Abstract
Aberrant histone lysine methylation that is controlled by histone lysine methyltransferases (KMTs) and demethylases (KDMs) plays significant roles in carcinogenesis. Infections by tumor viruses or parasites and exposures to chemical carcinogens can modify the process of histone lysine methylation. Many KMTs and KDMs contribute to malignant transformation by regulating the expression of human telomerase reverse transcriptase (hTERT), forming a fused gene, interacting with proto-oncogenes or being up-regulated in cancer cells. In addition, histone lysine methylation participates in tumor suppressor gene inactivation during the early stages of carcinogenesis by regulating DNA methylation and/or by other DNA methylation independent mechanisms. Furthermore, recent genetic discoveries of many mutations in KMTs and KDMs in various types of cancers highlight their numerous roles in carcinogenesis and provide rare opportunities for selective and tumor-specific targeting of these enzymes. The study on global histone lysine methylation levels may also offer specific biomarkers for cancer detection, diagnosis and prognosis, as well as for genotoxic and non-genotoxic carcinogenic exposures and risk assessment. This review summarizes the role of histone lysine methylation in the process of cellular transformation and carcinogenesis, genetic alterations of KMTs and KDMs in different cancers and recent progress in discovery of small molecule inhibitors of these enzymes.
Keywords: Histone lysine-specific methyltransferases, histone lysine-specific demethylases, cell transformation, carcinogenesis, gene mutations, inhibitors
1. INTRODUCTION
Epigenetic alterations have been shown to play a fundamental role during the process of carcinogenesis and thus epigenetic regulatory enzymes are increasingly recognized to be important targets for cancer prevention and therapy. DNA methylation and histone modifications are the two major epigenetic modifications involved in transcriptional regulation. DNA methylation is mediated by a group of DNA methyltransferase (DNMTs), while covalent histone modifications are more complex and controlled by diversified groups of enzymes that regulate histone methylation, acetylation, ubiquitylation, phosphorylation and sumoylation [1, 2]. So far, four drugs, including DNMT inhibitors 5-azacytidine (Vidaza) and decitabine (20-deoxy-5-azacytidine, Dacogen) and the histone deacetylase (HDAC) inhibitors suberoylanilide hydroxamic acid (SAHA, Zolinza) and romidepsin (Istodax) have been approved by the FDA for treatment of cancer [4–6]. However, these DNMT inhibitors (e. g. 5-azacytidine and 20-deoxy-5-azacytidine) are nucleoside analogs and have low specificity to DNMTs and tumor cells, whereas histone deacetylases have broad substrate specificity including many non-histone proteins that are not involved in epigenetic regulation [7, 8]. As such, there are currently no established epigenetic biomarkers that can accurately predict patients’ responses to DNMT and HDAC inhibitors in the clinic. Therefore, the “proof of principle” of “epigenetic therapies” using DNMT and HDAC inhibitors for treating cancer, as well as their tumor specificity, remains to be established. To support the potential effectiveness of epigenetic therapies, there is urgent need to identify novel and more specific targets of epigenetic regulation and their inhibitors.
In contrast to HDACs and DNMTs that globally regulate gene expression across different types of cells, recent studies have indicated that histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs) may alter gene expression that was specific to particular normal and cancer cell types [9, 10]. In addition, genetic alterations in KMTs and KDMs such as chromosomal translocations, gene mutations, fusion proteins, and resultant aberrant expression, are frequently observed in cancer [11–16]. These genetic alterations in KMTs and KDMs have recently been linked to their oncogenic properties via and loss of tumor suppressing functions, as well as linked to the developmental plasticity of cancer cells [11–16]. Moreover, infections by tumor viruses or parasites and exposure to carcinogens affect the levels of histone lysine methylation and the associated patterns of gene expression, which leads to tumorigenic transformation [17, 18]. Taken together, these observations support that targeting abberant KMTs and KDMs in cancer may achieve a higher degree of specificity in epigenetic therapy and accomplish prevention by blocking tumor specific epigenetic alterations or mutations. It is also conceivable that genetic alterations in KMTs and KDMs could serve as patient stratification biomarkers for future potential treatment with specific inhibitors of KMTs and KDMs. In this review, we summarize functional roles of KMTs and KDMs in cellular transformation and carcinogenesis and their genetic alterations in cancers, as well as the inhibitors of KMTs and KDMs. We will also discuss the challenges and opportunities for developing personalized medicine by targeting histone lysine methylation in appropriate patients.
2. HISTONE LYSINE METHYLTRANSFERASES AND DEMETHYLASES (KMTS AND KDMS)
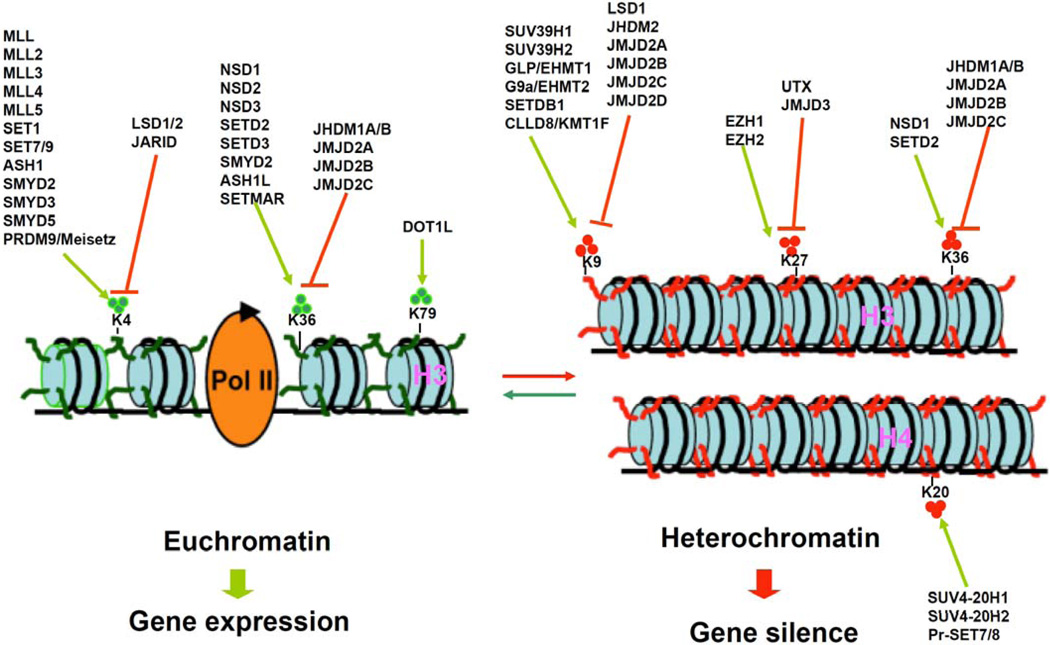
So far, there are more than 50 human KMTs and 30 KDMs that have been identified [19, 20]. KMTs catalyze the transfer of one to three methyl groups from S-adenosylmethionine (SAM) to specific lysine residues on histones. H3K4, H3K9, H3K27, H3K36, H3K79 and H4K20 are most commonly reported lysine residues which can become mono-, di-, or trimethylated. According to recent findings, H3K9, H3K27, and H4K20 methylation is associated mainly with repressed transcription, whereas methylation of H3K4 and H3K36 is associated with activated transcription [21]. The classification of KMTs and KDMs and mechanisms of histone lysine methylation are summarized as (Figs.1 and 2).
Fig 1.
Schematic presentation of histone lysine methylation and demethylation regulated by methyltransferases and demethylases for gene transcription. Currently known histone H3 and H4 lysine methyltrasferases (green arrows) and demethylases (red arrows). In general, methylation of lysines H3K4, H3K36, and H3K79 is associated with euchromatin and transcriptional activation, whereas methylation of lysines H3K9 and H3K27and H4K20 is related to heterochromatin and transcriptional repression. Trimethylation of H3K36 is also thought to be correlated with transcriptional repression.
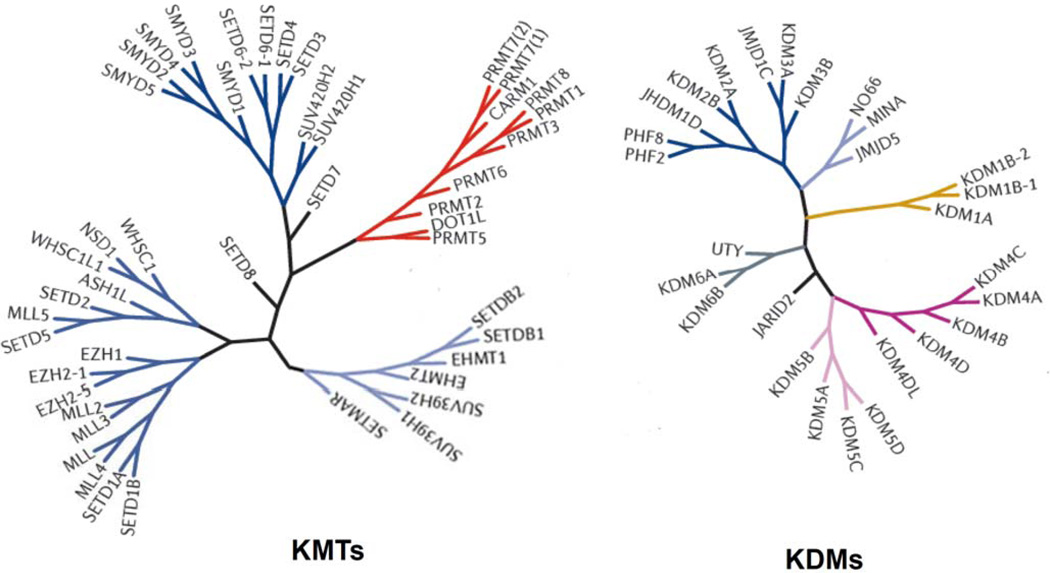
Fig 2.
Histone lysine methyltransferase (KMT) and demethylase (KDM) family tree diagram. KMTs and KDMs are clustered on branches on the basis of the similarity of their amino acid sequences.
Based on the sequence and structure of their catalytic domain, KMTs can be classified into two families: DOT1 like (DOT1L) and SET-domain-containing lysine methyltransferases [22]. Here we discuss five groups of KMTs that target different histone lysine marks. They have been reported to be current or potential drug targets in cancers and include: 1. Mixed-lineage leukemia gene 1 (MLL1)/KMT2A and SET and MYND domain containing protein 3 (SMYD3 /KMT3E) (H3K4 me); 2. Variegation 3–9 homolog 1and 2 (SUV39H1 and 2)/KMT1A/B and G9a/KMT1C (H3K9 me); 3. EZH2/KMT6A (H3K27 me); 4. Nuclear receptor-binding SET domain protein 2 (NSD2)/MMSET/WHSC1 and SMYD2 (H3K36 me); 5. DOT1L/KMT4 (H3K79 me).
Lysine-specific demethylase 1 (LSD1) was the first discovered KDM which revealed that the process of histone lysine methylation is reversible [23]. Up to now, there are two major families of KDMs that have been identified [24]. LSD1 belongs to KDM1 family that includes two members so far: KDM1A/LSD1 and KDM1B/LSD2. The LSD1 demethylase family removes a single methyl group via an amine oxidation process in the presence of a FAD cofactor. Because the amine oxidation process requires a protonated nitrogen at the ε-amino group of lysine, LSD1 cannot remove a trimethyl group from the methylated lysine. The second KDM family is Jumonji C (JmjC) domain containing protein family, which catalyzes the hydroxylation of a lysine methyl group via an α-keto-glutarate and Fe(II)-ion dependent reaction. There are seven subgroups in JmjC family with a total of 14 KDMs (KDM2A/B, KDM3A/B, KDM4A–D, KDM5A–D and KDM6A/B). Aberrant regulation of KDMs is also involved in cancer progression, however they have been much less extensively studied than KMTs. KDMs and KMTs work coordinately to maintain normal global histone lysine methylation levels and then regulate gene expression patterns.
3. HISTONE LYSINE METHYLATION IN CELL IMMORTALIZATION AND TRANSFORMATION
The process of neoplasia begins with cell transformation. Virus infection (e.g. human papilloma virus, Epstein-Barr virus, etc.), transfection with oncogenes (e.g. mutant Ras), and exposure to ionizing radiation or chemical carcinogens (e.g. cigarette smoke, nickel, arsenic, and chromium) can initiate and/or promote the process of cell transformation, leading to uncontrolled cell growth. The predominant mechanism for carcinogen induced cell transformation is currently associated with genetic damage with DNA alterations leading to point mutations of genes, translocations of genetic material between chromosomes, and gene duplication with amplification. However, the transition from normal cells to transformed cells requires an extensive reconfiguration of the genome’s expression program. The chain of events from carcinogen exposure to cell transformation is still largely unknown. Emerging evidence indicates that epigenetic mechanisms, including histone lysine methylation, are also involved in the process of cellular transformation or oncogenic reprogramming.
3.1. Infection, Carcinogen Exposure and Histone Lysine Methylation
In addition, infection by oncogenic viruses or Theileria parasites, or exposure to carcinogens, such as 2-amino-1-methyl-6-phenylimidazo [4, 5 β] pyridine (PhIP), 4-aminobiphenyl (4-ABP), nickel, chromate, and arsenite, can alter the expression of histone lysine methyltransferases in normal cells, leading to cellular transformation [17, 25–33]. Anderton et al [26] showed that oncogenic Epstein-Barr virus infection induced the expression of the H3K27me3 demethylase, KDM6B, in primary B cells. Hyland et al [27] reported that there were elevated levels of EZH2/KMT6A and KDM6A in human papillomavirus type 16 E6/E7-transformed keratinocytes. SMYD3/KMT3E dimethylates and trimethylates H3K4. SMYD3 has been shown to be the only upregulated histone methyltransferase upon Theileria infection and is associated with trimethylation of H3K4 at the MMP-9 promoter [17].
Bradley et al. [29] showed that carcinogens PhIP and 4-ABP increased nuclear localization of LSD1 and reduced mono-methylation of H3K4 in human mammary epithelial cells (HMECs). Costa and his associates [30–33] published a series of papers, showing that nickel, chromate, and arsenite increased di- and tri-methylated H3K4 and di-methylated H3K9, while arsenite decreased mono-methyl H3K4. The increase of H3K9me2 by arsenite is associated with increased expression of G9a/ KMT1C protein and mRNA [32]. In addition, nickel has been shown to inhibit JMJD1A/KDM3A activity [33, 34]. These results argue that KMTs and KDMs may be potential targets for oncogenic infections- and chemical carcinogen- mediated transformation. Potential mechanisms of immortalization and transformation that are associated with altered expression of KMTs and KDMs are discussed below.
3.2. Cell Immortalization
Activation of telomerase is essential for cellular immortalization and malignant transformation. The lack of telomerase activity in most normal cells is mainly due to the stable repression of the telomerase catalytic subunit, encoded by the human telomerase reverse transcriptase (hTERT). Atkinson et al. [35] reported that highly trimethylated H3-K4 was associated with active transcription of the hTERT gene in telomerase-proficient tumor cells. The hTERT is a direct target gene of a H3K4 demethylase SMYD3 [36]. Overexpression of SMYD3 in non-transformed cells results in the induction of hTERT expression and the increased cell proliferation and colony formation, whereas down-regulation of SMYD3 expression by small interference RNA (siRNA) leads to growth inhibition or apoptosis of colorectal carcinoma and hepatocellular carcinoma cells [36, 37]. Inhibition of LSD1by a pharmacological inhibitor or siRNA also induces the expression of hTERT in both normal human fibroblasts and cancer cells due to increased binding of LSD1 to hTERT proximal promoter and induction of dimethylated H3K4 [38]. Not dead yet (Ndy)-1/KDM2B and Ndy-2/KDM2A have Jumonji C-dependent histone H3K36 dimethyldemthylase or H3K4 trimethyl-demethylase activities. Pfau et al. [39] showed that overexpression of Ndy1 or 2 promoted the immortalization of mouse embryo fibroblasts (MEFs) in the absence of replicative senescence, and that knockdown of Ndy1 and expression of dominant-negative Ndy1 mutants induced senescence. The Ndy1 induced immortalization is dependent on JmjC domain and JmjC domain-mediated histone demethylation [40]. Taken together, these results highlight a potential mechanism of epigenetic control and modulation of the hTERT promoter via histone lysine methylation during the process of cellular immortalization.
3.3. Cellular Transformation
Histone methyltransferases can form a fusion partner gene or interact with proto-oncogenes or other methyltransferases to immortalize normal cells and transform non-malignant cells. The MLL1/KMT2A that encodes a H3K4 methyltransferase is frequently translocated in leukemia [41]. Due to chromosome translocation, the MLL1 N terminus can be ‘fused’ to the C terminus of over 50 different partners, leading to the loss of the H3K4 methyltransferase domain [41]. Many MLL1 fusion partners result in leukemic transformation by the involvement of transcriptional regulation through chromatin remodeling. The MLL1 fusion partners AF4, AF5, AF9, AF10 and ENL interact with the Dot1-like protein (DOT1L)/KMT4 that methylates H3K79 [41]. In addition, indirect interaction with DOT1L/KMT4 may be possible through common binding proteins, which has been documented between histone sites and many other MLL1 fusion partners including ABI1, EEN, EPS15 and ELL [41]. In the absence of DOT1L/KMT4, MLL1–AF10 has been shown to be unable to transform haematopoietic progenitors [42, 43]. Daigle et al. reported that EPZ004777, a potent and selective inhibitor of DOT1L/KMT4, selectively killed MLL1 rearranged MV4–11 and MOLM-13 cells by blocking cellular H3K79 methylation and inhibiting leukemogenic gene (e.g. Homeobox A9, HOX A9) expression [44]. These results suggest that DOT1L/ KMT4 has emerged as an important mediator of MLL1-fusion-mediated leukemic transformation, and acts as a “driver” target for treatment of MLL1 rearranged leukemia. Although cancer cells carry multiple genetic and epigenetic abnormalities, they can be highly dependent on the activity of a single oncogene for continued cell proliferation and survival. This phenomenon is called “oncogene addiction” [45]. Therefore, identification of the state of oncogene addiction, i.e. the 'Achilles' heel,' due to genetic alterations of KMTs and KDMs would be important for development of effective targeted therapy in specific cancers.
Nuclear SET domain containing protein 2 (NSD2) alone has been shown to transform p19ARF−/− MEFs in an H3K36me2-dependent manner [46]. In addition, the expression of catalytically active NSD2 in t(4;14)-negative myeloma cells can form xenograft tumors in nude mice [46]. Mutant NSD1 with inactivation of H3K36 methyltransferase activity inhibits myeloid progenitor immortalization via HOX A gene activation [47]. Ecotropic viral integration site 1(EVI1) is an oncoprotein aberrantly expressed in acute myeloid leukemia and myelodysplastic syndrome cells [48]. SUV39H1/KMT1A or G9a/ KMT1C can interact with EVI1 leading to the enhancement of EVI1 transcriptional repression [48]. Mulligan et al. reported that knockdown of G9a by siRNA induced transformation of HMECs expressing hTERT and SV40 Large T [49]. EZH2/KMT6A that catalyzes H3K27 methylation can transform the growth of a normal prostate epithelial cell line both in vitro and in vivo, and that siRNA knockdown of EZH2/KMT6A inhibits the proliferation of human papillomavirus positive cancer cells [50, 51]. These results suggest that EZH2/KMT6A also has oncogenic properties. A model that transformation is driven by increased H3K27 methylation as a consequence of EZH2/KMT6A overactivity has therefore been proposed recently [50, 51].
Jhdm1b/KDM2b encoding a Jumonji domain family H3K36me2 and H3K4me3 demethylase is first identified as a hotspot for proviral insertion in murine tumors generated by random mutagenesis of Moloney murine leukemia virus, and ectopic expression of Jhdm1b/KDM2b is sufficient to transform hematopoietic progenitors [52]. WHSC1L1/NSD3 has been found to be one of the most potently transforming oncogenes based on the number of altered phenotypes expressed by the cells. Conversely, knockdown of WHSC1L1/NSD3 in 8p11–12-amplified breast cancer cells results in cell growth inhibition [53].
This body of accumulated evidence shows that histone lysine methylation plays an essential role in the process of cellular transformation via oncogenic reprogramming. This observation suggests that genetic alterations of KMTs and of KDMs may drive the process of oncogenesis in a variety of cirumstances and that the KMTs and KDMs are important targets for cancer prevention and treatment.
4. HISTONE LYSINE METHYLATION AND DNA METHYLATION IN CARCINOGENESIS
Cancer prevention and therapy can be greatly improved by detection at its early stages. Epigenetic alterations, including DNA and histone lysine methylation, are detectable in the broad regions of normal tissues during the early stages of carcinogenesis before the initiation of tumorigenesis.
4.1. Carcinogen Induced Histone Methylation in Experimental Animals
Carcinogens, like N-methyl-N-nitrosurea (MNU), have been shown to modify amino acids and lead to predominantly methylated lysine and arginine residues in H3 histone proteins [54, 55]. In a well-established 17β-estradiol-induced mammary carcinogenesis in female August Copenhagen Irish (ACI) rats, epigenetic changes, including an increase in H3K9 and H3K27 trimethylation and de novo CpG island methylation at the Rassf1a promoter, as well as loss of global DNA methylation and LINE-1 hypomethylation and H4K20 tirmethylation, have been found in the mammary gland tissue as early as 2 days after exposure to 17β-estradiol [56]. In tamoxifen-induced hepatocarcinogenesis, female Fisher 344 rats fed with tamoxifen for different numbers of weeks showed that global DNA hypomethylation is increased and de novo DNA methytransferases DNMT3a and DNMT3b, as well as H4K20 trimethylation, are progressively decreased in liver at all-time points compared to non-target tissues (i.e. mammary gland, pancreas and spleen) [57, 58]. In 2-acetylaminofluorene (2-AAF) -induced preneoplastic livers of rats, increased H3K9 and H3K27 trimethylation in the promoter regions of Rassf1a, p16(INK4a), Socs1, Cdh1, and Cx26 tumor suppressor genes, and early Rassf1a and p16(INK4a) promoter CpG island hypermethylation, as well as a decrease of global and LINE-1-associated H4K20 trimethylation with time also are detected [59, 60]. In the methyl-deficient model of endogenous hepatocarcinogenesis in rats, the methyl-deficient diet results in a progressive loss of H4K20 and H3K9 trimethylation, which is accompanied by a decreased expression of Suv4–20h2/KMT5C and RIZ1/KMT8 and an increased expression of Suv39-h1/KMT1A/B in liver tumors [61]. These observations indicate that carcinogen-induced changes in histone methylation preceed known promotion mechanisms and phenotypic alterations during the process of carcinogenesis.
4.2. Histone Lysine Methylation and Gene Silencing
DNA methylation is associated with histone modifications. The functional link between patterns of DNA methylation and histone methylation is first demonstrated by the observation that DNMT1 inhibitor 5-aza-2’-deoxycytidine (5-AzadCyD, 5-Aza-CdR, decitabine) treatment of cancer cells leads to depletion of DNA methylation, a loss of H3K9 methylation, and a corresponding increase in H3K4 methylation [62, 63]. DNA methylation patterns are established and maintained by three DNA methyltransferases: DNMT3a, DNMT3b, and DNMT1. It has been shown that DNMT3a and its accessory protein DNMT3L contain an H3K4me0-interacting ATRX-Dnmt3-Dnmt3L (ADD) domain and H3K4 methyltransferase MLL1 contains a CpG-interacting Cys-X-X-Cys (CXXC) domain [64]. The interactions through these protein domains may couple H3K4 methylation reaction to unmethylated DNA [64]. In addition, H3K9 methyltransferase SETDB1 contains a putative methyl-CpG-binding domain (MBD) that also potentially links H3K4 methylation reaction to unmethylated DNA [64]. Ubiquitin-like with PHD and RING finger domains 1 (UHRF1) plays a role in maintaining DNA methylation in mammalian cells by targeting DNMT1 to DNA replication foci [65]. UHRF1 has been found to bind hemimethylated DNA, H3K9me3, and G9a/KMT1C [65]. In addition, persistent transcriptional repressive histone modifications, such as H3K9me3 and H3K27me3, are associated with DNMT1-mediated DNA methylation recovery after DNMT1 inhibitor treatment [66]. Furthermore, targeted deletion of LSD1 in embryonic stem cells results in a progressive loss of DNA methylation via degradation of DNMT1 protein [67].
Bivalent chromatin structures that are present with both activating H3K4 trimethylation and repressive H3K27 trimethylation [68] have also been identified in human tumors. Bivalent chromatin structures signify an important molecular characteristic of embryonic stem cells. In addition, genes affected by de novo DNA methylation during tumorigenesis are pre-marked by H3K27 trimethylation, suggesting an instructive role of H3K27 trimethylation in the establishment of cancer specific DNA methylation patterns [69–71]. The relationship between H3K27 trimethylation and de novo DNA methylation therefore may reflect the presence of a stem cell-like epigenetic program in cancer cells [72].
However, enriched H3K27 trimethylation at promoter regions can also lead to a tumor suppressor silencing mechanism that is independent of DNA methylation. Vallot et al. [73] have recently shown that multiple regional epigenetic silencing (MRES) is associated with progression of carcinoma in situ in bladder. MRES occurs not through DNA methylation, but via histone H3K9 and H3K27 methylation and histone H3K9 hypoacetylation. Taken together, these results indicate that histone lysine methylation may play a crucial role in tumor suppressor gene inactivation during the early stages of carcinogenesis via DNA methylation-dependent and /or -independent mechanisms.
5. ALTERNATIONS IN CANCER
Deregulation of KMTs and KDMs and their signaling networks in cancer can occur through several mechanisms that include gene mutations, amplification, translocations and aberrant expression. Understanding of these mechanisms will be helpful in identifying cancer specific targets and developing more effective epigenetic therapies. In this regard, we will summarize alterations of KMTs and KDMs in cancer classified by underlying mechanisms as Table 1.
Table 1.
Alterations of KMTs and KDMs in cancer
| Enzymes | Genetic Alteration | Substrates | Function | Cancer |
|---|---|---|---|---|
|
KMT2C MLL3 |
Mutations and deletion [93, 100, 101] |
H3K4me1, me2, and me3 |
A co-activator complex of nuclear receptors |
Medulloblastoma, Opisthorchis viverrini– related cholangio-carcinoma, acute myeloid leukemia, glioblastoma, melanoma, and colorectal, pancreatic, lung, bladder and breast cancers [93, 100, 101]. |
|
KMT2D MLL2 |
Recurrent mutations, duplications and trans- locations [93, 95, 98–100] |
H3K4me3 | A coactivator for Estrogen Receptor (ER) α [195] |
Medulloblastoma, non-Hodgkin lymphoma, multiple myeloma, renal cell carcinoma, lung cancer, prostate cancer [93, 95, 98–100] |
|
KMT3A SETD2 |
truncating or missense mutations [94, 103–106] |
H3K36me3 | Transcription activation, and binds to the promoters of adenovirus 12 E1A gene |
Early T-cell precursor acute lymphoblastic leukaemia, clear cell renal carcinoma [94, 103–106] |
|
KMT3B NSD1 |
Mutations, fuse to NUP98, microdeletion, and minimal common regions of gain [107] |
H3K36me1, me2, and me3 |
Transcriptional intermediary factor | Carcinoma of the upper aero-digestive tract, acute myeloid leukemia, ganglioglioma, lung adenocarcinoma in never smokers, acute lymphoblastic leukemia, neuroblastoma, and Wilms tumor [107]. |
|
KMT6A EZH2 |
Mutations and overexpression [74–90] |
H3K27me1, me2, and me3 |
Repressing expression of HOXC8, HOXA9, MYT1, CDKN2A and retinoic acid target genes |
A wide variety of cancers [74–90] |
|
KMT8 PRDM2 /RIZ1 |
Inactivating mutation and deletion, silencing [194–197] |
H3K9me3 | An ER co-activator [198 | hepatocellular carcinoma, oral squamous cell carcinomas, prostate cancer, breast cancer cells [194–197] |
|
KDM3B JMJD1B |
cryptic deletion or over- expression[105] |
H3K9me1 and me2 |
Synergistic interaction with CBP, inducing leukemogenic oncogene lmo2 expression [199] |
myeloid malignancies, prostate cancer [105, 199] |
|
KDM5C JARID1C |
inactivating mutations [94] | H3K4me3 | Transcriptional repression of neuronal genes |
clear cell renal carcinoma [94] |
|
KDM6A UTX |
Inactivating mutations [78, 91–95] |
H3K27 me2, and me3 |
HOX expression, recruitment of the PRC1 complex and monoubiquitination of histone H2A |
Squamous cell carcinomas, acute myeloid leukemias (AML), glioblastoma, breast, bladder and colorectal cancers [78, 91–95] |
|
KMT2A MLL1 |
Translocation, overexpression [110–112, 117] |
H3K4me1 | HOX and E2F3-dependent gene expression |
Breast cancer cells, prostate cancer, and glioblastoma multiforme [110–112, 117] |
|
NSD3 WHSC1L1 |
Translocation, amplification [114, 201] |
H3K36 me1 and me2 |
Gene expression in cell growth, motility, cell cycle, and apoptosis [202] |
Leukemia, pancreatic ductal adenocarcinoma, breast and lung cancers [114, 201, 202] |
|
NSD2 WHSC1 MMSET |
Translocation, overexpression [115,116] |
H3K36me1and me2 |
Interact with β-catenin [203] | multiple myeloma, bladder cancer neuroblastoma [115,116] |
|
KMT1C G9a |
Over expression [126] | H3K9me1 and me2, H3K27me |
DNA methylation, gene expression silence, and cell cycle [126, 214, 215] |
Hepatocellular carcinoma [126] |
|
KMT1E SETDB1 |
Amplification [127] | H3K9me3 | Silencing of euchromatic genes and interaction with DNMT3A [212] |
Melanoma [127] |
|
KMT2E MLL5 |
Over expression [216] | H3K4me and me2 |
A coactivator of RAR-alpha and cell cycle regulator |
AML, HPV16/18-associated cervical cancers [204, 205] |
|
KMT3C SMYD2 |
Over expression [123] | H3K4me H3K36me2 |
Rb and p53 methylation and cell cycle [121, 122] |
esophageal squamous cell carcinoma [123] |
|
KMT3E SMYD3 |
Over expression [118–120] |
H3K4me2 and me3, H4K5me, H4K20me |
MMP9 expression [217] | colorectal carcinoma, hepatocellular carcinoma, and breast cancer [118–120] |
|
KDM1A LSD1 |
Over expression [131–134] |
H3K4me and me2, H3K9me |
A coactivator of androgen receptor and snail/slug [133, 218] |
Bladder, prostate, lung and colorectal cancer [131–134] |
|
KDM3A JMJD1A |
Over expression [207, 208] |
H3K9me1 and me2 |
HOXA1 and CCND1 expression and G1S transition [207, 208] |
Bladder and lung cancers [207, 208] |
|
KDM5A JARID1A |
Overexpressed and gene fusion [219–221] |
H3K4me2 and me3 |
Transcription of HOX and repression of CXCL12 genes [143] |
Gastric cancer, leukemia and cervical cancer [219–219] |
|
KDM2B FBXL10 |
Down-regulation [211] | H3K36me2 H3K4me3 |
Binds the transcribed region of ribosomal RNA and represses the transcription of ribosomal RNA [149]. |
A hotspot for proviral insertion in murine tumors, Human brain tumors [211]. |
|
KDM4B JMJD2B |
Over expression [209, 210] |
H3K9me2 and me3 |
A co-factor of estrogen receptor [223] |
Bladder, lung and gastric cancer [209, 210] |
|
KDM5B JARID1B |
Over expression the copy number gain [144–146, 188] |
H3K4 me1, me2 and me3 |
Growth regulation, co-regulation of the E2F/RB1, BRCA1 and HOXA5 [223] |
Bladder, prostate, breast cancer melanoma, lung cancer [144–146, 188] |
|
KDM6B JMJD3 |
Overexpression [148] | H3K9 and H3K36 |
HOX gene expression and inflammatory response [150, 224] |
Prostate cancer [148] |
5.1. Mutations
Recent next-generation sequencing studies have greatly facilitated discovery of mutations of KMTs and KDMs in cancers and precancerous conditions. Acquired EZH2/ KMT6A mutations have been identified in a variety of cancers [74–78]. Heterozygous mutations at the tyrosine 641 (Y641), which is predicted to be replaced with a histidine, in the SET domain of the EZH2/KMT6A demonstrate enhanced catalytic activity for mono- to di- and di- to trimethylation, whereas wild-type EZH2/KMT6A shows greatest catalytic activity for monomethylation of H3K27 and relatively weak efficiency for the subsequent reactions [79–81]. Therefore, heterozygous Y641 mutants, when co-expressing with wild-type EZH2/KMT6A, increase levels of H3K27me3 and may be functionally equivalent to EZH2/ KMT6A overexpression [79–81]. Distinct from those of both wide-type EZH2/KMT6A and Y641 mutants, another EZH2 A677G mutant that has been identified in lymphoma cell lines and primary human B-cell lymphoma tumor specimens is equally efficient for methylating H3K27me0, H3K27me1, and H3K27me2 [82–85]. In contrast to description above, EZH2 mutations in myelodysplasia-myeloproliferative neoplasms – precancerous conditions - (10 to 13%), myelofibrosis (13%), and various subtypes of myelodysplastic syndromes (6%) are inactive and spread throughout the gene, which comprise missense, nonsense, and premature stop mutations [86–88]. All nonsense and stop codon mutations are predicted to result in a loss of EZH2/KMT6A methyltransferase function as the catalytic SET domain lies at the far C terminus [89]. Patients who are diagnosed with myeloid neoplasms and harbor EZH2 inactive mutations exhibit a poor survival as compared to those without mutations [90]. Taken together, these results suggest that either loss or gain of EZH/KMT6A function are associated with tumorigenesis.
UTX/KDM6A encoding an H3K27 demethylase was the first identified mutated histone demethylase gene in human cancer [91]. Mutations of UTX/KDM6A commonly occur in a wide variety of cancers, including leukemia, renal cell carcinoma (RCC), breast adenocarcinoma, lung cancer, pancreatic adenocarcinoma, bladder cancer, prostate cancer, and others [78, 91–95]. Point mutations affecting the functional jumonji C (jmjC) domain of UTX inactivate its H3K27 demethylase activity [92]. In addition, UTX/KDM6A has been reported as the most frequently mutated gene in transitional cell carcinoma (TCC) of the bladder (mutated in 21% of TCCs) and the second most frequently mutated gene (next to TP53) in lung cancer [93]. UTX/KDM6A mutations are present at the C-terminus and the N-terminus of the UTX protein but mostly in the region adjacent to the JmjC domain required for UTX activity [78]. Therefore, most mutations in UTX/KDM6A are thought to cause loss of function
MLL2/ KMT2D and MLL3/KMT2C are complexed with UTX, which function in a concerted transcriptional egulatory mechanism for many developmental genes, including the HOX gene family. This occurs by involvement of H3K27 demethylation and H3K4 methylation [96]. The HOX gene family collectively controls segment specificity and cell fate in the developing embryo. Each MLL family member is thought to target different subsets of HOX genes. MLL2 also is known to regulate the transcription of a diverse set of genes [97]. Somatic inactivating mutations in both MLL2 and MLL3 are found in various cancers, including lung cancer, RCC, medulloblastoma, glioblastoma, head and neck squamous cell cancers, pancreatic ductal adenocarcinoma, melanoma, leukemias, bladder cancer, Opisthorchis viverrini-related cholangiocarcinoma (CCA), and colorectal cancer [94, 98–100]. The observed pattern of monoallelic somatic inactivation of MLL2 in these cancers suggests a role for MLL2 as a haploinsufficient tumor suppressor. Frameshift mutations and deletion of MLL3 are found in more aggressive cancers [101]. In addition, targeted inactivation of MLL3 in mice results in ureteral epithelial tumors, and spontaneous tumorigenesis was exacerbated in p53 (+/−) mice [102]. These results suggest that MLL3 also functions as a tumor-suppressor gene.
H3K4 demethylase, JARID1C/KDM5C, has also been found to be mutated in human pancreatic cancers and RCC [94], supporting the importance of H3K4 methylation status in cancer. Additionally, mutations affecting a H3K36 methyltransferase SETD2/KMT3A, a H3K9 demethylase KDM3B, and a H3K4 demethylase JARID1C/KDM5C, in cancers have been reported and are associated with distinct gene expression patterns [94, 103–106]. NSD1 is mutated in several cancers, including carcinoma of the upper aerodigestive tract [107]. Multiple distinct and spatially separated inactivating mutations in SETD2/KMT3A, PTEN, and JARID1C/KDM5C are identified within a single tumor, suggesting potential convergent phenotypic evolution [104].
5.2. Chromosome Translocations
Besides gene mutations, other abnormalities implicate histone-modifying enzymes. NSD2/MMSET/WHSC1 is frequently overexpressed in multiple myelomas due to a recurrent t(4;14)(p16;q32) chromosomal translocation [108]. NSD1/KMT3B and NSD3 are fused with nucleoporin-98 (NUP98) due to the recurring t(5;11)(q35;p15.5) translocation in AML and in therapy and radiation-associated myelodysplastic syndrome with t(8;11)(p11;p15), respectively [109].
The MLL1/KMT2A gene may be with one of over 60 distinct partner genes through chromosomal translocations in various human acute leukemias, resulting in the formation of multiple MLL1 fusion protein with transforming activities [110]. The most frequent translocations are the t(4;11) and t(11;19) translocations, which are associated with the expression of MLL1–AF4 and MLL1–ENL, respectively in ALL [110]. Other translocations, such as the t(9;11), t(6;11), t(x;11) and t(11;19), result in expression of MLL1–AF9, MLL1–AF6, MLL1-FKHR-L1, and MLL1-ELL fusion proteins in AML, myelodysplasia or etoposide-therapy-related acute leukemia, respectively [110]. Different fusions can lead to different histone lysine methylation pathways during MLL1-induced leukemic transformation, which adds complexity to drug development efforts for targeting MLL1-mediated methylation in leukemia [110]. Mll knockout mice studies have significantly improved the understanding of the molecular consequences of 11q23 translocations [111]. Loss of MLL1 results in embryonic lethality and defects of axial skeleton and hematopoietic system, as well as abnormal HOX gene expression, which is known to play roles in oncogenesis and attenuated H3K4 methylation [111]. The efficacy of conventional chemotherapies for leukemia may be explained by translocations in the MLL locus at 11q2. Patients with certain cytogenetic abnormalities do not effectively respond these chemotherapies [112]. MLL1 has also been shown to be required for multidrug resistance 1 (MDR1) gene promoter methylation and the activation of MDR1 is accompanied by increased H3K4 methylation. This result suggests that MLL1 be served as a new target for circumvention of tumor multidrug resistance [113].
5.3. KMT and KDM Gene Amplification and Over-expression
NSD3 that maps to chromosome band 8p12 is amplified in several tumor cell lines and primary breast carcinomas, which is associated with poor prognosis [114]. NSD2/ MMSET overexpression has been reported in more than 15 different cancers compared to their respective normal tissues. In most cancer types, the expression levels of NSD2 positively correlate with tumor aggressiveness and prognosis [115]. In neuroblastoma, chemotherapy and retinoic acid can decrease the expression levels of NSD2, and NSD2 levels are associated with drug responses, suggesting the potential of NSD2 as a therapeutic target for a subset of neuroblastomas with unfavorable prognosis [116]. For some cases of myelodysplastic syndrome and AML, MLL1/KMT2A is present in increased copy number, which occurs as the result of additional copies of chromosome 11 or through MLL1 amplification [117]. The amplification of MLL1 is associated with the upregulation of at least some of the genes that are consistently expressed in leukemias with MLL1 rearrangements [117].
SMYD3/KMT3E that is responsible for H3K4 di-and trimethylation is frequently up-regulated in colorectal carcinoma (CRC), hepatocellular carcinoma (HCC), liver and breast cancers [118, 119]. The SMYD3-gene-transfected cells have showed increased proliferation rate and become more resistant to cell death, via transcription of several oncogenes (e.g. Wnt10b) and genes associated with cell adhesion (i.e. N-Myc, CrkL, L-selectin, CD31 and galectin-4) [118, 119]. Human cancer cells expressing both the full-length and an N-terminal cleaved form of SMYD3 exhibit higher methyltransferase activity than that of the full-length protein [120]. This result indicates that the N-terminus may play an important role in regulating enzymatic activity. SMYD2/KMT3C can methylate both histone (H2B, H3, and H4) and nonhistone proteins p53 and Rb [121, 122]. Overexpression of SMYD2 has been reported in the esophageal cell line KYSE150 and esophageal squamous cell carcinoma (ESCC) primary tumor samples. Genetic knockdown of SMYD2 leads to decreased ESCC cell proliferation via apoptosis and cell cycle regulation [123]. Taken together, these results make SMYD2 and 3 attractive targets for the development of novel anticancer drugs.
Several H3K9 methyltransferases, such as Suv39h1/ KMT1A, Suv39h2/KMT1B, G9a/KMT1C, and SETDB1/ KMT1E, are over-expressed in tumor versus normal tissues [124–126]. SETDB1/KMT1E has been found frequently amplified in malignant melanoma, and its expression accelerates melanoma formation in zebrafish [127]. Levels of G9a are significantly elevated in HCC compared to non-malignant liver tissues [126].
EZH2 overexpression has also been reported in various cancer types, such as prostate cancer, breast carcinoma, bladder carcinoma, lung cancer, pancreatic cancer, colon cancer, and others, whereas EZH2 expression is rarely detectable in all tested normal tissues [128]. The function of EZH2 in carcinogenesis is commonly associated with increased cell proliferation. In addition, EZH2 expression increases the invasiveness and metastatic potential of cancer cells and is associated with aggressive tumor subgroups [129, 130].
Expression levels of LSD1 are significantly elevated in human bladder cancer tissues compared to nonneoplastic bladder tissues [131, 132]. Tumor tissues with early stage bladder cancer exhibit particularly high LSD1 expression [131]. This result suggests that LSD1 may be one of the factors involved in the initiation process of carcinogenesis. LSD1 overexpression in prostate cancer, lung cancer and colorectal cancer cells, as well as estrogen receptor (ER)-negative tumors, has also been observed [132, 133]. In prostate cancer, the overexpression of LSD1 is associated with disease recurrence during therapy [133, 134].
The other family members of KDMs, such as JARID1A/KDM5A, JARID1B/KDM5B, JARID1C/KDM5C, JMJD2B/KDM4B, JMJD2C/KDM4C and JMJD3/KDM6B, are also up-regulated in various types of cancers [135–147]. Enhanced JARID1A expression has been shown to contribute to drug resistance in NSCLC cell lines, while JARID1B is thought to be required for melanoma maintenance [144]. JARID1B has also been reported to be highly expressed in 90% of ductal breast carcinomas and associated with aggressive phenotype of breast and prostate cancer [145–146]. JARID1C plays a role in the pathogenesis of human papillomavirus (HPV)-associated cancers as JARID1C has been identified as one of the papillomavirus E2 protein-dependent regulators for HPV oncogene expression [147]. The overexpression of JMJD2C increases the expression of Mdm2 oncogene but decreases the expression of p53, which is dependent on its demethylase activity [140]. The colocalization of JMJD2C with androgen receptor and LSD1 in both normal prostate and prostate carcinomas suggests that these two demethylases may cooperatively regulate androgen receptor-dependent gene transcription [141]. Few studies have shown the relation of JMJD3 with cancer progression, but in one report, JMJD3 has been shown to be upregulated in prostate cancer with higher expression in metastatic prostate cancer [148]. Preclinical studies have shown that inhibition of some of these enzymes can suppress tumorigenesis [143]. Therefore, a rationale for developing specific inhibitors for demethylases for targeted cancer therapy has been established [143].
In contrast to the above described KDMs that normally function as oncogenes, JHDM1B/KDM2B and UTX/KDM6A are more likely to be tumor suppressors. JHDM1B is responsible for removal of methyl groups from H3K36 me1/2, but not H3K36 me3. The expression of JHDM1B is significantly down-regulated in the most aggressive type of the primary brain tumors, glioblastoma multiforme, compared to normal brain tissue [149]. UTX can specifically remove methyl groups from H3K27 me2/3 and, thereby counteract PcG-mediated histone modification by EZH2 [150, 151]. Altered expression of UTX and UTX-bound target genes are commonly seen in human cancers. One study has linked the inactivation of UTX to transcriptional silencing of the RB pathway [152]. However, the detailed mechanism of UTX-mediated tumor suppression remains unclear.
Alterations of KMT or KDM activities lead to changes in global histone methylation levels and dysregulation of specific gene expressions. Global histone methylation levels have been analyzed between normal and cancer cells in the cultured cells and clinical specimens. For example, global loss of H4K20 me3 has been shown to be one of the hallmarks of human cancer [153]. Immunohistochemical studies of a well-characterized series of human breast carcinomas have demonstrated that H3K4 me2 and H4K20 me3 levels are significantly lower in tumor tissues than those in normal tissues, and correlate with clinicopathologic factors [154]. In prostate cancer, global levels of H3K4 me1 and H3K9 me2 and me3 are significantly reduced in cancer tissues compared to normal tissues. High levels of H3K4 me3, H3K9 me2 and me3, and H3K27 me1, me2 and me3 are associated with increased clinical-pathological parameters such as serum PSA, capsular invasion, seminal vesicle infiltration, lymph node involvement, and Gleason score [155, 156]. Global histone lysine methylation levels, including H3K4me1, H3K4me3, H4K20me1, H4K20me2, and H4K20me3, are lower in bladder cancer than in normal urothelial tissues and inversely correlated with pathological stages of human urinary bladder cancer [157]. In lung cancer, lower levels of H3K4 me2 appear to predict significantly poorer survival probabilities of patients [158, 159]. These observations suggest that specific and novel multiple histone methylation patterns are associated with cancer and may have important biological significance.
6. INHIBITORS OF KMTs AND KDMs
The emerging fundamental roles of altered KMTs and KDMs in cell transformation, carcinogenesis and tumorigenesis have implicated that development of inhibitors for these enzymes is a new frontier for drug discovery. However, so far, only a few compounds targeting KMTs and KDMs are available for preclinical and clinical development due to their toxicity. Most small molecule inhibitors for KMTs and KDMs are mainly used in basic research. Some of the first-generation inhibitors for KMTs and KDMs are derived from natural products. Small molecule inhibitors of KMTs and KDMs are summarized in Table 2.
Table 2.
KMT and KDM inhibitors
| Agent | Chemical Strucutre | Targets | Potency | Refs. |
|---|---|---|---|---|
| Sinefungin A | 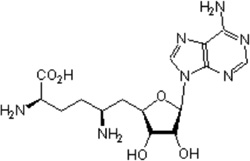 |
S-adenosyl-L- methionine (SAM, AdoMet) methyltransferase |
Ki =1.3 µM | [160] |
| Chaetocin | 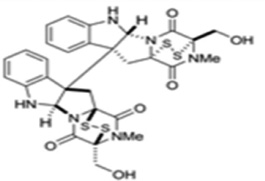 |
SU(VAR)3–9 | Ki = 0.6 µM | [161] |
| Gliotoxin | 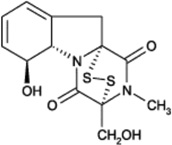 |
G9a | Ki = 6.4 µM | [164] |
| BIX-01294 | 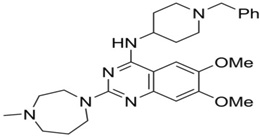 |
G9a, GLP | G9a: Ki = 180 nM GLP: Ki =34 nM |
[165,166] |
| E72 | 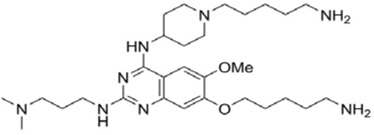 |
GLP | Ki = 100 nM | [211] |
| UNC0321 | 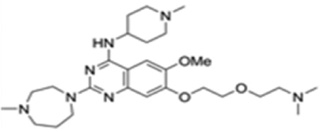 |
G9a, GLP | G9a: Ki < 15 nM GLP: Ki =15 nM |
[166] |
| UNC0638 | 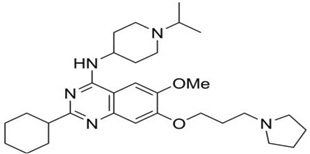 |
G9a, GLP | G9a: Ki < 15 nM GLP: Ki =19 ±1 nM |
[166] |
| UNC0646 | 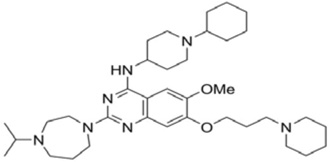 |
G9a | Ki = 6 nM | [212] |
| EPZ004777 | 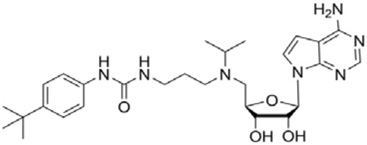 |
DOT1L | Ki = 0.3nM | [212] |
| AZ505 | 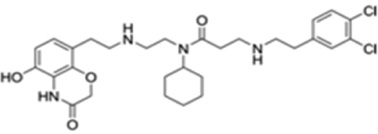 |
SMYD2 | Ki = 0.3 µM | [212] |
| trans-2- Phenylcyclopropyla mine |
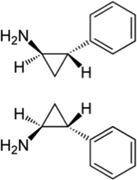 |
LSD1 | Ki = 242 µM | [179] |
| Polyamine analogues |
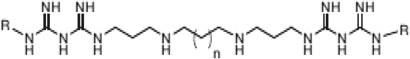 |
LSD1 | Ki <2.5 µM | [183–185] |
| CBB1007 | 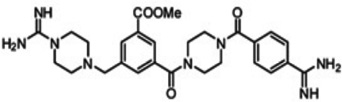 |
LSD1 | Ki = 5.27µM | [186] |
| N-oxalylglycine | 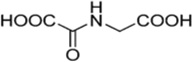 |
JMJD2C JMJD2A |
JMJD2C: Ki = 500 µM JMJD2A: Ki = 250 µM |
[187] |
| 2,4- pyridindicarboxylic acid (PDCA) |
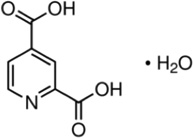 |
KDM5B KDM4A/C |
Ki = 3 ± 1 µM. | [188] |
| 8-hydroxyquinoline | 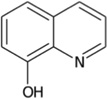 |
JMJD2 | Ki = 28 µM | [189] |
| 2,2’-bipyridine | 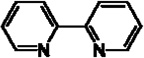 |
JMJD2E | Ki = 6 µM | [190] |
| GSK-343 | 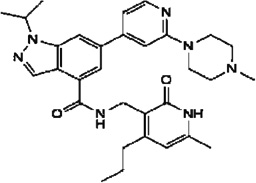 |
EZH2 | Ki = 4nM | [172] |
| GSK-126 | 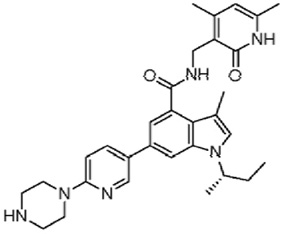 |
EZH2 | Ki = 3nM | [171] |
| Methylstat | 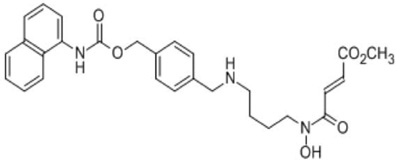 |
JMJD3, JMJD2E, JMJD2C |
Ki = 4.3 µM | [191] |
| GSK-J1 | 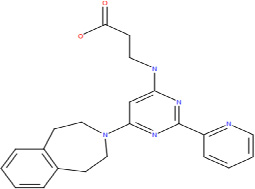 |
JMJD3, UTX | Ki = 60nM | [192] |
6. 1. KMT Inhibitors
KMT enzymes catalyze the transfer of one to three methyl groups from S-adenosylmethionine (SAM) to specific lysine residues on histones. Targeting the cofactor (SAM) binding site of protein methyltransferases appears to be the first approach for KMT inhibition. Sinefungin A, natural product isolated from Streptomyces spp., is the first SAM-competitive and nonselective inhibitor of KMTs identified [160]. Another natural KMT inhibitor, Chaetocin, was discovered by random screening of compound libraries. Chaetocin is a fungal metabolite and has been found to be an inhibitor of the Drosophila melanogaster Suv39 family including Suv39h1 with an IC50 of 0.8 µM [161]. Chaetocin does not inhibit E(Z) or SET7/9 at concentrations below 90 µM, suggesting its potential selectivity against certain KMTs. A recent attempt at total synthesis of (+)-chaetocin enantiomers has showed that they also have inhibitory activity towards G9a with IC50s of 2.4 µM and 1.7µM, respectively [162]. Chaetocin has also been reported to exhibit anti-myeloma activity, and can inhibit Suv39h1 in acute myeloid leukemia cells with hypermethylated tumor suppressor genes [163]. These results support the potential of developing chaetocin H3K9 methyltransferase inhibitors as therapeutics to target reactivation of silenced genes. Gliotoxin analogs with a disulfide bond, which are isolated from the fungus Penicillium sp. strain JMF034, show potent inhibitory activity of G9a and Suv39h1without affecting SET7/9 [164].
In another high throughput screen against a preselected chemical library, a highly selective small inhibitor of G9a, BIX-01294 (diazepinquinazolin-amine derivative) has been identified to lower bulk H3K9 me2 levels in mouse ES cells and fibroblasts, with levels restored upon removal of the inhibitor [165]. BIX-01294 binds at the protein substrate channel of G9A and GLP1. However, BIX-01294 is unable to induce significant changes in cellular morphology and its potency in affecting global histone methylation appeares to be very limited. Recently, second-generation inhibitors, such as E72, UNC321, UNC0638, and UNC0646 that are based on a 7-alkoxyamine tethered to the quinazoline core, have been developed with a marked improvement of potency and specificity against G9a/GLP. Among them, UNC0646 has also demonstrated improved potency of this quinazoline series in cell-based assays [166]. In addition, Yuan et al [167] reported that BRD4770, a compound from a focused library of 2-substituted benzimidazoles as a potential SAM mimetic, reduced cellular levels of di- and trimethylated H3K9, induced senescence and inhibition of cell growth in the pancreatic cancer cell line PANC-1.
The DOT1L inhibitor EPZ004777 is also a SAM analogue and binds to the SAM binding site. However, EPZ004777 shows more than 1,000 fold greater selectivity over other histone methyltransferases, including EZH2, SETD7, and WHSC1. EPZ004777 has been shown to kill mixed lineage leukemia cells with little effect on non-MLL-translocated cells [44]. In addition, EPZ004777 increases the survival of mice bearing tumors with MLL translocation [168]. By using structure- and mechanism-based design, Yao et al [169] identified several potent and specific DOT1L inhibitors with IC50 values as low as 38 nM.
EZH2 is essential for cancer stem cell self-renewal. A potent SAM hydrolase inhibitor, 3-Deazaneplanocin A (DZNep), has been shown to selectively inhibit EZH2, leading to H3K27 demethylation and induction of apoptosis in breast cancer cells but not in normal breast epithelial cells [170]. In addition, DZNeP demonstrates promising anti-tumor activity in vivo, and inhibits cancer cell invasion and tumor angiogenesis in prostate and brain cancers, respectively. Unlike DZNeP that inhibits EZH2 via degradation of Polycomb Repressive Complex 2(PRC2), GSK-343 and GSK-126 directly inhibit EZH2 methyltransferase activity by competing with the co-factor SAM with potencies of Kiapp = 0.5–4 nM [171, 172]. Both GSK343 and GSK126 are cell-active and over 1000-fold selective for EZH2 versus 20 other KMTs [171, 172].
SMYD2 exhibits oncogenic properties by repressing the functional activities of p53 and retinoblastoma protein. Therefore, SMYD2 is an attractive drug target for the development of small-molecule inhibitors. In a high-throughput screening assay, a potent and selective SMYD2 inhibitor, AZ505, has been identified and structural analysis shows that AZ505 is bound in the peptide binding groove of SMYD2 [173, 174].
6. 2. KDM1A/AOF2/LSD1 Inhibitors
LSD1 utilizes flavin adenosine dinucleotide (FAD) as a cofactor to demethylate mono-and dimethylated H3K4 [175]. LSD1 is also involved in the demethylation of H3K9 when associated with nuclear transcriptional factors (e.g. estrogen and androgen receptors), as well as in the demethylation of non-histone proteins such as p53, Stat3 and DNMT1 [175]. Monoamine oxidase (MAO) A and B and LSD1 show homology in their catalytic site in a flavin-dependent manner. MAO A and B are established targets for neurological disorders [176]. A large number of inhibitors have been synthesized and tested for their specificity toward MAO A or B [176, 177]. Recently a number of MAO inhibitors, such as clorgyline, pargyline, tranylcypromine and phenelzine have also been tested for LSD1 inhibitory activities [178]. Tranylcypromine demonstrates the highest potency with IC50 of 21 µM [179]. Further study showes that tranylcypromine treatment reduces neuroblastoma xenograft growth, but causes substantial toxicity in vivo [20]. By analysis of structure-activity relationships and subsequent extension of the chemical structure further into the lysine substrate pocket, a more potent and selective tranycypromine analogue against LSD1 (S2101) has been designed and synthesized [180]. Pargyline inhibits LSD1 activity when it is associated with the androgen receptor complex, suggesting that the inhibitory activity of pargyline may be due to the conformational change of LSD1 [181]. Willmann et al [182] recently reported that Namoline, a γ-pyrone from a focused, natural product inspired library, inhibited LSD1 with an IC50 of about 50 µM, and resulted in silencing of AR-regulated gene expression and a growth inhibitory effect on prostate cancer cells. Additionally, polyamine analogues (bisguanidine and biguanidine types) have been characterized as potent non-competitive inhibitors of LSD1 and shown to reactivate aberrantly silenced genes to suppress colon cancer development [183–185]. Based on molecular modeling, Wang et al [186] developed a novel small molecule LSD1 inhibitor (i.e. CBB1007) that inhibited the proliferation of pluripotent cancer cells including teratocarcinoma, embryonic carcinoma, and seminoma, or embryonic stem cells with minimum growth-inhibitory effects on non-pluripotent cancer or normal somatic cells. These results indicate that LSD1 inhibitors may be a new class of epigenetic drugs for cancer prevention and treatment.
6. 3. JmjC-Domain Demethylase Inhibitors
Jumonji domain-containing lysine demethlyases use Fe2+ and 2-oxoglutarate (2-OG) as co-substrates/co-factors. Specific inhibitors against JmjC-domain demethylases are designed to compete with 2-oxoglutarate and bind to the catalytic iron in the active site with selectivity over other human 2-OG oxygenases. N-oxalylglycine (NOG) as a 2-oxoglutarate analogue is a weaker inhibitor of JMJD2C and the catalytic core of JMJD2A [187]. Another 2-OG analog, 2, 4-pyridindicarboxylic acid (PDCA), inhibits the Jumonji domain-containing demethylases and other 2-OG-dependent oxygenases such as HIF prolyl hydroxylase 1 (HPH1; also known as EGLN2) and HPH2 (also known as EGLN1) [188]. More selective compounds have also been developed by extending the chemical structure of the Jumonji domain-containing demethylase inhibitor template to the iron in the substrate binding pocket [187]. These compounds, such as metal-chelating hydroxamic acids, have increased potency [187]. However, the molecular and physicochemical properties of the compound may limit its bioavailability [187]. Recently, a new generation of cell permeable Jumonji domain-containing demethylase inhibitors with subtype selectivity and more drug-like properties have been identified through high-throughput screening, which include 8-hydroxyquinolines and 2,2’-bipyridines [189]. In addition, some flavonoid- and catechol-type molecules, as well as HDAC inhibitor SAHA, have also been found to be JmjC KDM inhibitors [190].
Based on the crstal structures and the enzymatic mechanism of Jumonji C domain-containing histone demethylases (JHDMs), Wang et al. [191] designed and synthesized a small molecule inhibitor of JHDM, called methylstat, which contains a methyllysine mimic (substrate mimic), an α-ketoglutarate mimic (co-factor mimic) and a linker. Methylstat exhibits selective inhibitory activity against trimethyl-specific JHDMs in vitro and prevents myogenesis through inhibition of H3K27me3-demethylase UTX [191]. By studying high-resolution crystal structures of histone peptides in complex with JMJD3 in the presence of cofactor analogues and by determining the required JMJD3 residues for substrate recognition, Kruidenier et al. [192]have further identified a more potent and selective inhibitor (GSK-J1) of the H3K27 histone demethylases JMJD3 and UTX (IC50 = 60 nM for human JMJD3). GSK-J1 does not inhibit other chromatin-modifying enzymes or protein kinases. These JHDM inhibitors would be very useful tools for understanding roles of JMJD3 and UTX in gene transcription and epigenetic inheritance.
7. PERSPECTIVE AND CONCLUSION
An enormous amount of evidence recently has been emerging that histone lysine methylation plays a crucial role in cancer initiation and development. Infection by oncogenic viruses or parasites, or exposure to chemical carcinogens, can directly affect lysine histone methylation leading to cellular transformation, suggesting that certain KMTs and KDMs may be direct targets of oncogenic viruses and chemical carcinogens. However, the epigenetic mechanisms for the involvement of histone lysine methylation in cellular transformation and early carcinogenesis remain largely unexplored. Understanding the epigenetic mechanisms that lie behind the window of operational reversibility during early carcinogenesis, may allow chemopreventive strategies to be implemented to revert or halt these early carcinogen or infectious agent-induced changes prior to the development of cancer.
Recently, with second-generation sequencing, profound genetic alterations in KMTs and KDMs, including gene mutation, translocation and amplification, have been observed in a wide variety of cancers. As mutations or other genetic alterations (i.e. gene translocation and amplification) in KMTs and KDMs have the potential to reprogram the whole genome by altering the expression of hundreds of genes all at once, these diverse mutations or other genetic alterations can be associated with a uniform phenotype that may not be distinguishable on clinical grounds. Therefore, development of molecular diagnostics from genetic alterations of KMTs and KDMs could be useful for further stratifying cancer patients into subgroups for future selection of targeted therapies. In addition, cancer-specific epigenetic drug targets may be identified or validated through ongoing functional studies that investigate the mechanisms underlying the phenotypic plasticity of cancer cells.
However, function roles of KMTs and KDMs in cancer appear to be complex and there exists a cross-talk among them. Currently there is no single unique methylation mark that can predict a specific type of cancer. Regulation of gene activation/repression not only depends on the position of lysine methylation, but also the number of methylation residues. In addition, due to intratumor heterogeneity, multiple mutations or genetic alterations may co-exist and single tumor biopsy sampling may not be sufficient to portray tumor mutational landscapes. Such biological complexity points to challenges in developing targeted epigenetic therapies in the future. If multiple epigenetic targets must be inhibited to effectively prevent or treat cancer, then single-target screening strategies may ultimately fail, and screening a natural compound with the potential to affect many signaling pathways at once may be more productive.
Due to the wide diversity of chemical structures in natural products and the interaction between natural products and cellular targets during long-term process of natural evolution, natural products often provide a higher “hit rate” to targets than a random approach. Natural products therefore have served as a stepping stone for developing more specific KMT and KDM inhibitors. However, natural products have the limitation of being promiscuous inhibitors affecting a wide range of enzymatic targets. Further chemical modification of natural products for developing more selective inhibitors is often required. Targeted approaches have advantages of known mechanisms of action and selectivity for specific patient populations.
ACKNOWLEDGEMENTS
We apologize for not being able to cite all of the publications in the field due to the limitations of the length of the review. XZ is supported by NIH grants R01CA122558 and R21CA152804.
Glossary
- 2-AAF
2-acetylaminofluorene
- 4-ABP
4-aminobiphenyl
- ACI
August Copenhagen Irish
- ADD
ATRX-Dnmt3-Dnmt3L
- AR
androgen receptor
- AML
acute myelogenous leukemia
- CCA
cholangiocarcinoma
- CRC
colorectal carcinoma
- CXXC
Cys-X-X-Cys
- DNMT
DNA methyltransferase
- DZNep
3-Deazaneplanocin A
- DOT1L
DOT1 like
- ESCC
esophageal squamous cell carcinoma
- EVI1
Ecotropic viral integration site 1
- EZH2
enhancer of zeste homolog 2 (Drosophila)
- FAD
flavin adenosine dinucleotide
- H3K4 me
histone H3 lysine 4 mono-methylation
- H3K4me2
histone H3 lysine 4 di-methylation
- H3K4me3
histone H3 lysine 4 tri-methylation
- H3K9 me
histone H3 lysine 9 mono-methylation
- H3K9me2
histone H3 lysine 9 di-methylation
- H3K9me3
histone H3 lysine 9 tri-methylation
- H3K27 me
histone H3 lysine 27 mono-methylation
- H3K27me2
histone H3 lysine 27 di-methylation
- H3K27me3
histone H3 lysine 27 tri-methylation
- H3K36 me
histone H3 lysine 36 mono-methylation
- H3K36me2
histone H3 lysine 36 di-methylation
- H3K36me3
histone H3 lysine 36 tri-methylation
- H3K79 me
histone H3 lysine 79 mono-methylation
- H4K20me1
histone H4 lysine 20 mono-methylation
- H4K20me2
histone H4 lysine 20 di-methylation
- H4K20me3
histone H4 lysine 20 tri-methylation
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- HPH1
Hypoxia inducible factor prolyl hydroxylase 1
- HMECs
human mammary epithelial cells
- HPV
human papillomavirus
- HOX A9
Homeobox A9
- hTERT
human telomerase reverse transcriptase
- JHDMs
Jumonji C domain-containing histone demethylase
- JmjC
Jumonji C
- KDM
demethylases
- KMT
histone lysine methyltransferases
- LSD1
Lysine-specific demethylase 1
- MBD
methyl-CpG-binding domain
- Ndy
Not dead yet
- MAO
monoamine oxidase
- MEFs
mouse embryo fibroblasts
- MLL
Mixed-lineage leukemia gene
- MNU
N-methyl-N-nitrosurea
- MRES
multiple regional epigenetic silencing
- NOG
N-oxalylglycine
- NSD2
Nuclear receptor SET domain containing protein 2
- NUP98
nucleoporin-98
- 2-OG
2-oxoglutarate
- PDCA
2, 4-pyridindicarboxylic acid
- PhIP
2-amino-1-methyl-6-phenylimidazo [4, 5 β] pyridine
- RCC
renal cell carcinoma
- PRC2
Polycomb Repressive Complex 2
- SAHA
suberoylanilide hydroxamic acid
- SAM
S-adenosylmethionine
- SMYD3
SET and MYND domain containing protein 3
- SUV39H
Variegation 3–9 homolog
- siRNA
small interference RNA
- TCC
transitional cell carcinoma
- UTX
Ubiquitously transcribed tetratricopeptide repeat, X chromosome
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R, 3rd, Shen L, Nimer SD, Leavitt R, Raza A, Saba H. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 5.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of ; primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 6.VanderMolen KM, McCulloch W, Pearce CJ, Oberlies NH. Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): a natural product recently approved for cutaneous T-cell lymphoma. J Antibiot (Tokyo) 2011;64:525–531. doi: 10.1038/ja.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008;123:8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 9.Kubicek S, Gilbert JC, Fomina-Yadlin D, Gitlin AD, Yuan Y, Wagner FF, Holson EB, Luo T, Lewis TA, Taylor B, Gupta S, Shamji AF, Wagner BK, Clemons PA, Schreiber SL. Chromatin-targeting small molecules cause class-specific transcriptional changes in pancreatic endocrine cells. Proc Natl Acad Sci U S A. 2012;109:5364–5369. doi: 10.1073/pnas.1201079109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islam AB, Richter WF, Lopez-Bigas N, Benevolenskaya EV. Selective targeting of histone methylation. Cell Cycle. 2011;10:413–24. doi: 10.4161/cc.10.3.14705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popovic R, Licht JD. Emerging epigenetic targets and therapies in cancer medicine. Cancer Discov. 2012;2:405–413. doi: 10.1158/2159-8290.CD-12-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimi A, Kurokawa M. Key roles of histone methyltransferase and demethylase in leukemogenesis. J Cell Biochem. 2011;112:415–424. doi: 10.1002/jcb.22972. [DOI] [PubMed] [Google Scholar]
- 13.Hudlebusch HR, Santoni-Rugiu E, Simon R, Ralfkiær E, Rossing HH, Johansen JV, Jørgensen M, Sauter G, Helin K. The histone methyltransferase and putative oncoprotein MMSET is overexpressed in a large variety of human tumors. Clin Cancer Res. 2011;17:2919–2933. doi: 10.1158/1078-0432.CCR-10-1302. [DOI] [PubMed] [Google Scholar]
- 14.Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, Feng Z, Punt N, Daigle A, Bullinger L, Pollock RM, Richon VM, Kung AL, Armstrong SA. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20:66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Méreau H, De Rijck J, Cermáková K, Kutz A, Juge S, Demeulemeester J, Gijsbers R, Christ F, Debyser Z, Schwaller J. Impairing MLL-fusion gene-mediated transformation by dissecting critical interactions with the lens epithelium-derived growth factor (LEDGF/p75) Leukemia. 2013 doi: 10.1038/leu.2013.10. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Hormaeche I, Licht JD. Chromatin modulation by oncogenic transcription factors: new complexity, new therapeutic targets. Cancer Cell. 2007;11:475–478. doi: 10.1016/j.ccr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Cock-Rada AM, Medjkane S, Janski N, Yousfi N, Perichon M, Chaussepied M, Chluba J, Langsley G, Weitzman JB. SMYD3 promotes cancer invasion by epigenetic upregulation of the metalloproteinase MMP-9. Cancer Res. 2012;72:810–820. doi: 10.1158/0008-5472.CAN-11-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arita A, Shamy MY, Chervona Y, Clancy HA, Sun H, Hall MN, Qu Q, Gamble MV, Costa M. The effect of exposure to carcinogenic metals on histone tail modifications and gene expression in human subjects. J Trace Elem Med Biol. 2012;26:174–178. doi: 10.1016/j.jtemb.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spannhoff A, Hauser AT, Heinke R, Sippl W, Jung M. The emerging therapeutic potential of histone methyltransferase and demethylase inhibitors. Chem Med Chem. 2009;4:1568–1582. doi: 10.1002/cmdc.200900301. [DOI] [PubMed] [Google Scholar]
- 20.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11:384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- 21.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black JC, Whetstine J. R.Tipping the lysine methylation balance in disease. Biopolymers. 2013;99:127–35. doi: 10.1002/bip.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Heightman TD. Chemical biology of lysine demethylases. Curr Chem Genomics. 2011;5(Suppl 1):62–71. doi: 10.2174/1875397301005010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneda A, Matsusaka K, Aburatani H, Fukayama M. Epstein-barr virus infection as an epigenetic driver of tumorigenesis. Cancer Res. 2012;72:3445–3450. doi: 10.1158/0008-5472.CAN-11-3919. [DOI] [PubMed] [Google Scholar]
- 26.Anderton JA, Bose S, Vockerodt M, Vrzalikova K, Wei W, Kuo M, Helin K, Christensen J, Rowe M, Murray PG, Woodman CB. The H3K27me3 demethylase, KDM6B, is induced by Epstein-Barr virus and over-expressed in Hodgkin's Lymphoma. Oncogene. 2011;30:2037–2043. doi: 10.1038/onc.2010.579. [DOI] [PubMed] [Google Scholar]
- 27.Hyland PL, Mcdade SS, Mccloskey R, Dickson GJ, Arthur K, McCance DJ, Patel D. Evidence for alteration of EZH2, BMI1, and KDM6A and epigenetic reprogramming in human papillomavirus type 16 E6/E7-expressing keratinocytes. J Virol. 2011;85:10999–101006. doi: 10.1128/JVI.00160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo XG, Xi T, Guo S, Liu ZP, Wang N, Jiang Y, Zhang TC. Effects of SMYD3 overexpression on transformation, serum dependence, and apoptosis sensitivity in NIH3T3 cells. IUBMB Life. 2009;61:679–684. doi: 10.1002/iub.216. [DOI] [PubMed] [Google Scholar]
- 29.Bradley C, Van der meer R, Roodi N, Yan H, Chandrasekharan MB, Sun ZW, Mernaugh RL, Parl FF. Carcinogen-induced histone alteration in normal human mammary epithelial cells. Carcinogenesis. 2007;28:2184–2192. doi: 10.1093/carcin/bgm100. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Ke Q, Costa M. Alterations of histone modifications by cobalt compounds. Carcinogenesis. 2009;30:1243–1251. doi: 10.1093/carcin/bgp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chervona Y, Arita A, Costa M. Carcinogenic metals and the epigenome: understanding the effect of nickel, arsenic, and chromium. Metallomics. 2012;4:619–627. doi: 10.1039/c2mt20033c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou X, Sun H, Ellen TP, Chen H, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29:1831–1836. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Kluz T, Zhang R, Costa M. Hypoxia and nickel inhibit histone demethylase JMJD1A and repress Spry2 expression in human bronchial epithelial BEAS-2B cells. Carcinogenesis. 2010;31:2136–2144. doi: 10.1093/carcin/bgq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Ke Q, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol Cell Biol. 2006;26:3728–3737. doi: 10.1128/MCB.26.10.3728-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson SP, Hoare SF, Glasspool RM, Keith WN. Lack of telomerase gene expression in alternative lengthening of telomere cells is associated with chromatin remodeling of the hTR and hTERT gene promoters. Cancer Res. 2005;65:7585–7590. doi: 10.1158/0008-5472.CAN-05-1715. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Fang X, Ge Z, Jalink M, Kyo S, Björkholm M, Gruber A, Sjöberg J, Xu D. The telomerase reverse transcriptase (hTERT) gene is a direct target of the histone methyltransferase SMYD3. Cancer Res. 2007;67:2626–2631. doi: 10.1158/0008-5472.CAN-06-4126. [DOI] [PubMed] [Google Scholar]
- 37.Hamamoto R, Furukawa Y, Morita M, Iimura Y, Silva FP, Li M, Yagyu R, Nakamura Y. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol. 2004;6:731–740. doi: 10.1038/ncb1151. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Q, Liu C, Ge Z, Fang X, Zhang X, Strååt K, Björkholm M, Xu D. Lysine-specific demethylase 1 (LSD1) is required for the transcriptional repression of the telomerase reverse transcriptase (hTERT) gene. PLoS ONE. 2008;3:e1446. doi: 10.1371/journal.pone.0001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfau R, Tzatsos A, Kampranis SC, Serebrennikova OB, Bear SE, Tsichlis PN. Members of a family of JmjC domain-containing oncoproteins immortalize embryonic fibroblasts via a JmjC domain-dependent process. Proc Natl Acad Sci USA. 2008;105:1907–1912. doi: 10.1073/pnas.0711865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzatsos A, Pfau R, Kampranis SC, Tsichlis PN. Ndy1/KDM2B immortalizes mouse embryonic fibroblasts by repressing the Ink4a/Arf locus. Proc Natl Acad Sci USA. 2009;106:2641–2646. doi: 10.1073/pnas.0813139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–178. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Chang MJ, Wu H, Achille NJ, Reisenauer MR, Chou CW, Zeleznik-Le NJ, Hemenway CS, Zhang W, et al. Histone H3 lysine 79 methyltransferase Dot1 is required for immortalization by MLL oncogenes. Cancer Res. 2010;70:10234–10242. doi: 10.1158/0008-5472.CAN-10-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernt KM, Armstrong SA. A role for DOT1L in MLL-rearranged leukemias. Epigenomics. 2011;3:667–670. doi: 10.2217/epi.11.98. [DOI] [PubMed] [Google Scholar]
- 44.Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, Johnston LD, Scott MP, Smith JJ, Xiao Y, Jin L, Kuntz KW, Chesworth R, Moyer MP, Bernt KM, Tseng JC, Kung AL, Armstrong SA, Copeland RA, Richon VM, Pollock RM. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20:53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction--a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006;3:448–457. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- 46.Kuo AJ, Cheung P, Chen K, Zee BM, Kioi M, Lauring J, Xi Y, Park BH, Shi X, Garcia BA, Li W, Gozani O. NSD2 links dimethylation of histone H3 at lysine 36 to oncogenic programming. Mol Cell. 2011;44:609–620. doi: 10.1016/j.molcel.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang GG, Cai L, Pasillas MP, Kamps MP. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat Cell Biol. 2007;9:804–812. doi: 10.1038/ncb1608. [DOI] [PubMed] [Google Scholar]
- 48.Spensberger D, Delwel R. A novel interaction between the protooncogene Evi1 and histone methyltransferases, SUV39H1 and G9a. FEBS Lett. 2008;582:2761–2767. doi: 10.1016/j.febslet.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 49.Mulligan P, Westbrook TF, Ottinger M, Pavlova N, Chang B, Macia E, Shi YJ, Barretina J, Liu J, Howley PM, Elledge SJ, Shi Y. CDYL bridges REST and histone methyltransferases for gene repression and suppression of cellular transformation. Mol Cell. 2008;32:718–726. doi: 10.1016/j.molcel.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herrera-merchan A, Arranz L, Ligos JM, De Molina A, Dominguez O, Gonzalez S. Ectopic expression of the histone methyltransferase Ezh2 in haematopoietic stem cells causes myeloproliferative disease. Nat Commun. 2012;3:623. doi: 10.1038/ncomms1623. [DOI] [PubMed] [Google Scholar]
- 51.Karanikolas BD, Figueiredo ML, Wu L. Polycomb group protein enhancer of zeste 2 is an oncogene that promotes the neoplastic transformation of a benign prostatic epithelial cell line. Mol Cancer Res. 2009;7:1456–65. doi: 10.1158/1541-7786.MCR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J, Nguyen AT, Zhang Y. KDM2b/JHDM1b, an H3K36me2-specific demethylase, is required for initiation and maintenance of acute myeloid leukemia. Blood. 2011;7:3869–3880. doi: 10.1182/blood-2010-10-312736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang ZQ, Liu G, Bollig-fischer A, Giroux CN, Ethier SP. Transforming properties of 8p11-12 amplified genes in human breast cancer. Cancer Res. 2010;70:8487–8497. doi: 10.1158/0008-5472.CAN-10-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boffa LC, Bolognesi C. Methylating agents: their target amino acids in nuclear proteins. Carcinogenesis. 1985;6:1399–13401. doi: 10.1093/carcin/6.9.1399. [DOI] [PubMed] [Google Scholar]
- 55.Pinsky SD, Lee KE, Woolley PV3rd. Uptake and binding of 1-methyl-1-nitrosourea (MNU) and 1-methyl-3-nitro-1-nitrosoguanidine (MNNG) by the isolated guinea pig pancreas. Carcinogenesis. 1980;1:567–575. doi: 10.1093/carcin/1.7.567. [DOI] [PubMed] [Google Scholar]
- 56.Starlard-davenport A, Tryndyak VP, James SR, Karpf AR, Latendresse JR, Beland FA, Pogribny IP. Mechanisms of epigenetic silencing of the Rassf1a gene during estrogen-induced breast carcinogenesis in ACI rats. Carcinogenesis. 2010;31:376–381. doi: 10.1093/carcin/bgp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tryndyak VP, Muskhelishvili L, Kovalchuk O, Rodriguez-Juarez R, Montgomery B, Churchwell MI, Ross SA, Beland FA, Pogribny IP. Effect of long-term tamoxifen exposure on genotoxic and epigenetic changes in rat liver: implications for tamoxifen-induced hepatocarcinogenesis. Carcinogenesis. 2006;27:1713–1720. doi: 10.1093/carcin/bgl050. [DOI] [PubMed] [Google Scholar]
- 58.Kovalchuk O, Tryndyak VP, Montgomery B, Boyko A, Kutanzi K, Zemp F, Warbritton AR, Latendresse JR, Kovalchuk I, Beland FA, Pogribny IP. Estrogen-induced rat breast carcinogenesis is characterized by alterations in DNA methylation, histone modifications and aberrant microRNA expression. Cell Cycle. 2007;6:2010–2018. doi: 10.4161/cc.6.16.4549. [DOI] [PubMed] [Google Scholar]
- 59.Pogribny IP, Muskhelishvili L, Tryndyak VP, Beland FA. The role of epigenetic events in genotoxic hepatocarcinogenesis induced by 2-acetylaminofluorene. Mutat Res. 2011;722:106–113. doi: 10.1016/j.mrgentox.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Bagnyukova TV, Tryndyak VP, Montgomery B, Churchwell MI, Karpf AR, James SR, Muskhelishvili L, Beland FA, Pogribny IP. Genetic and epigenetic changes in rat preneoplastic liver tissue induced by 2-acetylaminofluorene. Carcinogenesis. 2008;29:638–646. doi: 10.1093/carcin/bgm303. [DOI] [PubMed] [Google Scholar]
- 61.Pogribny IP, Ross SA, Tryndyak VP, Pogribna M, Poirier LA, Karpinets TV. Histone H3 lysine 9 and H4 lysine 20 trimethylation and the expression of Suv4-20h2 and Suv-39h1 histone methyltransferases in hepatocarcinogenesis induced by methyl deficiency in rats. Carcinogenesis. 2006;27:1180–1186. doi: 10.1093/carcin/bgi364. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen CT, Weisenberger DJ, Velicescu M, Gonzales FA, Lin JC, Liang G, Jones PA. Histone H3-lysine 9 methylation is associated with aberrant gene silencing in cancer cells and is rapidly reversed by 5-aza-2’-deoxycytidine. Cancer Res. 2002;62:6456–6461. [PubMed] [Google Scholar]
- 63.Wozniak RJ, Klimecki WT, Lau SS, Feinstein Y, Futscher BW. 5-Aza-2'-deoxycytidine-mediated reductions in G9A histone methyltransferase and histone H3 K9 di-methylation levels are linked to tumor suppressor gene reactivation. Oncogene. 2007;26:77–90. doi: 10.1038/sj.onc.1209763. [DOI] [PubMed] [Google Scholar]
- 64.Hashimoto H, Vertino PM, Cheng X. Molecular coupling of DNA methylation and histone methylation. Epigenomics. 2010;2:657–669. doi: 10.2217/epi.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 66.Wong CM, Wong CC, Ng YL, Au SL, Ko FC, Ng IO. Transcriptional repressive H3K9 and H3K27 methylations contribute to DNMT1-mediated DNA methylation recovery. PLoS ONE. 20116:e16702. doi: 10.1371/journal.pone.0016702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Hevi S, Kurash JK, Lei H, Gay F, Bajko J, Su H, Sun W, Chang H, Xu G, Gaudet F, Li E, Chen T. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 2009;41:125–129. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 68.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 69.Ohm JE, Mcgarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad HP, Chen W, Daniel VC, Yu W, Berman DM, Jenuwein T, Pruitt K, Sharkis SJ, Watkins DN, Herman JG, Baylin SB. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, Eden E, Yakhini Z, Ben-Shushan E, Reubinoff BE, Bergman Y, Simon I, Cedar H. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet. 2007;39:232–236. doi: 10.1038/ng1950. [DOI] [PubMed] [Google Scholar]
- 71.Widschwendter M, Fiegl H, Egle D, Mueller-Holzner E, Spizzo G, Marth C, Weisenberger DJ, Campan M, Young J, Jacobs I, Laird PW. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 72.Ohm JE, Baylin SB. Stem cell chromatin patterns: an instructive mechanism for DNA hypermethylation? Cell Cycle. 2007;6:1040–1043. doi: 10.4161/cc.6.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vallot C, Stransky N, Bernard-pierrot I, Hérault A, Zucman-Rossi J, Chapeaublanc E, Vordos D, Laplanche A, Benhamou S, Lebret T, Southgate J, Allory Y, Radvanyi F. A novel epigenetic phenotype associated with the most aggressive pathway of bladder tumor progression. J Natl Cancer Inst. 2011;103:47–60. doi: 10.1093/jnci/djq470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lohr JG, Stojanov P, Lawrence MS, Auclair D, Chapuy B, Sougnez C, Cruz-Gordillo P, Knoechel B, Asmann YW, Slager SL, Novak AJ, Dogan A, Ansell SM, Link BK, Zou L, Gould J, Saksena G, Stransky N, Rangel-Escareño C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Hernández-Lemus E, Schwarz-Cruz y, Celis A, Imaz-Rosshandler I, Ojesina AI, Jung J, Pedamallu CS, Lander ES, Habermann TM, Cerhan JR, Shipp MA, Getz G, Golub TR. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA. 2012;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saramäki OR, Tammela TL, Martikainen PM, Vessella RL, Visakorpi T. The gene for polycomb group protein enhancer of zeste homolog 2 (EZH2) is amplified in late-stage prostate cancer. Genes Chromosomes Cancer. 2006;45:639–645. doi: 10.1002/gcc.20327. [DOI] [PubMed] [Google Scholar]
- 76.Yamada A, Fujii S, Daiko H, Nishimura M, Chiba T, Ochiai A. Aberrant expression of EZH2 is associated with a poor outcome and P53 alteration in squamous cell carcinoma of the esophagus. Int J Oncol. 2011;38:345–353. doi: 10.3892/ijo.2010.868. [DOI] [PubMed] [Google Scholar]
- 77.He LR, Liu MZ, Li BK, Jia WH, Zhang Y, Liao YJ, Chen YC, Zhang LJ, Guan XY, Zeng YX, Kung HF, Xie D. High expression of EZH2 is associated with tumor aggressiveness and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Int J Cancer. 2010;127:138–147. doi: 10.1002/ijc.25031. [DOI] [PubMed] [Google Scholar]
- 78.Jankowska AM, Makishima H, Tiu RV, Szpurka H, Huang Y, Traina F, Visconte V, Sugimoto Y, Prince C, O’Keefe C, Hsi ED, List A, Sekeres MA, Rao A, McDevitt MA, Maciejewski JP. Mutational spectrum analysis of chronic myelomonocytic leukemia includes genes associated with epigenetic regulation: UTX, EZH2, and DNMT3A. Blood. 2011;118:3932–3941. doi: 10.1182/blood-2010-10-311019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, Yap D, Humphries RK, Griffith OL, Shah S, Zhu H, Kimbara M, Shashkin P, Charlot JF, Tcherpakov M, Corbett R, Tam A, Varhol R, Smailus D, Moksa M, Zhao Y, Delaney A, Qian H, Birol I, Schein J, Moore R, Holt R, Horsman DE, Connors JM, Jones S, Aparicio S, Hirst M, Gascoyne RD, Marra MA. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181–185. doi: 10.1038/ng.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yap DB, Chu J, Berg T, Schapira M, Cheng SW, Moradian A, Morin RD, Mungall AJ, Meissner B, Boyle M, Marquez VE, Marra MA, Gascoyne RD, Humphries RK, Arrowsmith CH, Morin GB, Aparicio SA. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood. 2011;117:2451–2459. doi: 10.1182/blood-2010-11-321208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bödör C, O’riain C, Wrench D, Matthews J, Iyengar S, Tayyib H, Calaminici M, Clear A, Iqbal S, Quentmeier H, Drexler HG, Montoto S, Lister AT, Gribben JG, Matolcsy A, Fitzgibbon J. EZH2 Y641 mutations in follicular lymphoma. Leukemia. 2011;25:726–729. doi: 10.1038/leu.2010.311. [DOI] [PubMed] [Google Scholar]
- 82.Mccabe MT, Graves AP, Ganji G, Diaz E, Halsey WS, Jiang Y, Smitheman KN, Ott HM, Pappalardi MB, Allen KE, Chen SB, Della Pietra A, 3rd, Dul E, Hughes AM, Gilbert SA, Thrall SH, Tummino PJ, Kruger RG, Brandt M, Schwartz B, Creasy CL. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27) Proc Natl Acad Sci USA. 2012;109:2989–2994. doi: 10.1073/pnas.1116418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryan RJ, Nitta M, Borger D, Zukerberg LR, Ferry JA, Harris NL, Iafrate AJ, Bernstein BE, Sohani AR, Le LP. EZH2 codon 641 mutations are common in BCL2-rearranged germinal center B cell lymphomas. PLoS ONE. 2011;6:e28585. doi: 10.1371/journal.pone.0028585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salido M, Martinez-avilés L, Ademà V, Ferrer A, Espinet B, Garcia M, Salar A, Besses C, Florensa L, Serrano S, Bellosillo B, Solé F. Absence of mutations of the histone methyltransferase gene EZH2 in splenic b-cell marginal zone lymphoma. Leuk Res. 2011;35:e23–e24. doi: 10.1016/j.leukres.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 85.Sneeringer CJ, Scott MP, Kuntz KW, Knutson SK, Pollock RM, Richon VM, Copeland RA. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc Natl Acad Sci USA. 2010;107:20980–20985. doi: 10.1073/pnas.1012525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abdel-wahab O, Pardanani A, Patel J, Wadleigh M, Lasho T, Heguy A, Beran M, Gilliland DG, Levine RL, Tefferi A. Concomitant analysis of EZH2 and ASXL1 mutations in myelofibrosis, chronic myelomonocytic leukemia and blast-phase myeloproliferative neoplasms. Leukemia. 2011;25:1200–1202. doi: 10.1038/leu.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tönnissen ER, van der Heijden A, Scheele TN, Vandenberghe P, de Witte T, van der Reijden BA, Jansen JH. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42:665–677. doi: 10.1038/ng.620. [DOI] [PubMed] [Google Scholar]
- 88.Makishima H, Jankowska AM, Tiu RV, Szpurka H, Sugimoto Y, Hu Z, Saunthararajah Y, Guinta K, Keddache MA, Putnam P, Sekeres MA, Moliterno AR, List AF, McDevitt MA, Maciejewski JP. Novel homo- and hemizygous mutations in EZH2 in myeloid malignancies. Leukemia. 2010;24:1799–1804. doi: 10.1038/leu.2010.167. [DOI] [PubMed] [Google Scholar]
- 89.Park SW, Chung NG, Eom HS, Yoo NJ, Lee SH. Mutational analysis of EZH2 codon 641 in non-Hodgkin lymphomas and leukemias. Leuk Res. 2011;35:e6–e7. doi: 10.1016/j.leukres.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 90.Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV, Waghorn K, Zoi K, Ross FM, Reiter A, Hochhaus A, Drexler HG, Duncombe A, Cervantes F, Oscier D, Boultwood J, Grand FH, Cross NC. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42:722–736. doi: 10.1038/ng.621. [DOI] [PubMed] [Google Scholar]
- 91.van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C, Edkins S, Hardy C, O’Meara S, Teague J, Butler A, Hinton J, Latimer C, Andrews J, Barthorpe S, Beare D, Buck G, Campbell PJ, Cole J, Forbes S, Jia M, Jones D, Kok CY, Leroy C, Lin ML, McBride DJ, Maddison M, Maquire S, McLay K, Menzies A, Mironenko T, Mulderrig L, Mudie L, Pleasance E, Shepherd R, Smith R, Stebbings L, Stephens P, Tang G, Tarpey PS, Turner R, Turrell K, Varian J, West S, Widaa S, Wray P, Collins VP, Ichimura K, Law S, Wong J, Yuen ST, Leung SY, Tonon G, DePinho RA, Tai YT, Anderson KC, Kahnoski RJ, Massie A, Khoo SK, Teh BT, Stratton MR, Futreal PA. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009;41:521–533. doi: 10.1038/ng.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mar BG, Bullinger L, Basu E, Schlis K, Silverman LB, Döhner K, Armstrong SA. Sequencing histone-modifying enzymes identifies UTX mutations in acute lymphoblastic leukemia. Leukemia. 2012;26:1881–1893. doi: 10.1038/leu.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, Wu R, Chen C, Li X, Zhou L, He M, Li Z, Sun X, Jia W, Chen J, Yang S, Zhou F, Zhao X, Wan S, Ye R, Liang C, Liu Z, Huang P, Liu C, Jiang H, Wang Y, Zheng H, Sun L, Liu X, Jiang Z, Feng D, Chen J, Wu S, Zou J, Zhang Z, Yang R, Zhao J, Xu C, Yin W, Guan Z, Ye J, Zhang H, Li J, Kristiansen K, Nickerson ML, Theodorescu D, Li Y, Zhang X, Li S, Wang J, Yang H, Wang J, Cai Z. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43:875–888. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C, Teague J, Andrews J, Barthorpe S, Beare D, Buck G, Campbell PJ, Forbes S, Jia M, Jones D, Knott H, Kok CY, Lau KW, Leroy C, Lin ML, McBride DJ, Maddison M, Maguire S, McLay K, Menzies A, Mironenko T, Mulderrig L, Mudie L, O’Meara S, Pleasance E, Rajasingham A, Shepherd R, Smith R, Stebbings L, Stephens P, Tang G, Tarpey PS, Turrell K, Dykema KJ, Khoo SK, Petillo D, Wondergem B, Anema J, Kahnoski RJ, Teh BT, Stratton MR, Futreal PA. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–373. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, Ateeq B, Chun SY, Siddiqui J, Sam L, Anstett M, Mehra R, Prensner JR, Palanisamy N, Ryslik GA, Vandin F, Raphael BJ, Kunju LP, Rhodes DR, Pienta KJ, Chinnaiyan AM, Tomlins S. A.The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ansari KI, Mandal SS. Mixed lineage leukemia: roles in gene expression, hormone signaling and mRNA processing. FEBS J. 2010;277:1790–1804. doi: 10.1111/j.1742-4658.2010.07606.x. [DOI] [PubMed] [Google Scholar]
- 97.Morin RD, Mendez-lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M, Jackman S, Krzywinski M, Scott DW, Trinh DL, Tamura-Wells J, Li S, Firme MR, Rogic S, Griffith M, Chan S, Yakovenko O, Meyer IM, Zhao EY, Smailus D, Moksa M, Chittaranjan S, Rimsza L, Brooks-Wilson A, Spinelli JJ, Ben-Neriah S, Meissner B, Woolcock B, Boyle M, McDonald H, Tam A, Zhao Y, Delaney A, Zeng T, Tse K, Butterfield Y, Birol I, Holt R, Schein J, Horsman DE, Moore R, Jones SJ, Connors JM, Hirst M, Gascoyne RD, Marra MA. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, Harview CL, Brunet JP, Ahmann GJ, Adli M, Anderson KC, Ardlie KG, Auclair D, Baker A, Bergsagel PL, Bernstein BE, Drier Y, Fonseca R, Gabriel SB, Hofmeister CC, Jagannath S, Jakubowiak AJ, Krishnan A, Levy J, Liefeld T, Lonial S, Mahan S, Mfuko B, Monti S, Perkins LM, Onofrio R, Pugh TJ, Rajkumar SV, Ramos AH, Siegel DS, Sivachenko A, Stewart AK, Trudel S, Vij R, Voet D, Winckler W, Zimmerman T, Carpten J, Trent J, Hahn WC, Garraway LA, Meyerson M, Lander ES, Getz G, Golub TR. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pleasance ED, Stephens PJ, O’meara S, McBride DJ, Meynert A, Jones D, Lin ML, Beare D, Lau KW, Greenman C, Varela I, Nik-Zainal S, Davies HR, Ordoñez GR, Mudie LJ, Latimer C, Edkins S, Stebbings L, Chen L, Jia M, Leroy C, Marshall J, Menzies A, Butler A, Teague JW, Mangion J, Sun YA, McLaughlin SF, Peckham HE, Tsung EF, Costa GL, Lee CC, Minna JD, Gazdar A, Birney E, Rhodes MD, McKernan KJ, Stratton MR, Futreal PA, Campbell PJ. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang XX, Fu L, Li X, Wu X, Zhu Z, Fu L, Dong JT. Somatic mutations of the mixed-lineage leukemia 3 (MLL3) gene in primary breast cancers. Pathol Oncol Res. 2011;17:429–433. doi: 10.1007/s12253-010-9316-0. [DOI] [PubMed] [Google Scholar]
- 101.Watanabe Y, Castoro RJ, Kim HS, North B, Oikawa R, Hiraishi T, Ahmed SS, Chung W, Cho MY, Toyota M, Itoh F, Estecio MR, Shen L, Jelinek J, Issa JP. Frequent alteration of MLL3 frameshift mutations in microsatellite deficient colorectal cancer. PLoS ONE. 2011;6:e23320. doi: 10.1371/journal.pone.0023320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee J, Kim DH, Lee S, Yang QH, Lee DK, Lee SK, Roeder RG, Lee JW. A tumor suppressive coactivator complex of p53 containing ASC-2 and histone H3-lysine-4 methyltransferase MLL3 or its paralogue MLL4. Proc Natl Acad Sci USA. 2009;106:8513–8518. doi: 10.1073/pnas.0902873106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M, Lu C, Chen SC, Wei L, Collins-Underwood JR, Ma J, Roberts KG, Pounds SB, Ulyanov A, Becksfort J, Gupta P, Huether R, Kriwacki RW, Parker M, McGoldrick DJ, Zhao D, Alford D, Espy S, Bobba KC, Song G, Pei D, Cheng C, Roberts S, Barbato MI, Campana D, Coustan-Smith E, Shurtleff SA, Raimondi SC, Kleppe M, Cools J, Shimano KA, Hermiston ML, Doulatov S, Eppert K, Laurenti E, Notta F, Dick JE, Basso G, Hunger SP, Loh ML, Devidas M, Wood B, Winter S, Dunsmore KP, Fulton RS, Fulton LL, Hong X, Harris CC, Dooling DJ, Ochoa K, Johnson KJ, Obenauer JC, Evans WE, Pui CH, Naeve CW, Ley TJ, Mardis ER, Wilson RK, Downing JR, Mullighan CG. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.MacKinnon RN, Kannourakis G, Wall M, Campbell LJ. A cryptic deletion in 5q31.2 provides further evidence for a minimally deleted region in myelodysplastic syndromes. Cancer Genet. 2011;204:187–194. doi: 10.1016/j.cancergen.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 106.Lohr JG, Stojanov P, Lawrence MS, Auclair D, Chapuy B, Sougnez C, Cruz-Gordillo P, Knoechel B, Asmann YW, Slager SL, Novak AJ, Dogan A, Ansell SM, Link BK, Zou L, Gould J, Saksena G, Stransky N, Rangel-Escareño C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Hernández-Lemus E, Schwarz-Cruz y, Celis A, Imaz-Rosshandler I, Ojesina AI, Jung J, Pedamallu CS, Lander ES, Habermann TM, Cerhan JR, Shipp MA, Getz G, Golub TR. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A. 2012;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cross NC. Histone modification defects in developmental disorders and cancer. Oncotarget. 2012;3:3–4. doi: 10.18632/oncotarget.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hudlebusch HR, Santoni-rugiu E, Simon R, Ralfkiær E, Rossing HH, Johansen JV, Jørgensen M, Sauter G, Helin K. The histone methyltransferase and putative oncoprotein MMSET is overexpressed in a large variety of human tumors. Clin Cancer Res. 2011;17:2919–2933. doi: 10.1158/1078-0432.CCR-10-1302. [DOI] [PubMed] [Google Scholar]
- 109.Jaju RJ, Fidler C, Haas OA, Strickson AJ, Watkins F, Clark K, Cross NC, Cheng JF, Aplan PD, Kearney L, Boultwood J, Wainscoat JS. A novel gene, NSD1, is fused to NUP98 in the t(5;11)(q35;p15.5) in de novo childhood acute myeloid leukemia. Blood. 2001;98:1264–1277. doi: 10.1182/blood.v98.4.1264. [DOI] [PubMed] [Google Scholar]
- 110.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 111.Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- 112.Behm FG, Raimondi SC, Frestedt JL, Liu Q, Crist WM, Downing JR, Rivera GK, Kersey JH, Pui CH. Rearrangement of the MLL gene confers a poor prognosis in childhood acute lymphoblastic leukemia, regardless of presenting age. Blood. 1996;87:2870–2877. [PubMed] [Google Scholar]
- 113.Huo H, Magro PG, Pietsch EC, Patel BB, Scotto KW. Histone methyltransferase MLL1 regulates MDR1 transcription and chemoresistance. Cancer Res. 2010;70:8726–35. doi: 10.1158/0008-5472.CAN-10-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Angrand PO, Apiou F, Stewart AF, Dutrillaux B, Losson R, Chambon P. NSD3, a new SET domain-containing gene, maps to 8p12 and is amplified in human breast cancer cell lines. Genomics. 2001;74:79–88. doi: 10.1006/geno.2001.6524. [DOI] [PubMed] [Google Scholar]
- 115.Kassambara A, Klein B, Moreaux J. MMSET is overexpressed in cancers: link with tumor aggressiveness. Biochem Biophys Res Commun. 2009;379:840–855. doi: 10.1016/j.bbrc.2008.12.093. [DOI] [PubMed] [Google Scholar]
- 116.Hudlebusch HR, Skotte J, Santoni-rugiu E, Zimling ZG, Lees MJ, Simon R, Sauter G, Rota R, De Ioris MA, Quarto M, Johansen JV, Jørgensen M, Rechnitzer C, Maroun LL, Schrøder H, Petersen BL, Helin K. MMSET is highly expressed and associated with aggressiveness in neuroblastoma. Cancer Res. 2011;71:4226–4235. doi: 10.1158/0008-5472.CAN-10-3810. [DOI] [PubMed] [Google Scholar]
- 117.Zatkova A, Merk S, Wendehack M, Bilban M, Muzik EM, Muradyan A, Haferlach C, Haferlach T, Wimmer K, Fonatsch C, Ullmann R. AML/MDS with 11q/MLL amplification show characteristic gene expression signature and interplay of DNA copy number changes. Genes Chromosomes Cancer. 2009;48:510–520. doi: 10.1002/gcc.20658. [DOI] [PubMed] [Google Scholar]
- 118.Hamamoto R, Furukawa Y, Morita M, Iimura Y, Silva FP, Li M, Yagyu R, Nakamura Y. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol. 2004;6:731–740. doi: 10.1038/ncb1151. [DOI] [PubMed] [Google Scholar]
- 119.Luo XG, Ding Y, Zhou QF, Ye L, Wang SZ, Xi T. SET and MYND domain-containing protein 3 decreases sensitivity to dexamethasone and stimulates cell adhesion and migration in NIH3T3 cells. J Biosci Bioeng. 2007;103:444–450. doi: 10.1263/jbb.103.444. [DOI] [PubMed] [Google Scholar]
- 120.Silva FP, Hamamoto R, Kunizaki M, Tsuge M, Nakamura Y, Furukawa Y. Enhanced methyltransferase activity of SMYD3 by the cleavage of its N-terminal region in human cancer cells. Oncogene. 2008;27:2686–2692. doi: 10.1038/sj.onc.1210929. [DOI] [PubMed] [Google Scholar]
- 121.Brown MA, Sims RJ, Gottlieb PD, Tucker PW. Identification and characterization of Smyd2: a split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol Cancer. 2006;5:26. doi: 10.1186/1476-4598-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang J, Perez-burgos L, Placek BJ, Sengupta R, Richter M, Dorsey JA, Kubicek S, Opravil S, Jenuwein T, Berger SL. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;444:629–632. doi: 10.1038/nature05287. [DOI] [PubMed] [Google Scholar]
- 123.Komatsu S, Imoto I, Tsuda H, Kozaki KI, Muramatsu T, Shimada Y, Aiko S, Yoshizumi Y, Ichikawa D, Otsuji E, Inazawa J. Overexpression of SMYD2 relates to tumor cell proliferation and malignant outcome of esophageal squamous cell carcinoma. Carcinogenesis. 2009;30:1139–1146. doi: 10.1093/carcin/bgp116. [DOI] [PubMed] [Google Scholar]
- 124.Peters AH, O’carroll D, Scherthan H, Mechtler K, Sauer S, Schöfer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 125.Ozdag H, Teschendorff AE, Ahmed AA, Hyland SJ, Blenkiron C, Bobrow L, Veerakumarasivam A, Burtt G, Subkhankulova T, Arends MJ, Collins VP, Bowtell D, Kouzarides T, Brenton JD, Caldas C. Differential expression of selected histone modifier genes in human solid cancers. BMC Genomics. 2006;7:90. doi: 10.1186/1471-2164-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kondo Y, Shen L, Suzuki S, Kurokawa T, Masuko K, Tanaka Y, Kato H, Mizuno Y, Yokoe M, Sugauchi F, Hirashima N, Orito E, Osada H, Ueda R, Guo Y, Chen X, Issa JP, Sekido Y. Alterations of DNA methylation and histone modifications contribute to gene silencing in hepatocellular carcinomas. Hepatol Res. 2007;37:974–983. doi: 10.1111/j.1872-034X.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 127.Ceol CJ, Houvras Y, Jane-valbuena J, Bilodeau S, Orlando DA, Battisti V, Fritsch L, Lin WM, Hollmann TJ, Ferré F, Bourque C, Burke CJ, Turner L, Uong A, Johnson LA, Beroukhim R, Mermel CH, Loda M, Ait-Si-Ali S, Garraway LA, Young RA, Zon LI. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–517. doi: 10.1038/nature09806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takawa M, Masuda K, Kunizaki M, Daigo Y, Takagi K, Iwai Y, Cho HS, Toyokawa G, Yamane Y, Maejima K, Field HI, Kobayashi T, Akasu T, Sugiyama M, Tsuchiya E, Atomi Y, Ponder BA, Nakamura Y, Hamamoto R. Validation of the histone methyltransferase EZH2 as a therapeutic target for various types of human cancer and as a prognostic marker. Cancer Sci. 2011;102:1298–1305. doi: 10.1111/j.1349-7006.2011.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bryant RJ, Cross NA, Eaton CL, Hamdy FC, Cunliffe VT. EZH2 promotes proliferation and invasiveness of prostate cancer cells. Prostate. 2007;67:547–556. doi: 10.1002/pros.20550. [DOI] [PubMed] [Google Scholar]
- 130.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR Kumar-Sinha C, Sanda MG Ghosh D, Pienta KJ Sewalt RG, Otte AP Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 131.Kauffman EC, Robinson BD, Downes MJ, Powell LG, Lee MM, Scherr DS, Gudas LJ, Mongan NP. Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Mol Carcinog. 2011;50:931–944. doi: 10.1002/mc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hayami S, Kelly JD, Cho HS, Yoshimatsu M, Unoki M, Tsunoda T, Field HI, Neal DE, Yamaue H, Ponder BA, Nakamura Y, Hamamoto R. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int J Cancer. 2011;128:574–586. doi: 10.1002/ijc.25349. [DOI] [PubMed] [Google Scholar]
- 133.Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J, Metzger E, Schüle R, Buettner R. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 2006;66:11341–11347. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- 134.Lim S, Janzer A, Becker A, Zimmer A, Schüle R, Buettner R, Kirfel J. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31:512–20. doi: 10.1093/carcin/bgp324. [DOI] [PubMed] [Google Scholar]
- 135.Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- 136.Ehrbrecht A, Müller U, Wolter M, Hoischen A, Koch A, Radlwimmer B, Actor B, Mincheva A, Pietsch T, Lichter P, Reifenberger G, Weber RG. Comprehensive genomic analysis of desmoplastic medulloblastomas: identification of novel amplified genes and separate evaluation of the different histological components. J Pathol. 2006;208:554–563. doi: 10.1002/path.1925. [DOI] [PubMed] [Google Scholar]
- 137.Italiano A, Attias R, Aurias A, Pérot G, Burel-Vandenbos F, Otto J, Venissac N, Pedeutour F. Molecular cytogenetic characterization of a metastatic lung sarcomatoid carcinoma: 9p23 neocentromere and 9p23-p24 amplification including JAK2 and JMJD2C. Cancer Genet Cytogenet. 2006;167:122–130. doi: 10.1016/j.cancergencyto.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 138.Vinatzer U, Gollinger M, Müllauer L, Raderer M, Chott A, Streubel B. Mucosa-associated lymphoid tissue lymphoma: novel translocations including rearrangements of ODZ2, JMJD2C, and CNN3. Clin Cancer Res. 2008;14:6426–6431. doi: 10.1158/1078-0432.CCR-08-0702. [DOI] [PubMed] [Google Scholar]
- 139.Yang ZQ, Imoto I, Fukuda Y, Pimkhaokham A, Shimada Y, Imamura M, Sugano S, Nakamura Y, Inazawa J. Identification of a novel gene, GASC1, within an amplicon at 9p23–24 frequently detected in esophageal cancer cell lines. Cancer Res. 2000;60:4735–9. [PubMed] [Google Scholar]
- 140.Ishimura A, Terashima M, Kimura H, Akagi K, Suzuki Y, Sugano S, Suzuki T. Jmjd2c histone demethylase enhances the expression of Mdm2 oncogene. Biochem Biophys Res Commun. 2009;389:366–371. doi: 10.1016/j.bbrc.2009.08.155. [DOI] [PubMed] [Google Scholar]
- 141.Wissmann M, Yin N, Müller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Günther T, Buettner R, Metzger E, Schüle R. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 142.Chi P, Allis CD, Wang GG. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10:457–469. doi: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blair LP, Cao J, Zou MR, Sayegh J, Yan Q. Epigenetic Regulation by Lysine Demethylase 5 (KDM5) Enzymes in Cancer. Cancers (Basel) 2011;3:1383–1404. doi: 10.3390/cancers3011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong KK, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu PJ, Sundquist K, Baeckstrom D, Poulsom R, Hanby A, Meier-Ewert S, Jones T, Mitchell M, Pitha-Rowe P, Freemont P, Taylor-Papadimitriou J. A novel gene (PLU-1) containing highly conserved putative DNA/chromatin binding motifs is specifically up-regulated in breast cancer. J Biol Chem. 1999;274:15633–15645. doi: 10.1074/jbc.274.22.15633. [DOI] [PubMed] [Google Scholar]
- 146.Xiang Y, Zhu Z, Han G, Ye X, Xu B, Peng Z, Ma Y, Yu Y, Lin H, Chen AP, Chen CD. JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc Natl Acad Sci USA. 2007;104:19226–19231. doi: 10.1073/pnas.0700735104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smith JA, White EA, Sowa ME, Powell ML, Ottinger M, Harper JW, Howley PM. Genome-wide siRNA screen identifies SMCX, EP400, and Brd4 as E2-dependent regulators of human papillomavirus oncogene expression. Proc Natl Acad Sci USA. 2010;107:3752–3757. doi: 10.1073/pnas.0914818107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xiang Y, Zhu Z, Han G, Lin H, Xu L, Chen CD. JMJD3 is a histone H3K27 demethylase. Cell Res. 2007;17:850–857. doi: 10.1038/cr.2007.83. [DOI] [PubMed] [Google Scholar]
- 149.Frescas D, Guardavaccaro D, Bassermann F, Koyama-nasu R, Pagano M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature. 2007;450:309–313. doi: 10.1038/nature06255. [DOI] [PubMed] [Google Scholar]
- 150.Agger K, Cloos PA, Christensen J, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–7444. doi: 10.1038/nature06145. [DOI] [PubMed] [Google Scholar]
- 151.Hong S, Cho YW, Yu LR, Yu H, Veenstra TD, Ge K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci USA. 2007;104:18439–18444. doi: 10.1073/pnas.0707292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsai MC, Wang JK, Chang HY. Tumor suppression by the histone demethylase UTX. Cell Cycle. 2010;9:2043–2054. doi: 10.4161/cc.9.11.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fraga MF, Ballestar E, Villar-garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Pérez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MA, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 154.Elsheikh SE, Green AR, Rakha EA, Powe DG, Ahmed RA, Collins HM, Soria D, Garibaldi JM, Paish CE, Ammar AA, Grainge MJ, Ball GR, Abdelghany MK, Martinez-Pomares L, Heery DM, Ellis IO. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res. 2009;69:3802–3809. doi: 10.1158/0008-5472.CAN-08-3907. [DOI] [PubMed] [Google Scholar]
- 155.Ellinger J, Kahl P, Von der, gathen J, Rogenhofer S, Heukamp LC, Gütgemann I, Walter B, Hofstädter F, Büttner R, Müller SC, Bastian PJ, von Ruecker A. Global levels of histone modifications predict prostate cancer recurrence. Prostate. 2010;70:61–69. doi: 10.1002/pros.21038. [DOI] [PubMed] [Google Scholar]
- 156.Ellinger J, Kahl P, Von der, gathen J, Heukamp LC, Gütgemann I, Walter B, Hofstädter F, Bastian PJ, von Ruecker A, Müller SC, Rogenhofer S. Global histone H3K27 methylation levels are different in localized and metastatic prostate cancer. Cancer Invest. 2012;30:92–97. doi: 10.3109/07357907.2011.636117. [DOI] [PubMed] [Google Scholar]
- 157.Schneider AC, Heukamp LC, Rogenhofer S, Fechner G, Bastian PJ, von Ruecker A, Müller SC, Ellinger J. Global histone H4K20 trimethylation predicts cancer-specific survival in patients with muscle-invasive bladder cancer. BJU Int. 2011;108(8 Pt 2):E290–E296. doi: 10.1111/j.1464-410X.2011.10203.x. [DOI] [PubMed] [Google Scholar]
- 158.Seligson DB, Horvath S, Mcbrian MA, Mah V, Yu H, Tze S, Wang Q, Chia D, Goodglick L, Kurdistani SK. Global levels of histone modifications predict prognosis in different cancers. Am J Pathol. 2009;174:1619–1628. doi: 10.2353/ajpath.2009.080874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barlési F, Giaccone G, Gallegos-ruiz MI, Loundou A, Span SW, Lefesvre P, Kruyt FA, Rodriguez JA. Global histone modifications predict prognosis of resected non small-cell lung cancer. J Clin Oncol. 2007;25:4358–4364. doi: 10.1200/JCO.2007.11.2599. [DOI] [PubMed] [Google Scholar]
- 160.Barbés C, Sánchez J, Yebra MJ, Robert-Geró M, Hardisson C. Effects of sinefungin and S-adenosylhomocysteine on DNA and protein methyltransferases from Streptomyces and other bacteria. FEMS Microbiol Lett. 1990;57:239–243. [PubMed] [Google Scholar]
- 161.Greiner D, Bonaldi T, Eskeland R, Roemer E, Imhof A. Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3–9. Nat Chem Biol. 2005;1:143–155. doi: 10.1038/nchembio721. [DOI] [PubMed] [Google Scholar]
- 162.Iwasa E, Hamashima Y, Fujishiro S, Higuchi E, Ito A, Yoshida M, Sodeoka M. Total synthesis of (+)-chaetocin and its analogues: their histone methyltransferase G9a inhibitory activity. J Am Chem Soc. 2010;132:4078–4089. doi: 10.1021/ja101280p. [DOI] [PubMed] [Google Scholar]
- 163.Lakshmikuttyamma A, Scott SA, Decoteau JF, Geyer CR. Reexpression of epigenetically silenced AML tumor suppressor genes by SUV39H1 inhibition. Oncogene. 2010;29:576–88. doi: 10.1038/onc.2009.361. [DOI] [PubMed] [Google Scholar]
- 164.Sun Y, Takada K, Takemoto Y, Yoshida M, Nogi Y, Okada S, Matsunaga S. Gliotoxin analogues from a marine-derived fungus, Penicillium sp., and their cytotoxic and histone methyltransferase inhibitory activities. J Nat Prod. 2012;75:111–124. doi: 10.1021/np200740e. [DOI] [PubMed] [Google Scholar]
- 165.Kubicek S, O’sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, Jenuwein T. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol Cell. 2007;25:473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 166.Vedadi M, Barsyte-lovejoy D, Liu F, Rival-Gervier S, Allali-Hassani A, Labrie V, Wigle TJ, Dimaggio PA, Wasney GA, Siarheyeva A, Dong A, Tempel W, Wang SC, Chen X, Chau I, Mangano TJ, Huang XP, Simpson CD, Pattenden SG, Norris JL, Kireev DB, Tripathy A, Edwards A, Roth BL, Janzen WP, Garcia BA, Petronis A, Ellis J, Brown PJ, Frye SV, Arrowsmith CH, Jin J. A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells. Nat Chem Biol. 2011;7:566–574. doi: 10.1038/nchembio.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yuan Y, Wang Q, Paulk J, Kubicek S, Kemp MM, Adams DJ, Shamji AF, Wagner BK, Schreiber SL. A small-molecule probe of the histone methyltransferase g9a induces cellular senescence in pancreatic adenocarcinoma. ACS Chem Biol. 2012;7:1152–1157. doi: 10.1021/cb300139y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bernt KM, Armstrong SA. Targeting epigenetic programs in MLL-rearranged leukemias. Hematology Am Soc Hematol Educ Program. 2011;2011:354–60. doi: 10.1182/asheducation-2011.1.354. [DOI] [PubMed] [Google Scholar]
- 169.Yao Y, Chen P, Diao J, Cheng G, Deng L, Anglin JL, Prasad BV, Song Y. Selective inhibitors of histone methyltransferase DOT1L: design, synthesis, and crystallographic studies. J Am Chem Soc. 2011;133:16746–16759. doi: 10.1021/ja206312b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A, 3rd, Diaz E, LaFrance LV, Mellinger M, Duquenne C, Tian X, Kruger RG, McHugh CF, Brandt M, Miller WH, Dhanak D, Verma SK, Tummino PJ, Creasy CL. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 172.Konze KD, Ma A, Li F, Barsyte-Lovejoy D, Parton T, Macnevin CJ, Liu F, Gao C, Huang XP, Kuznetsova E, Rougie M, Jiang A, Pattenden SG, Norris JL, James LI, Roth BL, Brown PJ, Frye SV, Arrowsmith CH, Hahn KM, Wang GG, Vedadi M, Jin J. An Orally Bioavailable Chemical Probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem Biol. 2013 doi: 10.1021/cb400133j. Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Crea F, Fornaro L, Bocci G, Sun L, Farrar WL, Falcone A, Danesi R. EZH2 inhibition: targeting the crossroad of tumor invasion and angiogenesis. Cancer Metastasis Rev. 2012;31(3–4):753–761. doi: 10.1007/s10555-012-9387-3. [DOI] [PubMed] [Google Scholar]
- 174.Ferguson AD, Larsen NA, Howard T, Pollard H, Green I, Grande C, Cheung T, Garcia-Arenas R, Cowen S, Wu J, Godin R, Chen H, Keen N. Structural basis of substrate methylation and inhibition of SMYD2. Structure. 2011;19:1262–1273. doi: 10.1016/j.str.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 175.Lynch JT, Harris WJ, Somervaille TC. LSD1 inhibition: a therapeutic strategy in cancer? Expert Opin Ther Targets. 2012 doi: 10.1517/14728222.2012.722206. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 176.Edmondson DE, Binda C, Mattevi A. Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B. Arch Biochem Biophys. 2007;464:269–276. doi: 10.1016/j.abb.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 178.Rotili D, Mai A. Targeting Histone Demethylases: A New Avenue for the Fight against Cancer. Genes Cancer. 2011;2:663–679. doi: 10.1177/1947601911417976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schmidt DM, Mccafferty DG. trans-2-Phenylcyclopro-pylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry. 2007;46:4408–4416. doi: 10.1021/bi0618621. [DOI] [PubMed] [Google Scholar]
- 180.Mimasu S, Umezawa N, Sato S, Higuchi T, Umehara T, Yokoyama S. Structurally designed trans-2-phenylcyclopropylamine derivatives potently inhibit histone demethylase LSD1/KDM1. Biochemistry. 2010;49:6494–6503. doi: 10.1021/bi100299r. [DOI] [PubMed] [Google Scholar]
- 181.Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AH, Günther T, Buettner R, Schüle R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–9. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 182.Willmann D, Lim S, Wetzel S, Metzger E, Jandausch A, Wilk W, Jung M, Forne I, Imhof A, Janzer A, Kirfel J, Waldmann H, Schüle R, Buettner R. Impairment of prostate cancer cell growth by a selective and reversible lysine-specific demethylase 1 inhibitor. Int J Cancer. 2012;131:2704–2709. doi: 10.1002/ijc.27555. [DOI] [PubMed] [Google Scholar]
- 183.Huang Y, Greene E, Murray Stewart T, Goodwin AC, Baylin SB, Woster PM, Casero RA., Jr Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc Natl Acad Sci USA. 2007;104:8023–8028. doi: 10.1073/pnas.0700720104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang Y, Marton LJ, Woster PM, Casero RA. Polyamine analogues targeting epigenetic gene regulation. Essays Biochem. 2009;46:95–110. doi: 10.1042/bse0460007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huang Y, Stewart TM, Wu Y, Baylin SB, Marton LJ, Perkins B, Jones RJ, Woster PM, Casero RA., Jr Novel oligoamine analogues inhibit lysine-specific demethylase 1 and induce reexpression of epigenetically silenced genes. Clin Cancer Res. 2009;15:7217–7228. doi: 10.1158/1078-0432.CCR-09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang J, Lu F, Ren Q, Sun H, Xu Z, Lan R, Liu Y, Ward D, Quan J, Ye T, Zhang H. Novel histone demethylase LSD1 inhibitors selectively target cancer cells with pluripotent stem cell properties. Cancer Res. 2011;71:7238–7249. doi: 10.1158/0008-5472.CAN-11-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hamada S, Suzuki T, Mino K, Koseki K, Oehme F, Flamme I, Ozasa H, Itoh Y, Ogasawara D, Komaarashi H, Kato A, Tsumoto H, Nakagawa H, Hasegawa M, Sasaki R, Mizukami T, Miyata N. Design, synthesis, enzyme-inhibitory activity, and effect on human cancer cells of a novel series of jumonji domain-containing protein 2 histone demethylase inhibitors. J Med Chem. 2010;53:5629–5638. doi: 10.1021/jm1003655. [DOI] [PubMed] [Google Scholar]
- 188.Kristensen LH, Nielsen AL, Helgstrand C, Lees M, Cloos P, Kastrup JS, Helin K, Olsen L, Gajhede M. Studies of H3K4me3 demethylation by KDM5B/Jarid1B/PLU1 reveals strong substrate recognition in vitro and identifies 2,4-pyridine-dicarboxylic acid as an in vitro and in cell inhibitor. FEBS J. 2012;279:1905–1914. doi: 10.1111/j.1742-4658.2012.08567.x. [DOI] [PubMed] [Google Scholar]
- 189.King ON, Li XS, Sakurai M, Kawamura A, Rose NR, Ng SS, Quinn AM, Rai G, Mott BT, Beswick P, Klose RJ, Oppermann U, Jadhav A, Heightman TD, Maloney DJ, Schofield CJ, Simeonov A. Quantitative high-throughput screening dentifies 8-hydroxyquinolines as cell-active histone demethylase inhibitors. PLoS ONE. 2010;5:e15535. doi: 10.1371/journal.pone.0015535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lohse B, Kristensen JL, Kristensen LH, Agger K, Helin K, Gajhede M, Clausen RP. Inhibitors of histone demethylases. Bioorg Med Chem. 2011;19:3625–3636. doi: 10.1016/j.bmc.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 191.Luo X, Liu Y, Kubicek S, Myllyharju J, Tumber A, Ng S, Che KH, Podoll J, Heightman TD, Oppermann U, Schreiber SL, Wang X. A selective inhibitor and probe of the cellular functions of Jumonji C domain-containing histone demethylases. J Am Chem Soc. 2011;133:9451–9466. doi: 10.1021/ja201597b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kruidenier L, Chung CW, Cheng Z, Liddle J, Che K, Joberty G, Bantscheff M, Bountra C, Bridges A, Diallo H, Eberhard D, Hutchinson S, Jones E, Katso R, Leveridge M, Mander PK, Mosley J, Ramirez-Molina C, Rowland P, Schofield CJ, Sheppard RJ, Smith JE, Swales C, Tanner R, Thomas P, Tumber A, Drewes G, Oppermann U, Patel DJ, Lee K, Wilson DM. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–418. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mo R, Rao SM, Zhu YJ. Identification of the MLL2 complex as a coactivator for estrogen receptor alpha. J Biol Chem. 2006;281:15714–15720. doi: 10.1074/jbc.M513245200. [DOI] [PubMed] [Google Scholar]
- 194.Piao GH, Piao WH, He Y, Zhang HH, Wang GQ, Piao Z. Hyper-methylation of RIZ1 tumor suppressor gene is involved in the early tumorigenesis of hepatocellular carcinoma. Histol Histopathol. 2008;23:1171–1185. doi: 10.14670/HH-23.1171. [DOI] [PubMed] [Google Scholar]
- 195.Hasegawa Y, Matsubara A, Teishima J, Seki M, Mita K, Usui T, Oue N, Yasui W. DNA methylation of the RIZ1 gene is associated with nuclear accumulation of p53 in prostate cancer. Cancer Sci. 2007;98:32–36. doi: 10.1111/j.1349-7006.2006.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tam W, Gomez MF, Chadburn A, Knowles DM, Houldsworth J. Lack of A563G (I188V) missense mutation in RIZ/ PRDM2 in human diffuse large B-cell lymphomas. Genes Chromosomes Cancer. 2007;46:416–8. doi: 10.1002/gcc.20409. [DOI] [PubMed] [Google Scholar]
- 197.Geli J, Nord B, Frisk T, Edström Elder E, Ekström TJ, Carling T, Bäckdahl M, Larsson C. Deletions and altered expression of the RIZ1 tumour suppressor gene in 1p36 in pheochromocytomas and abdominal paragangliomas. Int J Oncol. 2005;26:1385–91. [PubMed] [Google Scholar]
- 198.Abbondanza C, De rosa C, D’arcangelo A, Pacifico M, Spizuoco C, Piluso G, Di Zazzo E, Gazzerro P, Medici N, Moncharmont B, Puca GA. Identification of a functional estrogen-responsive enhancer element in the promoter 2 of PRDM2 gene in breast cancer cell lines. J Cell Physiol. 2012;227:964–975. doi: 10.1002/jcp.22803. [DOI] [PubMed] [Google Scholar]
- 199.Kim JY, Kim KB, Eom GH, Choe N, Kee HJ, Son HJ, Oh ST, Kim DW, Pak JH, Baek HJ, Kook H, Hahn Y, Kook H, Chakravarti D, Seo SB. KDM3B Is the H3K9 Demethylase Involved in Transcriptional Activation of lmo2 in Leukemia. Mol Cell Biol. 2012;32:2917–2933. doi: 10.1128/MCB.00133-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Björkman M, Ostling P, Härmä V, Virtanen J, Mpindi JP, Rantala J, Mirtti T, Vesterinen T, Lundin M, Sankila A, Rannikko A, Kaivanto E, Kohonen P, Kallioniemi O, Nees M. Systematic knockdown of epigenetic enzymes identifies a novel histone demethylase PHF8 overexpressed in prostate cancer with an impact on cell proliferation, migration and invasion. Oncogene. 2012;31:3444–3456. doi: 10.1038/onc.2011.512. [DOI] [PubMed] [Google Scholar]
- 201.Taketani T, Taki T, Nakamura H, Taniwaki M, Masuda J, Hayashi Y. NUP98-NSD3 fusion gene in radiation-associated myelodysplastic syndrome with t (8;11)(p11; p15) and expression pattern of NSD family genes. Cancer Genet Cytogenet. 2009;190:108–112. doi: 10.1016/j.cancergencyto.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 202.Zhou Z, Thomsen R, Kahns S, Nielsen AL. The NSD3L histone methyltransferase regulates cell cycle and cell invasion in breast cancer cells. Biochem Biophys Res Commun. 2010;398:565–570. doi: 10.1016/j.bbrc.2010.06.119. [DOI] [PubMed] [Google Scholar]
- 203.Toyokawa G, Cho HS, Masuda K, Yamane Y, Yoshimatsu M, Hayami S, Takawa M, Iwai Y, Daigo Y, Tsuchiya E, Tsunoda T, Field HI, Kelly JD, Neal DE, Maehara Y, Ponder BA, Nakamura Y, Hamamoto R. Histone lysine methyltransferase Wolf-Hirschhorn syndrome candidate 1 is involved in human carcinogenesis through regulation of the Wnt pathway. Neoplasia. 2011;13:887–898. doi: 10.1593/neo.11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yew CW, Lee P, Chan WK, Lim VK, Tay SK, Tan TM, Deng LW. A novel MLL5 isoform that is essential to activate E6 and E7 transcription in HPV16/18-associated cervical cancers. Cancer Res. 2011;71:6696–66707. doi: 10.1158/0008-5472.CAN-11-1271. [DOI] [PubMed] [Google Scholar]
- 205.Deng LW, Chiu I, Strominger JL. MLL 5 protein forms intranuclear foci, and overexpression inhibits cell cycle progression. Proc Natl Acad Sci U S A. 2004;101:757–62. doi: 10.1073/pnas.2036345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li H, Rauch T, Chen ZX, Szabó PE, Riggs AD, Pfeifer GP. The histone methyltransferase SETDB1 and the DNA methyltransferase DNMT3A interact directly and localize to promoters silenced in cancer cells. J Biol Chem. 2006;281:19489–19500. doi: 10.1074/jbc.M513249200. [DOI] [PubMed] [Google Scholar]
- 207.Yamada D, Kobayashi S, Yamamoto H, Tomimaru Y, Noda T, Uemura M, Wada H, Marubashi S, Eguchi H, Tanemura M, Doki Y, Mori M, Nagano H. Role of the Hypoxia-Related Gene, JMJD1A, in Hepatocellular Carcinoma: Clinical Impact on Recurrence after Hepatic Resection. Ann Surg Oncol. 2012;19:355–364. doi: 10.1245/s10434-011-1797-x. [DOI] [PubMed] [Google Scholar]
- 208.Cho HS, Toyokawa G, Daigo Y, Hayami S, Masuda K, Ikawa N, Yamane Y, Maejima K, Tsunoda T, Field HI, Kelly JD, Neal DE, Ponder BA, Maehara Y, Nakamura Y, Hamamoto R. The JmjC domain-containing histone demethylase KDM3A is a positive regulator of the G1/S transition in cancer cells via transcriptional regulation of the HOXA1 gene. Int J Cancer. 2012;131:E179–E189. doi: 10.1002/ijc.26501. [DOI] [PubMed] [Google Scholar]
- 209.Li W, Zhao L, Zang W, Liu Z, Chen L, Liu T, Xu D, Jia J. Histone demethylase JMJD2B is required for tumor cell proliferation and survival and is overexpressed in gastric cancer. Biochem Biophys Res Commun. 2011;416:372–378. doi: 10.1016/j.bbrc.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 210.Toyokawa G, Cho HS, Iwai Y, Yoshimatsu M, Takawa M, Hayami S, Maejima K, Shimizu N, Tanaka H, Tsunoda T, Field HI, Kelly JD, Neal DE, Ponder BA, Maehara Y, Nakamura Y, Hamamoto R. The histone demethylase JMJD2B plays an essential role in human carcinogenesis through positive regulation of cyclin-dependent kinase 6. Cancer Prev Res (Phila) 2011;4:2051–2061. doi: 10.1158/1940-6207.CAPR-11-0290. [DOI] [PubMed] [Google Scholar]
- 211.Fukuda T, Tokunaga A, Sakamoto R, Yoshida N. Fbxl10/Kdm2b deficiency accelerates neural progenitor cell death and leads to exencephaly. Mol Cell Neurosci. 2011;46:14–24. doi: 10.1016/j.mcn.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 212.Matthieu S. Structural Chemistry of Human SET Domain Protein Methyltransferases. Curr Chem Genomics. 2011;5:85–94. doi: 10.2174/1875397301005010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.He Y, Korboukh I, Jin J, Huang J. “Targeting protein lysine methylation and demethylation in cancers”. Acta Biochim Biophys Sin (Shanghai) 2012;44:70–79. doi: 10.1093/abbs/gmr109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dong KB, Maksakova IA, Mohn F, Leung D, Appanah R, Lee S, Yang HW, Lam LL, Mager DL, Schübeler D, Tachibana M, Shinkai Y, Lorincz MC. DNA methylation in ES cells requires the lysine methyltransferase G9a but not its catalytic activity. EMBO J. 2008;27:2691–2701. doi: 10.1038/emboj.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ikegami K, Iwatani M, Suzuki M, Tachibana M, Shinkai Y, Tanaka S, Greally JM, Yagi S, Hattori N, Shiota K. Genomewide and locus-specific DNA hypomethylation in G9a deficient mouse embryonic stem cells. Genes Cells. 2007;12:1–11. doi: 10.1111/j.1365-2443.2006.01029.x. [DOI] [PubMed] [Google Scholar]
- 216.Damm F, Oberacker T, Thol F, Surdziel E, Wagner K, Chaturvedi A, Morgan M, Bomm K, Göhring G, Lübbert M, Kanz L, Fiedler W, Schlegelberger B, Heil G, Schlenk RF, Döhner K, Döhner H, Krauter J, Ganser A, Heuser M. Prognostic importance of histone methyltransferase MLL5 expression in acute myeloid leukemia. J Clin Oncol. 2011;29:682–689. doi: 10.1200/JCO.2010.31.1118. [DOI] [PubMed] [Google Scholar]
- 217.Cock-Rada AM, Medjkane S, Janski N, Yousfi N, Perichon M, Chaussepied M, Chluba J, Langsley G, Weitzman JB, et al. SMYD3 promotes cancer invasion by epigenetic upregulation of the metalloproteinase MMP-9. Cancer Res. 2012;72:810–820. doi: 10.1158/0008-5472.CAN-11-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ferrari-Amorotti G, Fragliasso V, Esteki R, Prudente Z, Soliera AR, Cattelani S, Manzotti G, Grisendi G, Dominici M, Pieraccioli M, Raschellà G, Chiodoni C, Colombo MP, Calabretta B. Inhibiting interactions of lysine demethylase LSD1 with Snail/Slug blocks cancer cell invasion. Cancer Res. 2013;73:235–245. doi: 10.1158/0008-5472.CAN-12-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zeng J, Ge Z, Wang L, Li Q, Wang N, Björkholm M, Jia J, Xu D. The histone demethylase RBP2 Is overexpressed in gastric cancer and its inhibition triggers senescence of cancer cells. Gastroenterology. 2010;138:981–992. doi: 10.1053/j.gastro.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 220.Hidalgo A, Baudis M, Petersen I, Arreola H, Piña P, Vázquez-Ortiz G, Hernández D, González J, Lazos M, López R, Pérez C, García J, Vázquez K, Alatorre B, Salcedo M. Microarray comparative genomic hybridization detection of chromosomal imbalances in uterine cervix carcinoma. BMC Cancer. 2005;5:77. doi: 10.1186/1471-2407-5-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.van Zutven LJ, Onen E, Velthuizen SC, et al. Identification of NUP98 abnormalities in acute leukemia: JARID1A (12p13) as a new partner gene. Genes Chromosomes Cancer. 2006;45:437–446. doi: 10.1002/gcc.20308. [DOI] [PubMed] [Google Scholar]
- 222.Kawazu M, Saso K, Tong KI, McQuire T, Goto K, Son DO, Wakeham A, Miyagishi M, Mak TW, Okada H. Histone demethylase JMJD2B functions as a co-factor of estrogen receptor in breast cancer proliferation and mammary gland development. PLoS One. 2011;6:e17830. doi: 10.1371/journal.pone.0017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nijwening JH, Geutjes EJ, Bernards R, Beijersbergen RL. The histone demethylase Jarid1b (Kdm5b) is a novel component of the Rb pathway and associates with E2f–target genes in MEFs during senescence. PLoS One. 2011;6:e25235. doi: 10.1371/journal.pone.0025235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, Bucci G, Caganova M, Notarbartolo S, Casola S, Testa G, Sung WK, Wei CL, Natoli G. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28:3341–3352. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]