Abstract
Purpose
Two phase I, single-agent studies were conducted to determine the dose and regimen of obatoclax, an antagonist of all BCL-2 antiapoptotic proteins, for evaluation in phase II trials. The two studies, GX001 and GX005, evaluated the safety and tolerability of weekly 1-hour and 3-hour infusions of obatoclax, respectively.
Experimental Design
Eligible patients in both studies were adults with solid tumor or lymphoma and performance status 0–1 for whom standard therapies were not appropriate. In the GX001 study an accelerated dose titration design was initially used with subsequent cohorts of three to six patients with 40% dose increments between levels. In the GX005 study three to six patients entered at each dose level with 40% dose increments between levels.
Results
Thirty-five patients were enrolled in studies GX001 (n = 8) and GX005 (n = 27). Clinically significant central nervous system (CNS) toxicity was observed using the 1-hour infusion schedule. The obatoclax maximum tolerated dose (MTD) in GX001 was 1.25 mg/m2 due to these infusional CNS events. The 3-hour infusion schedule studied in GX005 had improved tolerability, and the obatoclax MTD was 20 mg/m2. One patient in GX005 with relapsed non-Hodgkin’s lymphoma achieved partial response of 2 months’ duration, and one patient with relapsed non-Hodgkin’s lymphoma had stable disease for 18 months.
Conclusions
The 1-hour infusion schedule of obatoclax was associated with neuropsychiatric dose-limiting toxicities at relatively low doses (MTD, 1.25 mg/m2). The 3-hour i.v. infusion of obatoclax administered once weekly to patients with solid tumors was better tolerated (MTD, 20 mg/m2), and evidence of clinical activity was observed.
Defects in apoptotic pathways are an essential element of tumor pathogenesis (1). One common cancer-causing defect arises from the overproduction of the antiapoptotic protein BCL-2 and related family members, including BCL-XL and MCL-1, which inactivate the apoptotic pathway in many types of cancer cells. Blocking proapoptotic signaling leads to the survival of genetically unstable cells, thus promoting resistance to immune effectors, radiation, and most cytotoxic agents.
The BCL-2 family consists of both antiapoptotic proteins (such as BCL-2) and proapoptotic proteins (such as BH3-only BIM and the apoptotic effectors BAX and BAK). The antiapoptotic proteins of the BCL-2 family are frequently overexpressed in a heterogeneous pattern across tumor types. Thus, antagonists of all the BCL-2 antiapoptotic proteins are likely to be active across a wide range of cancers.
Obatoclax (GX15-070, Gemin X Pharmaceuticals) is a small-molecule antagonist of all of the antiapoptotic BCL-2 family members. The inhibition of antiapoptotic BCL-2 family proteins by obatoclax sensitizes tumor cells to the proapoptotic stress signals inherent in cancer cells, inducing apoptosis. Obatoclax has been shown to induce cell death in a wide range of cancer cell lines in vitro, including non–small cell lung cancer, mantle cell lymphoma, multiple myeloma, acute myeloid leukemia, and melanoma (2–6). In animal toxicology studies, the major acute toxicities seen were transient and reversible neurobehavioral symptoms consisting of reduced activity, uncoordinated movements, tremors, and somnolence.
Results are reported here from two phase I, open-label, single-agent studies conducted to determine the dose and regimen of obatoclax to be studied in phase II trials in patients with solid tumors and lymphoma. Both studies had similar study designs, patient populations, and clinical evaluations. The initial study (GX001) was designed to evaluate weekly 1-hour infusions of obatoclax. However, clinically significant central nervous system (CNS) toxicity occurred at low doses. The second study (GX005) was designed to evaluate whether 3-hour infusions of obatoclax would be better tolerated.
Patients and Methods
Trial design
The two phase I, open-label, single-arm studies (GX001 and GX005) were approved by the institutional review boards at each participating medical center. All patients provided written informed consent before enrollment. The studies were conducted in accordance with the Declaration of Helsinki.
These dose-finding studies were designed to evaluate the initial safety and pharmacokinetics of i.v. obatoclax administered once weekly in patients with solid tumors or lymphoma. Obatoclax was administered using a 1-hour infusion duration in the initial study (GX001) and a 3-hour infusion duration in the second study (GX005). Patients continued treatment as long as obatoclax was well tolerated and there was no evidence of disease progression.
Patients
Eligible patients were required to have histologically or cytologically confirmed solid tumor or lymphoma and no appropriate standard therapy. There were no limitations on the duration or type of prior therapy, but acute toxicities from those therapies must have resolved to ≤grade 1. Eligibility requirements included age of at least 18 years, Eastern Cooperative Oncology Group performance status 0–1, and adequate hepatic and renal function [total bilirubin within normal institutional limits; aspartate aminotransferase/alanine aminotransferase ≤2.5 × the institutional upper limit of normal (ULN) or ≤5 × ULN in the presence of documented liver metastases; creatinine within ULN or calculated creatinine clearance ≥50 mL/min/1.73 m2).
Exclusion criteria included patients receiving any other therapies for cancer treatment, having brain metastases or history of seizure disorders, having a history of allergic reactions to polyethylene glycol 300 or polysorbate 20, or having an uncontrolled, intercurrent illness.
Dose escalation
In the GX001 study an accelerated dose titration design was used with single patient cohorts and doubling of the dose until >grade 1 treatment-related toxicity was observed. The initial starting dose in the GX001 study was based on the lethal dose for 10% of the population in rats (>12 mg/m2). The starting dose of obatoclax was 1.25 mg/m2. Subsequent cohorts were to include three to six patients with 40% dose increments between dose levels. A single intrapatient dose escalation was allowed after 8 weeks at the patient’s initial dose. In the GX005 study, a standard dose titration design was implemented with three to six patients entered at each dose level with 40% dose increments between levels. In the GX005 study, which utilized 3-hour infusions of obatoclax, the starting dose of obatoclax was 5.0 mg/m2 because doses of up to 7 mg/m2 over 1 hour had already been evaluated in the GX001 study. No intrapatient dose escalation was permitted in the GX005 study. In both studies, dose escalations between patient cohorts were to occur after ≥1 patient assigned to the prior dose level had been observed for 2 weeks and all other patients had been observed for at least 1 week.
Toxicities were graded based on the National Cancer Institute Common Toxicity Criteria, version 3.0. In light of the CNS symptoms seen in animal studies, the dose-limiting toxicity (DLT) definition included CNS symptoms. DLT was defined as ataxia or somnolence/depressed level of consciousness interfering with the ability to discharge the patient from the treatment center, grade 4 neutropenia of any duration with fever, grade 4 neutropenia without fever for at least 7 days, grade 4 thrombocytopenia or lymphocytopenia, or grade 3 or 4 nonhematologic toxicity not ameliorated by symptom-directed therapy. If at least two of three to six assessable patients at a dose level experienced a DLT, the dose escalation was stopped, and the prior dose level was considered the maximum tolerated dose (MTD) to be further evaluated in phase II studies.
Safety and efficacy measurements
Study visits were scheduled weekly during the treatment phase and 30 days after the last dose. Safety assessments, including physical and neurologic examinations, vital signs, performance status, hematology, urinalysis, serum chemistries, electrocardiograms, pulmonary function tests, and review of adverse events and concomitant medications, were done throughout the study.
Tumor evaluations were done every eight weeks. Response and progression were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST; ref. 7) for all patients.
Pharmacokinetic analyses
Blood samples for determination of plasma concentrations of obatoclax were collected on day 1 of weeks 1 and 5 in the GX001 study and on day 1 of weeks 1, 2, 3, 5, and 6 in the GX005 study.
Plasma concentrations of obatoclax were measured using a validated liquid chromatography with mass spectrometer method. The assay measures total obatoclax concentration and does not distinguish between free obatoclax and obatoclax bound to protein. Curves of obatoclax concentration versus time in plasma were constructed for each patient and analyzed using noncompartmental techniques. Peak concentration (Cmax) and corresponding time (Tmax) area under the concentration-versus-time curve through 24 hours (AUC0–24 hr), AUC through the last measurement (AUC0–∞), terminal half-life (t1/2), plasma clearance (Cl), and volume of distribution at steady state (Vss) were calculated.
Pharmacodynamic evaluation
As a result of cellular apoptosis, fragments of DNA are released into the circulation and levels of oligonucleosomal DNA can be measured. Plasma samples for determination of oligonucleosomal DNA concentrations were collected on days 1 to 8 in both the GX001 and the GX005 studies. The samples were analyzed using the Cell Death ELISA (Roche Applied Science; ref. 8).
Results
Patient population
Thirty-five patients were enrolled in the GX001 (n = 8) and GX005 (n = 27) studies between August 2004 and December 2006 (Table 1). The most common tumors were colon cancer and non-Hodgkin’s lymphoma, and all 35 patients had received prior chemotherapy. Enrolled patients had been heavily pretreated, receiving a median of 5 prior regimens (range, 1–12).
Table 1.
Baseline characteristics
| Characteristic statistic | GX001 (n = 8) | GX005 (n = 27) | All patients (n = 35) |
|---|---|---|---|
| Age (years) | |||
| Median | 63.5 | 59.0 | 60.0 |
| Range | 25–67 | 24–71 | 24–71 |
| Sex, male, n (%) | 4 (50) | 18 (67) | 22 (63) |
| Primary cancer, n (%) | |||
| Colon CA | 1 (13) | 4 (15) | 5 (14) |
| NHL | 1 (13) | 4 (15) | 5 (14) |
| Poorly differentiated adenocarcinoma | 1 (13) | 3 (11) | 4 (11) |
| Sarcoma | 2 (25) | 2 (7) | 4 (11) |
| Prostate CA | 1 (13) | 2 (7) | 3 (9) |
| Hodgkin’s lymphoma | 0 | 2 (7) | 2 (6) |
| Pancreatic CA | 0 | 2 (7) | 2 (6) |
| Other | 2* (25) | 8† (30) | 10 (29) |
| Patients with prior Chemotherapy | 8 (100.0) | 27 (100.0) | 35 (100.0) |
| No. of prior regimens | |||
| Median | 3.5 | 5.0 | 5.0 |
| Range | 1–10 | 1–12 | 1–12 |
Abbreviations: NHL, non-Hodgkin’s lymphoma; CA, cancer.
One each of cholangiocarcinoma and esophageal carcinoma.
One each of basal cell carcinoma, bladder cancer, duodenal carcinoma, hepatoma, small cell lung cancer, squamous cancer of nasopharynx, thymoma, and thyroid cancer.
Dose levels studied and toxicities observed
The dose levels evaluated, number of treatment weeks, dose escalations, and dose reductions are summarized in Table 2. The majority of patients (25 of 35, or 71%) received 4 to 8 weeks of therapy. Two patients in study GX005 received >24 weeks of therapy, including one patient with non-Hodgkin’s lymphoma at the 7.0 mg/m2 dose level who received 72 weeks of therapy. Dose reductions due to toxicity were required in 6 patients (17%), including 2 of 6 patients at the two highest dose levels administered over 1 hour and 4 of 18 patients at the three highest dose levels administered over 3 hours. Median duration of treatment for all 8 patients dosed on the 1-hour infusion schedule was 6.3 weeks and that for all 27 patients dosed on the 3-hour infusion schedule was 7.1 weeks.
Table 2.
Exposure to obatoclax by dose level
| Obatoclax infusion schedule and dose (mg/m2) | No. of patients | No. of treatment weeks (range) | Dose escalations* | Dose reductions |
|---|---|---|---|---|
| GX001, 1-hour infusion: | ||||
| 1.25 | 1 | 14 | 1.25→2.5 mg/m2 (n = 1) | No |
| 2.5 | 1 | 8 | No | No |
| 5.0 | 3 | 5–28 | No | 5→3.75 mg/m2 (n = 1) |
| 7.0 | 3 | 3–12 | No | 7→5.25 mg/m2 (n = 1) |
| GX005, 3-hour infusion: | ||||
| 5.0 | 3 | 4–8 | NA | No |
| 7.0 | 3 | 8–72 | NA | No |
| 10.0 | 3 | 8–20 | NA | No |
| 14.0 | 6 | 4–24 | NA | 14→10 mg/m2 (n = 2) |
| 20.0 | 6 | 4–8 | NA | No |
| 28.0 | 6 | 4–32 | NA | 28→21 mg/m2 (n = 2) |
Abbreviation: NA, not applicable.
Dose escalations were not permitted in study GX005.
The most common toxicity was infusion-related somnolence (91%), often accompanied by dizziness (60%) and/ or euphoria (57%; Table 3). Other neurologic symptoms included abnormal coordination (31%) and gait disturbance (26%). Neurologic symptoms, which were primarily grade 1 or 2 in severity, resolved promptly following the end of the infusion. There was no apparent difference in the incidence of these neurologic symptoms across obatoclax dose levels or infusion durations.
Table 3.
Clinical adverse events occurring in ≥15% of all patients by infusion schedule and dose level
| Event | GX001, 1-hour infusion, n (%)
|
GX-005, 3-hour infusion, n (%)
|
Overall total (n = 35) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obatoclax dose level
|
Obatoclax dose level
|
||||||||||||
| 1.25 (n = 1) | 2.5 (n = 1) | 5.0 (n = 3) | 7.0 (n = 3) | Total (n = 8) | 5.0 (n = 3) | 7.0 (n = 3) | 10.0 (n = 3) | 14.0 (n = 6) | 20.0 (n = 6) | 28.0 (n = 6) | Total (n = 27) | ||
| Somnolence | 1 (100) | 1 (100) | 3 (100) | 2 (67) | 7 (88) | 2 (67) | 3 (100) | 3 (100) | 6 (100) | 5 (83) | 6 (100) | 25 (93) | 32 (91) |
| Dizziness | 1 (100) | 0 | 67 | 1 (33) | 4 (50) | 0 | 2 (67) | 3 (100) | 5 (83) | 3 (50) | 4 (67) | 17 (63) | 21 (60) |
| Euphoric mood | 0 | 0 | 3 (100) | 1 (33) | 4 (50) | 0 | 2 (67) | 3 (100) | 5 (83) | 2 (33) | 4 (67) | 16 (59) | 20 (57) |
| Nausea | 1 (100) | 0 | 1 (33) | 1 (33) | 3 (38) | 1 (33) | 2 (67) | 2 (67) | 3 (50) | 3 (50) | 5 (83) | 16 (59) | 20 (54) |
| Fatigue | 1 (100) | 1 (100) | 2 (67) | 3 (100) | 7 (88) | 0 | 2 (67) | 1 (33) | 3 (50) | 2 (33) | 1 (17) | 9 (33) | 16 (46) |
| Vomiting | 1 (100) | 0 | 1 (33) | 1 (33) | 3 (38) | 1 (33) | 0 | 1 (33) | 3 (50) | 3 (50) | 4 (67) | 12 (44) | 15 (43) |
| Headache | 0 | 0 | 0 | 1 (33) | 1 (13) | 1 (33) | 2 (67) | 1 (33) | 4 (67) | 1 (17) | 5 (83) | 14 (52) | 15 (43) |
| Diarrhea | 0 | 0 | 1 (33) | 1 (33) | 2 (25) | 1 (33) | 2 (67) | 2 (67) | 1 (17) | 2 (33) | 1 (17) | 9 (33) | 11 (31) |
| Dyspnea | 0 | 0 | 1 (33) | 3 (100) | 4 (50) | 1 (33) | 33 | 0 | 2 (33) | 1 (17) | 2 (33) | 7 (26) | 11 (31) |
| Coordination abnormal | 0 | 0 | 0 | 1 (33) | 1 (13) | 0 | 0 | 1 (33) | 1 (17) | 3 (50) | 5 (83) | 10 (37) | 11 (31) |
| Cough | 0 | 0 | 0 | 1 (33) | 1 (13) | 0 | 2 (67) | 1 (33) | 2 (33) | 3 (50) | 2 (33) | 10 (37) | 11 (31) |
| Constipation | 0 | 0 | 0 | 2 (67) | 2 (25) | 1 (33) | 1 (33) | 2 (67) | 1 (17) | 1 (17) | 2 (33) | 8 (30) | 10 (29) |
| Abdominal pain | 0 | 0 | 0 | 3 (100) | 3 (38) | 0 | 1 (33) | 2 (67) | 3 (50) | 1 (17) | 0 | 7 (26) | 10 (29) |
| Back pain | 0 | 0 | 1 (33) | 0 | 1 (13) | 1 (33) | 0 | 2 (67) | 3 (50) | 2 (33) | 0 | 8 (30) | 9 (26) |
| Gait disturbance | 1 (100) | 0 | 0 | 0 | 1 (13) | 0 | 1 (33) | 1 (33) | 3 (50) | 1 (17) | 2 (33) | 8 (30) | 9 (26) |
| Arthralgia | 0 | 0 | 2 (67) | 1 (33) | 3 (38) | 0 | 1 (33) | 2 (67) | 1 (17) | 1 (17) | 0 | 5 (19) | 8 (23) |
| Anorexia | 1 (100) | 0 | 1 (33) | 67 | 4 (50) | 1 (33) | 0 | 1 (33) | 1 (17) | 1 (17) | 0 | 4 (15) | 8 (23) |
| Confusional state | 1 (100) | 0 | 0 | 0 | 1 (13) | 0 | 0 | 1 (33) | 1 (17) | 1 (17) | 3 (50) | 6 (22) | 7 (20) |
| Hyperhidrosis | 0 | 0 | 0 | 1 (33) | 1 (13) | 0 | 2 (67) | 1 (33) | 1 (17) | 0 | 2 (33) | 6 (22) | 7 (20) |
| Pyrexia | 1 (100) | 0 | 1 (33) | 0 | 2 (25) | 0 | 0 | 1 (33) | 0 | 1 (17) | 3 (50) | 5 (19) | 7 (17) |
| Chest pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33) | 3 (50) | 1 (17) | 1 (17) | 6 (22) | 7 (17) |
| Anemia | 0 | 1 (100) | 1 (33) | 1 (33) | 3 (38) | 0 | 1 (33) | 2 (67) | 0 | 0 | 0 | 3 (11) | 7 (17) |
| O2 saturation ↓ | 0 | 0 | 67 | 2 (67) | 4 (50) | 0 | 0 | 0 | 1 (17) | 1 (17) | 0 | 2 (7) | 7 (17) |
The most commonly reported toxicity of grade 3 or 4 severity was fatigue (4 patients, 11%). Although 86% of the reported events of somnolence and abnormal coordination were grade 1 and 2, grade 3 neurologic symptoms included somnolence and abnormal coordination, each in three patients (9%).
Grade 3 or 4 laboratory-based toxicities were uncommon. The most common abnormalities were lymphopenia and hyponatremia noted in 20% and 14% of patients, respectively. There was no clear dose relationship in the incidence of these laboratory findings.
Overall, 28 of 35 patients (80%) were withdrawn from the study due to disease progression, 4 patients withdrew consent for further treatment, and 3 withdrew due to toxicity.
DLT and MTD
Table 4 presents DLTs by infusion schedule and dose level. Using the 1-hour infusion schedule, the obatoclax MTD was 1.25 mg/m2. DLT events in the GX001 trial utilizing 1-hour infusions of obatoclax occurred both during and after the first 4 weeks of treatment, and the MTD determination was based on all DLT events. DLT was noted at the 2.5 mg/m2 dose level manifest as grade 3 somnolence occurring after previously tolerated doses of 1.25 and 2.5 mg/m2 in a patient who underwent dose escalation. Similarly at the 5.0 mg/m2 dose level, a DLT of somnolence was reported after previously tolerated doses at 5.0 mg/m2. One patient who received the 7.0 mg/m2 dose experienced a DLT at the second dose.
Table 4.
Dose-limiting toxicity by infusion schedule and dose level
| Obatoclax infusion schedule and dose (mg/m2) | Event | Dose no. | Duration (days) | Serious | Action with obatoclax |
|---|---|---|---|---|---|
| GX001, 1-hour infusion: | |||||
| 2.5* | Somnolence | 12 | <1 | No | None |
| 5.0 | Somnolence | 5 | <1 | No | Dose reduced |
| 7.0 | Coordination abnormal | 2 | <1 | No | Dose reduced |
| GX005, 3-hour infusion: | |||||
| 14.0 | Extrapyramidal disorder | 1 | <1 | No | Dose reduced |
| 28.0 | Somnolence | 1 | <1 | No | Dose reduced |
| Tonic clonic movements | NA† | <1 | Yes | NA | |
| 28.0 | Coordination abnormal | 3 | <1 | No | None |
| 28.0 | Coordination abnormal, dizziness | 11 | <1 | No | Dose reduced |
The patient’s initial dose was 1.25 mg/m2 which was increased to 2.5 mg/m2 prior to the occurrence of this event.
The event occurred 18 days posttreatment.
Extending the infusion schedule to 3 hours improved tolerability, and the obatoclax MTD in the second study (GX005) was 20 mg/m2. At the highest dose level tested (28 mg/m2) using the 3-hour infusion schedule, two of the six patients experienced DLTs (grade 3 neurologic symptoms) with the first dose. A third patient at 28 mg/m2 had a grade 3 infusional CNS event during cycle 3.
Pharmacokinetics
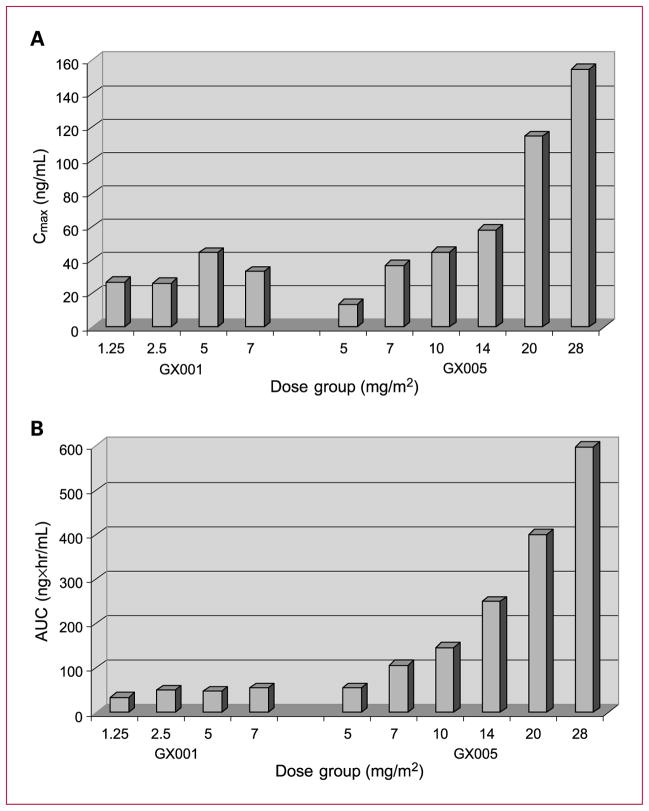
In protocol GX001, the pharmacokinetics were assessed at doses ranging from 1.25 to 7 mg/m2 administered weekly as a 1-hour infusion. The pharmacokinetics of obatoclax exhibited a triexponential concentration-time profile (Supplementary Table S1). A rapid decline of plasma obatoclax concentrations following the end of i.v. administration, with a mean half-life (t1/2α) of approximately 0.26 to 0.41 hour suggested an extensive protein binding or drug distribution occurring once obatoclax was injected into the systemic circulation. Obatoclax was eliminated out of the systemic circulation with a mean terminal half-life (t1/2z) of approximately 10.6 to 45.5 hours. The systemic exposure of obatoclax seemed to increase with dose, as determined by the estimated Cmax and AUC(0-∞) values over the dose range of 1.25 to 7 mg/m2 (Fig. 1); however, the exposure of obatoclax was less than dose proportional. Obatoclax administered weekly in the dose range of 1.25 to 7 mg/m2 resulted in only a slight accumulation of obatoclax in plasma. Both Cl and Vss were high (mean Cl values ranged from 46.3 to 135.2 L/h/m2; mean Vss values ranged from 221.1 to 1981.2 L/m2) and seemed to be dose dependent (Supplementary Table S1).
Fig. 1.
Mean Cmax and AUC by dose group in GX001 and GX005. A, mean Cmax by dose group in the GX001 and GX005 studies. B, mean AUC by dose group in the GX001 and GX005 studies.
In protocol GX005, the pharmacokinetic profile of obatoclax following a 3-hour infusion was characterized by an apparent triphasic decline following Cmax, with rapid distribution and slow elimination phases. The terminal elimination half-life was estimated at approximately 34 hours (Supplementary Table S2). Total body Cl was rapid and relatively constant across dose levels. The Vss prediction indicated rapid and thorough distribution that was fairly consistent across dose levels, which indicated linear kinetics. The increase in Cmax and AUC with dose was approximately linear (Fig. 1). Accumulation ratios generally indicated that there was no systemic accumulation of obatoclax following five weeks of dosing.
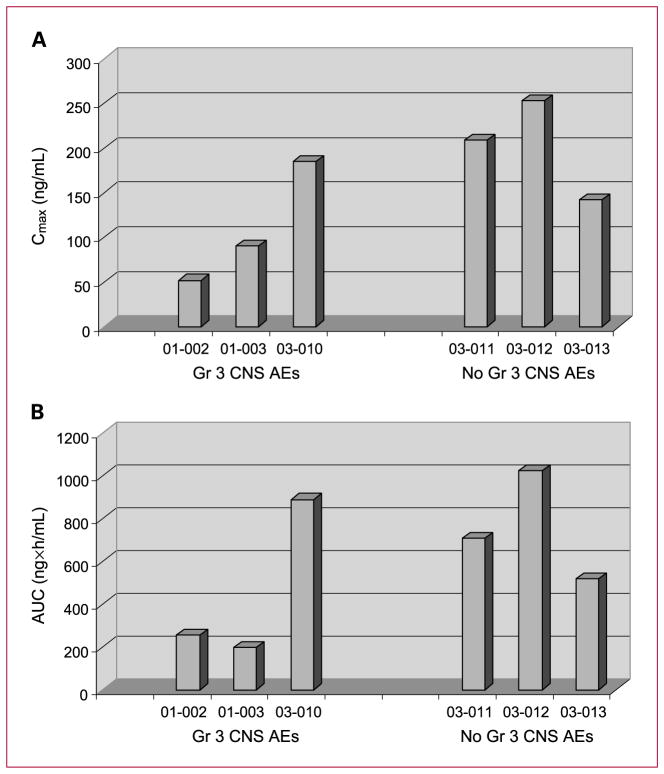
Of the six patients who received the maximum dose of 28 mg/m2 by 3-hour infusion, three had grade 3 infusional CNS adverse events during the study, two of which occurred during week 1. Pharmacokinetic data obtained during the first two weeks of treatment did not suggest a correlation between individual patient’s Cmax or AUC(0-∞) and the occurrence of infusional CNS adverse events (Fig. 2).
Fig. 2.
Cmax, AUC, and CNS adverse events (AEs) in patients receiving 28 mg/m2. A, Cmax and CNS adverse events in patients receiving 28 mg/m2. B, AUC and CNS adverse events in patients receiving 28 mg/m2.
Clinical efficacy
In the GX001 study, best response to treatment based on RECIST was stable disease observed in 2 of 8 (25%) patients. In the GX005 study, best response on treatment was partial response achieved in one patient with stage IV large cell lymphoma in the 28 mg/m2 dose group who received 32 weeks of therapy (all but the first dose at a reduced dose of 21 mg/m2). Duration of the partial response was two months. Five patients had confirmed stable disease as best response on study, with prolonged stabilization of disease observed in one patient with stage IV large cell lymphoma who had stable disease through 72 weeks of therapy at a dose of 7 mg/m2.
Pharmacodynamic evaluation
Levels of oligonucleosomal DNA were evaluated at all dose levels in studies GX001 and GX005. However, there was no clear increase of oligonucleosomal DNA with increasing doses of obatoclax. Oligonucleosomal DNA levels increased above baseline values within 48 hours in all six patients in the highest dosage group (28 mg/m2), but neither the absolute value nor the fold-increase over baseline was higher in the patient who achieved a partial response than in the other patients at the 28 mg/m2 dose level (data not shown).
Discussion
Study GX001 was the first clinical study conducted with obatoclax. The study evaluated the initial safety and pharmacokinetic profile of obatoclax administered as a 60-minute infusion once weekly to patients with solid tumors. The study was terminated early after eight patients were enrolled in four dose cohorts at dose levels ranging from 1.25 to 7.0 mg/m2. Early termination occurred because of infusional DLTs (somnolence and abnormal coordination) following multiple infusions at the same dose that was previously well tolerated.
Because animal studies had suggested that extending the infusion time would decrease the CNS symptoms associated with the obatoclax infusion, a longer infusion time was evaluated. Study GX005, in which obatoclax was administered as a 3-hour infusion once weekly in patients with solid tumors, showed that CNS symptoms were diminished by prolonging the infusion, leading to escalation to higher doses of obatoclax. A total of 27 patients were enrolled with dose escalation proceeding from 5 to 28 mg/m2. At the highest dose level evaluated (28 mg/m2), two of six patients experienced DLTs with the first dose and the 20 mg/m2 dose level was declared the MTD at this schedule. All DLTs were neuropsychiatric events.
Preclinical studies revealed that test animals experienced transient and reversible neurobehavioral signs. These symptoms were expected to be observed in humans, and infusion of obatoclax over 1 and 3 hours was associated with neurologic and psychiatric events, primarily reports of somnolence (91%), dizziness (60%), euphoric mood (57%), headache (43%), and abnormal coordination (31%). Most events were grade 1 or 2 in severity and were transient with resolution that same day. Patients who experienced these types of events with the first dose tended to experience the events following subsequent doses. Interestingly, these events were not reported with every dose in a patient who had previously experienced the events.
In both animals and humans, the clinical symptoms during infusion indicate that obatoclax crosses the blood-brain barrier. The BCL-2 antiapoptotic protein BCL-XL is highly expressed in CNS neurons, suggesting that CNS symptoms may be due to target effects. Another BCL-2 family antagonist, ABT-737, has been shown to alter the recovery of synaptic responses in vitro after repetitive synaptic activity (9), but ABT-737 is too large to cross the blood-brain barrier. Thus, it is possible that the CNS effects of obatoclax may be target effects. Laboratory evaluation of the direct effects of obatoclax on synaptic responses of CNS neurons is in progress.
The pharmacokinetic profile of obatoclax when administered as a 3-hour i.v. infusion was characterized by a rapid distribution phase and a slow terminal elimination phase. The majority of obatoclax was eliminated from the plasma by 4 to 5 hours after the end of infusion. The increase in Cmax and AUC with dose was approximately linear. There was no systemic accumulation of obatoclax following 2 weeks of dosing. At the 28 mg/m2 dose level, pharmacokinetic data did not suggest a correlation between exposure and CNS adverse events. Nevertheless CNS symptoms are clearly related to overall levels of exposure, as grade 3 infusional CNS adverse events occur only in higher-dosage groups.
At doses up to 28 mg/m2 administered over 3 hours once weekly, best response on treatment based on the investigator’s assessment was a partial response reported in one patient with stage IV lymphoma who had received multiple prior therapies. Activity was also observed in one patient with stage IV lymphoma who had received multiple prior therapies and who experienced prolonged stable disease during 72 weeks of therapy.
In another study of obatoclax in patients with chronic lymphocytic leukemia (CLL; ref. 10), the peak plasma concentration of oligonucleosomal DNA increased with dose. In this study, largely in patients with colon cancer and non-Hodgkin’s lymphoma, correlations between dose or response and levels of oligonucleosomal DNA were not apparent. This suggests that differences in the plasma levels of oligonucleosomal DNA released after obatoclax treatment may depend on tumor type, or, alternatively, that obatoclax may have been more effective in inducing apoptosis in patients with CLL than in patients with colon cancer and non-Hodgkin’s lymphoma.
One previously published phase I study (10) evaluated obatoclax administered as both 1-hour and 3-hour i.v. infusions in patients with advanced CLL. In that study 1-hour infusions were also found to be associated with CNS adverse events, and 3-hour infusions were better tolerated. The MTD for 3-hour infusions was 28 mg/m2, slightly higher than the MTD reached in patients with solid tumors and lymphomas in this study. Another previously published phase I obatoclax study (11) evaluated obatoclax administered by 24-hour infusion. CNS adverse events were less prominent than with shorter infusions, as indicated by escalation to 28 mg/m2, the highest dose planned, without DLT.
In conclusion, the 1-hour infusion schedule of obatoclax was associated with neuropsychiatric DLTs at relatively low doses with an MTD of 1.25 mg/m2. The MTD of obatoclax administered using a 3-hour i.v. infusion once weekly to patients with solid tumors was 20 mg/m2. Evidence of clinical activity was observed in two patients with stage IV lymphoma who experienced partial response and prolonged stabilization of their disease, respectively. Further studies with this promising agent should be done in both lymphomas and solid tumors at a weekly dose of 20 mg/m2 administered i.v. over 3 hours.
Supplementary Material
Translational Relevance.
Overexpression of the antiapoptotic protein BCL-2 and related family members, which inactivate the apoptotic pathway, is a common finding in many types of cancer. Blocking proapoptotic signaling leads to resistance to cancer therapies, such as radiation and cytotoxic chemotherapy. Thus, antagonists of all the BCL-2 antiapoptotic proteins are likely to be active across a wide range of cancers and would be expected to contribute to the reversal of resistance to cancer therapies. Obatoclax (GX15-070, Gemin X Pharmaceuticals) is a small-molecule antagonist of all of the antiapoptotic BCL-2 family members. The two phase I, single-agent studies presented here were the first phase I studies to evaluate the safety and tolerability of weekly 1-hour and 3-hour infusions of obatoclax. These data support the use of the 3-hour infusion schedule of obatoclax in additional clinical studies that will evaluate how best to use this promising agent.
Acknowledgments
This study was supported by Gemin X Pharmaceuticals, Malvern, PA. 19355.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Presented in part at 2006 Meeting of the American Society of Clinical Oncology.
Disclosure of Potential Conflicts of Interest
J.L. Marshall: honoraria from speakers bureau and ownership interest/ patents, Roche, Genentech, and Amgen.
References
- 1.Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15–070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61:525–34. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Galán P, Roué G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15–070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–9. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 4.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Pre-clinical studies of the pan-Bcl inhibitor obatoclax (GX015–070) in multiple myeloma. Blood. 2007;109:5430–8. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 5.Konopleva M, Watt J, Contractor R, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15–070 (obatoclax) Cancer Res. 2008;68:3413–20. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang CC, Wroblewski D, Yang F, Hersey P, Zhang XD. Human melanoma cells under endoplasmic reticulum stress are more susceptible to apoptosis induced by the BH3 mimetic obatoclax. Neoplasia. 2009;9:945–55. doi: 10.1593/neo.09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Holdenrieder S, Stieber P, Bodenmuller H, et al. Nucleosomes in serum as a marker for cell death. Clin Chem Lab Med. 2001;39:596–605. doi: 10.1515/CCLM.2001.095. [DOI] [PubMed] [Google Scholar]
- 9.Hickman FA, Hardwick JM, Kaczmarek LK, Jonas EA. Bcl-XL inhibitor ABT-737 reveals a dual role for Bcl- XL in synaptic transmission. J Neurophysiol. 2008;99:1515–22. doi: 10.1152/jn.00598.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien SM, Claxton DF, Crump M, et al. Phase I study of obatoclax mesylate (GX15–070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schimmer AQD, O’Brien S, Kantarjian H, et al. A Phase I study of the pan Bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2009;14:8295–301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.