighing less than 10 kg (14 children). As shown in Table 3 and Figure 3B, the WHO recommendations seem to correct this subtherapeutic dosage in part, because more than 71% of patients were above the efficacy target; however, the youngest children were still underexposed. Simulations of doses of 75 and 100 mg for the bodyweight ranges 3–6 and 6–10 kg, respectively, lead to Cmin values above the efficacy target in 96 and 97% of cases, respectively (Table 3).
Figure 3.
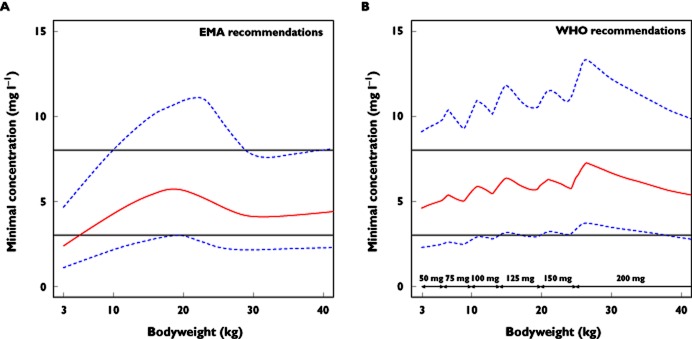
Calculated nevirapine (NVP) minimal concentrations (continuous line) and 90% confidence intervals (dashed lines) vs. bodyweight in children, following the European Medicines Agency (EMA) dosing recommendations (A) and the World Health Organization (WHO) dosing recommendations (B). The horizontal continuous lines represent the minimal concentration targets for efficacy (3 mg l−1) and toxicity (8 mg l−1), respectively
Table 3.
Percentage of patients with minimal concentrations below, above or within the therapeutic interval (3–8 mg l−1) following EMA dosing recommendations, WHO dosing recommendations or simulations of the present study
| Weight band | Recommendation | <3 mg l−1 (%) | In the range (%) | >8 mg l−1 (%) |
|---|---|---|---|---|
| 3–6 kg | EMA, 7 mg kg−1 BID | 57 | 42 | 1 |
| WHO, 50 mg BID | 19 | 73 | 8 | |
| Simulation, 75 mg BID | 4 | 62 | 34 | |
| 6–10 kg | EMA, 7 mg kg−1 BID | 27 | 70 | 3 |
| WHO, 75 mg BID | 11 | 71 | 18 | |
| Simulation, 100 mg BID | 3 | 64 | 33 |
Abbreviations are as follows: BID, twice daily; EMA, European Medicines Agency; and WHO, World Health Organization.
Discussion
Nevirapine concentrations were satisfactorily described by a one-compartment model with linear absorption and elimination, including the effects of bodyweight on CL/F and V/F and PMA on the relative bioavailability. The following observations support the model used: (i) the pharmacokinetic parameters were standardized for a 70 kg bodyweight adult and the apparent clearance estimate was 3.9 l h−1 (70 kg)−1, close to previously published values in adults 28, 29; (ii) NVP half-life and AUC0–12 h following a 200 mg BID NVP dosage were, respectively, around 25 h and 51 mg h l−1, results similar to other estimates in adults 28, 30, 31; and (iii) the mean apparent clearance estimate in our population (CL/F = 1.7 l h−1) was also in agreement with the previous paediatric population study (1.9 l h−1) of Nikanjam et al., which also found an age effect after allometric scaling of the apparent parameters 32. In the present study, the effect of PMA was significant on the basis of a decrease in the OFV, although there was no substantial reduction in the corresponding BSV; however, this effect was retained in the model for the following reasons: (i) it improved the diagnostic plots; (ii) it allowed prediction of the adult values; and (iii) it was also reported by another group 32. The effect of PMA on the bioavailability of NVP in children showed that at 210 weeks of life (4 years), 90% of the adult value was reached.
Based on EMA and WHO dosing recommendations, the probability of observing concentrations below the target of 3 mg l−1 is higher following the EMA than the WHO dosing recommendations. Indeed, following the WHO dosing recommendations the probability of observing concentrations below the efficacy target was less than 6% for patients weighing more than 10 kg; however, the probabilities were 19 and 11% in the 3–6 and 6–10 kg weight ranges, respectively. Thus, the WHO dosing recommendations appeared to be sufficient only for patients weighing more than 10 kg, which is in agreement with recent studies 13, 19, . Fillekes et al. have conducted a study in 15 children from 3 to 6 kg treated with Triomune Baby® and concluded that 27% of them had subtherapeutic NVP levels. This percentage was higher in children aged less than 5 months 20.
In order to avoid subtherapeutic dosage, which could lead to virological failure, higher dosing recommendations are suggested in the youngest children; 75 mg BID for the 3–6 kg weight range and 100 mg BID for the 6–10 kg group were simulated. These dose simulations resulted in 96 and 97% of patients with Cmin values above 3 mg l−1, whereas 34% (3–6 kg) and 33% (6–10 kg) were above 8 mg l−1, which has been considered as a potential toxic level 13, 14, 33. However, this toxicity threshold has not been properly defined because no significant relationship between high NVP concentrations and the occurrence of toxicity has been demonstrated either in adult or in pediatric patients. Poerksen et al. have conducted a study with 74 HIV-infected children treated with split Triomune 30® tablets and reported that 39% had Cmin values above 8 mg l−1 without clinical signs of drug toxicity 13. The balance between efficacy and toxicity for the youngest children from 3 to 10 kg suggests higher dosing recommendations; however, some caution must be exercised to avoid toxicity.
In conclusion, the WHO dosing recommendations seem appropriate in children weighing more than 10 kg, but doses could be increased to 75 and 100 mg BID in the 3–6 and 6–10 kg weight ranges, respectively, to obtain more than 95% of children with a minimal concentration above 3 mg l−1. These suggestions should be confirmed prospectively.
Acknowledgments
Nevirapine plasma measurements were performed at the ‘Laboratoire de Pharmacologie’, Hôpital Saint-Vincent-de-Paul, Paris.
We acknowledge the Paediatric European Network Treatment AIDS Laboratory Network (PENTA LABNET) for their financial support.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Boehringer Ingelheim Pharmaceuticals, Inc. Viramune, highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc; 2011. Available at http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Viramune/Viramune.pdf (last accessed 10 December 2012) [Google Scholar]
- 2.European Medicines Agency. Viramune: EPAR product information. European Medicines Agency. 2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000183/WC500051481.pdf (last accessed 10 December 2012) [Google Scholar]
- 3.Luzuriaga K, Bryson Y, McSherry G, Robinson J, Stechenberg B, Scott G, Lamson M, Cort S, Sullivan JL. Pharmacokinetics, safety, and activity of nevirapine in human immunodeficiency virus type 1-infected children. J Infect Dis. 1996;174:713–721. doi: 10.1093/infdis/174.4.713. [DOI] [PubMed] [Google Scholar]
- 4.Verweel G, Sharland M, Lyall H, Novelli V, Gibb DM, Dumont G, Ball C, Wilkins E, Walters S, Tudor-Williams G. Nevirapine use in HIV-1-infected children. AIDS. 2003;17:1639–1647. doi: 10.1097/00002030-200307250-00008. [DOI] [PubMed] [Google Scholar]
- 5.Benaboud S, Ekouévi DK, Urien S, Rey E, Arrivé E, Blanche S, Gray G, Sim KL, Avit D, McIntyre J, Nerrienet E, Dabis F, Tréluyer J-M, Hirt D. Population pharmacokinetics of nevirapine in HIV-1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2011;55:331–337. doi: 10.1128/AAC.00631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirochnick M, Dorenbaum A, Blanchard S, Cunningham CK, Gelber RD, Mofenson L, Culnane M, Sullivan JL. Predose infant nevirapine concentration with the two-dose intrapartum neonatal nevirapine regimen: association with timing of maternal intrapartum nevirapine dose. J Acquir Immune Defic Syndr. 2003;33:153–156. doi: 10.1097/00126334-200306010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Mirochnick M, Nielsen-Saines K, Pilotto JH, Pinto J, Jiménez E, Veloso VG, Parsons T, Watts DH, Moye J, Mofenson LM, Camarca M, Bryson Y. Nevirapine concentrations in newborns receiving an extended prophylactic regimen. J Acquir Immune Defic Syndr. 2008;47:334–337. [PubMed] [Google Scholar]
- 8.Shetty AK, Coovadia HM, Mirochnick MM, Maldonado Y, Mofenson LM, Eshleman SH, Fleming T, Emel L, George K, Katzenstein DA, Wells J, Maponga CC, Mwatha A, Jones SA, Abdool Karim SS, Bassett MT. Safety and trough concentrations of nevirapine prophylaxis given daily, twice weekly, or weekly in breast-feeding infants from birth to 6 months. J. Acquir Immune Defic Syndr. 2003;34:482–490. doi: 10.1097/00126334-200312150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Belew Y. Clinical review. Food and Drug Administration. 2007 Available at http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm072777.pdf (last accessed 10 December 2012) [Google Scholar]
- 10.European Medicines Agency. Viramune assessment report. European Medicines Agency. 2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000183/WC500117064.pdf (last accessed 10 December 2012) [Google Scholar]
- 11.De Vries-Sluijs TEMS, Dieleman JP, Arts D, Huitema ADR, Beijnen JH, Schutten M, Van der Ende ME. Low nevirapine plasma concentrations predict virological failure in an unselected HIV-1-infected population. Clin Pharmacokinet. 2003;42:599–605. doi: 10.2165/00003088-200342060-00009. [DOI] [PubMed] [Google Scholar]
- 12.la Porte CJL, Back D, Blaschke T, Boucher CAB, Fletcher CV. Updated guidelines to perform therapeutic monitoring for antiretroviral agents. Rev Antivir Ther. 3:4–12. [Google Scholar]
- 13.Poerksen G, Pollock L, Moons P, Chesshyre E, Burger D, Khoo S, Molyneux E. Steady-state nevirapine, lamivudine and stavudine levels in Malawian HIV-infected children on antiretroviral therapy using split Triomune 30 tablets. Antivir Ther. 2010;15:343–350. doi: 10.3851/IMP1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollock L, Else L, Poerksen G, Molyneux E, Moons P, Walker S, Fraser W, Back D, Khoo S. Pharmacokinetics of nevirapine in HIV-infected children with and without malnutrition receiving divided adult fixed-dose combination tablets. J Antimicrob Chemother. 2009;64:1251–1259. doi: 10.1093/jac/dkp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duong M, Buisson M, Peytavin G, Kohli E, Piroth L, Martha B, Grappin M, Chavanet P, Portier H. Low trough plasma concentrations of nevirapine associated with virologic rebounds in HIV-infected patients who switched from protease inhibitors. Ann Pharmacother. 2005;39:603–609. doi: 10.1345/aph.1E563. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: Towards Universal Access, 2010 Revision. World Health Organization; 2010. Available at http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf (last accessed 10 December 2012) [PubMed] [Google Scholar]
- 17.Corbett AH, Hosseinipour MC, Nyirenda J, Kanyama C, Rezk NL, Mkupani P, Sichali D, Tien H, Kashuba AD, Mwansambo C, Weigel R, Kazembe P. Pharmacokinetics of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian children. Antivir Ther. 2010;15:83–90. doi: 10.3851/IMP1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis JC, L'homme RFA, Ewings FM, Mulenga V, Bell F, Chileshe R, Molyneux E, Abernethy J, Van Oosterhout JJG, Chintu C, Walker AS, Gibb DM, Burger DM. Nevirapine concentrations in HIV-infected children treated with divided fixed-dose combination antiretroviral tablets in Malawi and Zambia. Antivir Ther. 2007;12:253–260. [PubMed] [Google Scholar]
- 19.L'homme RFA, Kabamba D, Ewings FM, Mulenga V, Kankasa C, Thomason MJ, Walker AS, Chintu C, Burger DM, Gibb DM. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on paediatric fixed-dose combination tablets. AIDS. 2008;22:557–565. doi: 10.1097/QAD.0b013e3282f4a208. [DOI] [PubMed] [Google Scholar]
- 20.Fillekes Q, Mulenga V, Kabamba D, Kankasa C, Thomason MJ, Cook A, Ferrier A, Chintu C, Walker AS, Gibb DM, Burger DM. Pharmacokinetics of nevirapine in HIV-infected infants weighing 3 kg to less than 6 kg taking paediatric fixed dose combination tablets. AIDS. 2012;26:1795–1800. doi: 10.1097/QAD.0b013e32835705fd. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal. 2005;49:1020–1038. [Google Scholar]
- 22.Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 23.Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 24.Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90:154–166. doi: 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria; 2010. [Google Scholar]
- 27.Capparelli EV, Aweeka F, Hitti J, Stek A, Hu C, Burchett SK, Best B, Smith E, Read JS, Watts H, Nachman S, Thorpe EM, Jr, Spector SA, Jimenez E, Shearer WT, Foca M, Mirochnick M. Chronic administration of nevirapine during pregnancy: impact of pregnancy on pharmacokinetics. HIV Med. 2008;9:214–220. doi: 10.1111/j.1468-1293.2008.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Heeswijk RP, Veldkamp AI, Mulder JW, Meenhorst PL, Wit FW, Lange JM, Danner SA, Foudraine NA, Kwakkelstein MO, Reiss P, Beijnen JH, Hoetelmans RM. The steady-state pharmacokinetics of nevirapine during once daily and twice daily dosing in HIV-1-infected individuals. AIDS. 2000;14:F77–82. doi: 10.1097/00002030-200005260-00001. [DOI] [PubMed] [Google Scholar]
- 29.Vanprapar N, Cressey TR, Chokephaibulkit K, Muresan P, Plipat N, Sirisanthana V, Prasitsuebsai W, Hongsiriwan S, Chotpitayasunondh T, Eksaengsri A, Toye M, Smith ME, McIntosh K, Capparelli E, Yogev R. A chewable pediatric fixed-dose combination tablet of stavudine, lamivudine, and nevirapine: pharmacokinetics and safety compared with the individual liquid formulations in human immunodeficiency virus-infected children in Thailand. Pediatr Infect Dis J. 2010;29:940–944. doi: 10.1097/INF.0b013e3181e2189d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Maat MMR, Huitema ADR, Mulder JW, Meenhorst PL, Van Gorp ECM, Beijnen JH. Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. Br J Clin Pharmacol. 2002;54:378–385. doi: 10.1046/j.1365-2125.2002.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moltó J, Valle M, Miranda C, Cedeño S, Miranda J, Santos JR, Negredo E, Vilaró J, Costa J, Clotet B. Once- or twice-daily dosing of nevirapine in HIV-infected adults: a population pharmacokinetics approach. J Antimicrob Chemother. 2008;62:784–792. doi: 10.1093/jac/dkn268. [DOI] [PubMed] [Google Scholar]
- 32.Nikanjam M, Kabamba D, Cressey TR, Burger D, Aweeka FT, Acosta EP, Spector SA, Capparelli EV. Nevirapine exposure with WHO pediatric weight band dosing: enhanced therapeutic concentrations predicted based on extensive international pharmacokinetic experience. Antimicrob Agents Chemother. 2012;56:5374–5380. doi: 10.1128/AAC.00842-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recommandations du groupe d'experts. Rapport 2010 sur la prise en charge médicale des personnes infectées par le VIH sous la direction du Pr. Patrick Yéni. 2010. Available at: http://www.sante.gouv.fr/rapport-2010-sur-la-prise-en-charge-medicale-des-personnes-infectees-par-le-vih-sous-la-direction-du-pr-patrick-yeni.html (last accessed 10 December 2012)
- 34.Chokephaibulkit K, Plipat N, Cressey TR, Frederix K, Phongsamart W, Capparelli E, Kolladarungkri T, Vanprapar N. Pharmacokinetics of nevirapine in HIV-infected children receiving an adult fixed-dose combination of stavudine, lamivudine and nevirapine. AIDS. 2005;19:1495–1499. doi: 10.1097/01.aids.0000183625.97170.59. [DOI] [PubMed] [Google Scholar]


