Abstract
Background
Skeletal muscle is one of the most important economic traits in agricultural animals, especially in pigs. In the modern pig industry, lean type pigs have undergone strong artificial selection for muscle growth, which has led to remarkable phenotypic variations compared with fatty type pigs, making these different breeds an ideal model for comparative studies.
Results
Here, we present comprehensive gene expression profiling for the white (longissimus dorsi muscle) and the red (psoas major muscle) skeletal muscles among male and female fatty Rongchang, feral Tibetan and lean Landrace pigs, using a microarray approach. We identified differentially expressed genes that may be associated the phenotypic differences of porcine muscles among the breeds, between the sexes and the anatomical locations. We also used a clustering method to identify sets of functionally coexpressed genes that are linked to different muscle phenotypes. We showed that, compared with the white muscles, which primarily modulate metabolic processes, the red muscles show a tendency to be a risk factor for inflammation and immune-related disorders.
Conclusions
This analysis presents breed-, sex- and anatomical location-specific gene expression profiles and further identified genes that may be associated with the phenotypic differences in porcine muscles among breeds, between the sexes and the anatomical locations.
Keywords: Gene expression, Microarray, Muscle, Pig
Background
Skeletal muscle is the most abundant tissue, comprising approximately 50% of the total body mass in mammals [1]. It is not only a motor organ, but also part of the endocrine system, participating in the regulation of whole body metabolism [2]. Skeletal muscle, as a highly heterogeneous tissue, is composed of a variety of functionally diverse myofibre types [3]; mainly the red (type I and IIa) and the white (type IIb) fibers. Red skeletal muscles, such as the psoas major muscles (PMM), have a higher percentage of capillaries, myoglobin, lipids and mitochondria [4], making them a better aerobic machine than the paler-appearing white muscle [5]. White skeletal muscles, such as the longissimus doris muscles (LDM) [4], are required for anaerobic glycolytic metabolism to support the high transient energy demand [6].
Deciphering the different gene expression patterns between the different tissues would aid in our understanding of their distinct metabolic features. Mo et al. identified various candidate genes involved in cell adhesion, energy balance, muscle atrophy and myogenesis by comparing patterns of gene expression in three independent mouse models of Kennedy disease/spinal bulbar muscular atrophy [7]. Wolfs et al. reported that coexpressed immune and metabolic genes are associated with plasma high density lipoprotein and glucose levels by comparing genome-wide transcription profiling of subcutaneous and visceral adipose tissues obtained from obese patients [8]. Previous reports also suggested that ethnic group and sex are also the important factors that affect physiological and biochemical features of skeletal muscles in mammals [9-12].
Pigs are important agricultural animals and ideal biomedical models [13]. In the modern pig industry, pigs have undergone strong artificial selection for lean meat or adipose production, which has led to remarkable phenotypic variations, making these different breeds a perfect model for comparative studies [14,15]. Using a microarray approach, Bai et al. noted that most differentially expressed genes between porcine PMM and LDM were of mitochondrial origin [16]. Li et al. (2010) reported that the differentially expressed genes between the LDM and soleus muscle of Chinese Meishan pigs were mainly over-represented in various signaling pathways (particularly TGF-β, MAPK, Wnt, mTOR and insulin pathways) [17]. Nonetheless, the different gene expression profiles associated with breed and sex in skeletal muscle tissues has been long overdue, and elucidation of this information will benefit the development of strategies for skeletal muscle manipulation.
Here, using a microarray technology, we present a comprehensive survey of gene expression profiles between two phenotypically distinct skeletal muscles and sexes of three well-defined pig breeds displaying distinct muscle phenotypes. This study will contribute to our understanding of the molecular process of muscle fiber type formulation and provide a theoretical basis for breed and meat quality improvement in pigs.
Results and discussion
Phenotypic measurements
Our previous report, based on the same individuals, demonstrated that the myofibre cross-sectional area (CSA) and myofibre ratio were significant different between the two skeletal tissues, between the male and female and among the three breeds [18] (Additional file 1: Figure S1). In addition, 24 representative metabolism indicators in serum also revealed the same ranking from the leaner Landrace, the wild Tibetan and the fatty Rongchang pigs [18] (Additional file 2: Table S1).
Functional enrichment analysis of differentially expressed genes
Out of 4,309 high-confidence and well annotated probe-targeted genes (Additional file 3: Tables S2), we identified five (0.12%), 444 (10.3%) and 1,359 (31.54%) differentially expressed genes (DEGs) between the sexes and the two tissues, and among the three breeds (P < 0.05, three-way ANOVA, n = 3 per breed per sex per tissue) (Additional file 4: Tables S3), respectively. These DEGs could discriminate the different breeds, sexes and tissues (Figure 1). The high number of DEGs among three pig breeds implies distinct muscle features among different pig breeds. In addition, the biological replicates correlated with each other (average Spearman’s r = 0.99, Figure 1), which suggested experimental reliability and further highlighted the low variation in gene expression profiles across different individuals.
Figure 1.
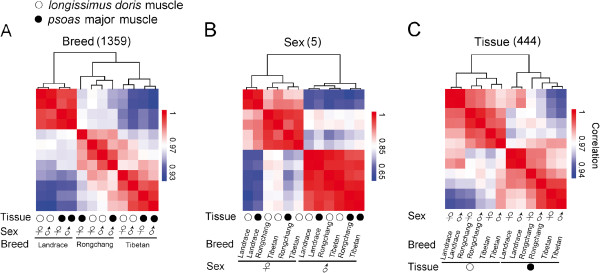
Heat map matrix of Spearman correlations among samples. (A) The counts of 1,359 DEGs among the three breeds. (B) The counts of five DEGs between the sexes. (C) The counts of 444 DEGs between the two tissues.
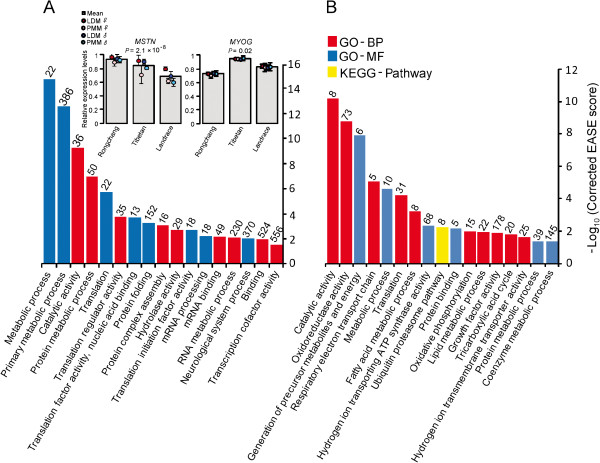
We found that the breed-specific DEGs were significantly enriched in the Gene Ontology (GO) categories of protein metabolism (i.e. protein metabolic process, translation, protein folding and protein complex assembly) and RNA metabolism (i.e. mRNA processing and RNA metabolic process) (Figure 2A). Various well-known genes involved in growth and development of skeletal muscles were identified. For example, myostatin (MSTN), a secreted transforming growth factor (TGF) beta protein family member, inhibits the differentiation and growth of muscle and Akt-induced protein synthesis [19]. The expression level of MSTN was highest in Rongchang pigs and lowest in Landrace pigs, which is consistent with the breeds’ characteristics (Figure 2A). Myogenin (MYOG) transforms potential mesoderm cells to sarcoblasts, and has a critical role in the terminal differentiation of the specified muscle cells [20,21]. Among the three breeds, the expression levels of MYOG were highest in Tibetan pigs and lowest in Rongchang pigs (Figure 2A). This result suggests that the breed-specific differences in muscle were mainly related to the protein translation process, which is consistent with previous studies [22-24]. Additionally, we found breed-specific DEGs that were over-represented in the neurological system process (370 DEGs, P = 0.01), which highlights the important roles of myoblast lineage and innervations in the diversification of skeletal muscle fiber types.
Figure 2.
Analysis of DEGs. (A) Gene Ontology (GO) categories enriched for breed-specific DEGs and relative expression level of MSTN and MYOG genes involved in breed-specific DEGs. LDM and PMM mean longissimus dorsi muscle and psoas major muscle, respectively. Datas are means ± SD. The significance of differences among samples was determined by One-way ANOVA. (B) Gene Ontology (GO) categories enriched for tissue-specific DEGs. The EASE score, which indicated the significance of the comparison, was calculated by Benjamini-corrected modified Fisher’s exact test. BP, biological process; MF, molecular function.
Tissue-specific DEGs were significantly enriched in energy metabolism related processes (i.e. generation of precursor metabolites and energy, respiratory electron transport chain, fatty acid metabolic process, oxidative phosphorylation, lipid metabolic process, tricarboxylic acid cycle and coenzyme metabolic process) (Figure 2B), which is consistent with the distinct features of energy expenditure regulation between the LDM and PMM [25]. Energy availability is important in the formation of mature muscle fibers and is essential for muscle proliferation and differentiation. Louis et al. reported that the energy content of cultured satellite cells is related to the hypertrophy of myofibres in vitro, which indicated a direct connection between energy metabolism and myogenesis [26]. Cagnazzo et al. also demonstrated that myogenic differentiation and energy metabolism were directly connected processes [27]. Genes involved in energy metabolism were identified. For example, MDH1, PDK3 and GOT1 play important roles in sympathetic-induced metabolism, which is involved in modulating the activity of glyceroneogenesis [28]. MDH1, PDK3 and GOT1 showed lower gene expression levels in the LDM than in PMM (Additional file 5: Figure S2), which agreed with previous reports [29-32]. We also found that tissue-specific DEGs were over-represented in the ubiquitin-proteasome pathway (Figure 2B), which plays a critical role in the adaptation of skeletal muscle to persistent decreases or increases in muscle activity. The ubiquitin-proteasome pathway is constitutively active in muscle and continually regulates protein turnover [33].
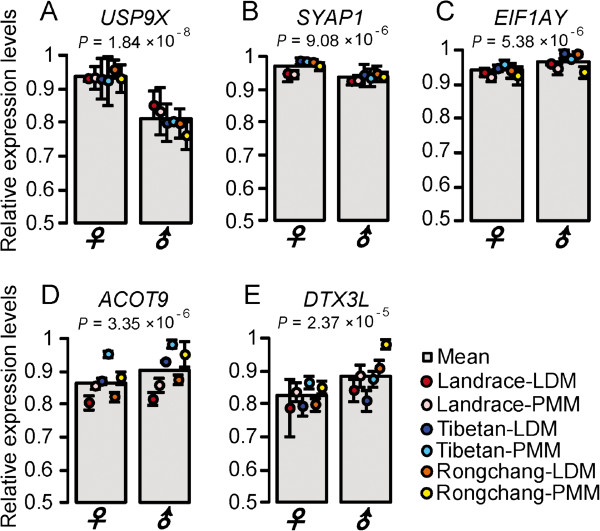
We only identified five DEGs between the sexes, of which two are X-linked genes (ubiquitin specific peptidase 9 (USP9X) and synapse associated protein 1 (SYAP1)) that exhibited higher expression levels in females than in males (P < 10-5, Student’s t-test; Figure 3A and Figure 3B). USP9X, as a novel mTORC1 and −2 binding partner, negatively regulates mTOR activity and further affects the differentiation of skeletal muscle [34]. SYAP1 plays an important role in cancer formation [35]. By contrast, a Y-linked gene, eukaryotic translation initiation factor 1A (EIF1AY) exhibited significantly higher expression in males than in females (P = 5.38 × 10-6, Student’s t-test; Figure 3C), which could affect the maximal rate of protein biosynthesis [36]. Additionally, two DEGs are located in the autosome: acyl-CoA thioesterase 9 (ACOT9) and the deltex 3-like (DTX3L), which exhibited higher mRNA expression levels in males than in females (P < 10-4, Student’s t-test; Figure 3D and Figure 3E). ACOT9, as an important enzyme involved in fatty acid metabolism, is located in the mitochondrion and provides energy through the citric acid cycle [37]. The higher mRNA expression level of ACOT9 in males reflects the fact that male muscles have a higher capacity for anaerobic metabolism and generate a higher maximum power output than female muscles [11]. DTX3L plays an important role in the Notch signaling pathway and controls myogenesis; its higher expression in male muscles is consistent with male pigs having more and larger muscles than the females [38].
Figure 3.
Relative expression levels of sex-specific DEGs. (A)USP9X gene; (B)SYAP1 gene; (C)EIF1AY gene; (D)ACOT9 gene; (E)DTX3L gene. LDM and PMM mean longissimus dorsi muscle and psoas major muscle, respectively. Data are means ± SD. The significance of differences among samples was determined by Student’s t-test.
Validation of gene expression changes by Quantitative PCR (Q-PCR)
Six genes (ADIPOR1, ADIPOR2, CAV1, CAV2, INSIG1, and MDH1) were selected to confirm their expression patterns using Q-PCR. The results indicated that the expression patterns of these genes were consistent with the microarray (average Pearson’s r = 0.86; Additional file 6: Figure S3).
Analysis of coexpressed gene modules
To extract more biological information within the genome-wide expression data set that could not be provided by individual, we constructed coexpressed gene modules and performed association analysis with the phenotypic traits, as did previous reports [8].
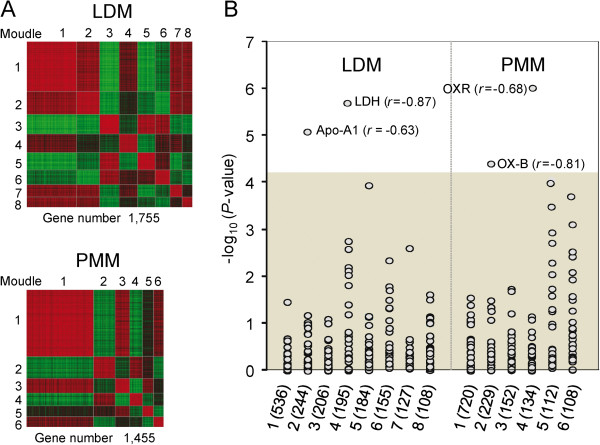
We identified eight and six gene modules for LDM and PMM (more than 100 genes per module), representing 1,755 and 1,455 genes, respectively (Additional file 7: Table S4A and 4B). Expressions of genes within a single gene module are strongly correlated, whereas genes that belong to different modules generally show no significant coexpression (Figure 4A). As shown in Additional file 8: Table S5, eight gene modules of LDM and PMM significantly overlapped with each other (P < 0.01, Fisher’s exact tests), which implies that similar gene expression patterns are involved in basic physiological and biochemical processes of skeletal muscle.
Figure 4.
Analysis of coexpressed gene modules in LDM and PMM. (A) Heat map of coexpressed gene modules in longissimus dorsi muscle (LDM) and psoas major muscle (PMM). Gene pairs strongly positively or negatively correlated are shown in red or green, respectively. (B) Correlations between coexpressed gene modules in two muscle tissues and phenotypic traits. -Log P-values for Spearman correlation coefficients between the values of the modules and the different phenotypic traits are shown. The gray shadow represents a highly stringent Bonferroni corrected P-value of 0.05.
We identified two coexpressed gene modules in LDM that were significantly negatively correlated with the amount of apolipoprotein A1 (Apo-A1) (Spearman’s r = −0.63, P =8.71 × 10-6) and lactate dehydrogenase (LDH) (Spearman’s r = −0.87, P =2.05 × 10-6, Figure 4B) in serum, which are primarily involved in metabolic processes. Apo-A1 is a major protein component of high density lipoprotein in serum and has been suggested to be tightly linked to muscle differentiation [39]. LDH is a marker of the oxidative and glycolytic capacities of the muscle, and converts pyruvate to lactate when oxygen is absent or in short supply [40]. The genes within these two gene modules were mainly enriched in the categories of protein metabolic process (94 genes, P = 5.03 × 10-4), cellular metabolic process (100 genes, P = 5.22 × 10-6), cellular nitrogen compound metabolic process (60 genes, P = 0.048) and primary metabolic process (127 genes, P = 4.55 × 10-8) (Table 1). These findings confirmed the report that the LDM is mainly associated with metabolic rate [41].
Table 1.
Gene Ontology (GO) categories enriched for coexpressed gene modules that correlated with phenotypic traits
| Tissues (gene module no.) | Correlated trait | Functional category | Term description | P value | Involved gene no. |
|---|---|---|---|---|---|
| LDM (2) |
Apo-A1 |
GO-BP |
Metabolic process |
1.06 × 10-7 |
139 |
| GO-BP |
Primary metabolic process |
4.55 × 10-8 |
127 |
||
| GO-BP |
Protein metabolic process |
5.03 × 10-4 |
94 |
||
| GO-BP |
Carbohydrate metabolic process |
0.028 |
82 |
||
| GO-BP |
Protein modification process |
0.016 |
73 |
||
| GO-BP |
Protein amino acid phosphorylation |
0.016 |
65 |
||
| LDM (4) |
LDH |
GO-BP |
Cellular metabolic process |
5.22 × 10-6 |
100 |
| GO-BP |
Metabolic process |
5.58 × 10-5 |
105 |
||
| GO-BP |
Cellular process |
2.47 × 10-4 |
128 |
||
| GO-BP |
Cellular nitrogen compound metabolic process |
0.048 |
60 |
||
| GO-BP |
Primary metabolic process |
0.016 |
93 |
||
| PMM (2) |
OX-B |
GO-BP |
Immune system process |
2.12 × 10-5 |
29 |
| GO-BP |
Inflammatory response |
0.001 |
16 |
||
| GO-BP |
Response to external stimulus |
0.001 |
26 |
||
| GO-BP |
Response to wounding |
0.001 |
20 |
||
| GO-BP |
Immune response |
0.001 |
22 |
||
| GO-BP |
Regulation of immune system process |
0.002 |
16 |
||
| GO-BP |
Regulation of response to stimulus |
0.004 |
17 |
||
| GO-BP |
Positive regulation of immune system process |
0.006 |
12 |
||
| GO-BP |
Regulation of immune response |
0.012 |
12 |
||
| GO-BP |
Lymphocyte activation |
0.021 |
11 |
||
| GO-BP |
Cell activation |
0.031 |
12 |
||
| GO-BP |
Leukocyte activation |
0.033 |
11 |
||
| GO-BP |
Positive regulation of lymphocyte activation |
0.049 |
8 |
||
| GO-MF |
Receptor binding |
2.19 × 10-5 |
30 |
||
| PMM (4) |
OXR |
GO-BP |
Immune response |
0.019 |
9 |
| GO-BP |
Immune system process |
1.79 × 10-6 |
16 |
||
| GO-BP |
Cellular defense response |
0.027 |
6 |
||
| GO-BP |
Response to stimulus |
4.87 × 10-4 |
12 |
||
| GO-BP | Cell-matrix adhesion | 2.24 × 10-3 | 10 |
We also found that two coexpressed gene modules in PMM were significantly negatively correlated with amount of orexin-B (OX-B) (Spearman’s r = −0.81, P =5.75 × 10-5) and the orexin receptor (OXR) (Spearman’s r = −0.68, P = 1.04 × 10-6, Figure 4B) in serum, which are representative indicators for the inflammatory process and the immune system in serum. The genes within these two gene modules were mainly enriched in the categories of the immune system process (29 genes, P = 2.12 × 10-5), inflammatory response (16 genes, P = 0.001), immune response (22 genes, P = 0.001), lymphocyte activation (11 genes, P = 0.02), leukocyte activation (11 genes, P = 0.03), and cellular defense response (6 genes, P = 0.02) (Table 1), which suggests that the PMM is a metabolic risk factor. This finding is consistent with evidence that shows that the PMM is supplied by venous blood from the lumbar spine and has lymphatics overlying the muscle from nearby intra-abdominal organs, making it highly susceptible to contiguous infection and inflammation from organs such as the colon, appendix, terminal ileum and several intra-abdominal structures [42-44].
Conclusions
The analysis presented the gene expression profiles and identified DEGs that may be related to the phenotypic differences in porcine muscles among breeds, between the sexes and the anatomical locations. The results provide a basis for further exploration of the molecular process of muscle fiber type formulation, and may also help the further development of biomarkers for important economic traits (i.e. pork quality and yield) in pigs.
Methods
Sample preparation
Three females and three males at 210-days-old for each of the leaner Landrace pigs, the wild Tibetan pigs and the fatty Rongchang pigs were used in this study as previously described [18]. Animals were humanely sacrificed, according to the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, revised in June 2004) and approved by the Institutional Animal Care and Use Committee in the College of Animal Science and Technology, Sichuan Agricultural University, Sichuan, China. The longissimus dorsi muscle (LDM, typical white muscle) near the last 3rd or 4th rib and the intermediate section of psoas major muscle (PMM, typical red muscle) were rapidly separated from each carcass. Samples were frozen in liquid nitrogen, and stored at −80°C until RNA extraction. For more information, please refer to Li et al. [18].
Measurements of skeletal muscle-related phenotype
Measurements of concentrations of 24 serum-circulating indicators of metabolism, myofibre cross-sectional area and myofibre ratio (type I vs. II) are from our previous report based on same individuals. For more information, please refer to Li et al. [18].
Extraction of RNA
Total RNA was extracted from 36 samples using TRIzol (Invitrogen). RNA was purified and DNase treated using an RNeasy column (Qiagen) according to the manufacturer’s instructions. The quantity of each RNA sample was examined by the NanoDrop ND-1000 spectrophotometer (Nano Drop) at 260/280 nm (ratio > 2.0). The integrity of total RNA also passed analysis with the Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Agilent Technologies) with RIN number > 6 (7.6 ± 0.3, n = 36).
Microarray analyses
Agilent Oligo microarrays were used to determine global gene expression of 36 samples. Individual microarrays were performed for each sample. Hybridization, washing, and scanning were done according to standard Agilent protocols. Generated array images were loaded into Feature Extraction Software (Agilent Technologies) for feature data extraction, and data analysis was performed with MultiExperiment Viewer (MeV) [45]. Array data have been uploaded to NCBI’s Gene Expression Omnibus (GEO) [accession number GSE30343]. For more information, please refer to Li et al.[18].
To obtain high-confidence gene expression data, we mapped 43,603 probes (60 mer in length) to the pig reference genome allowing up to one mismatch, and further filtered unannotated pig target sequences which resulting 4,309 genes were used in subsequent analysis. (Tables S2). To identify differentially expressed mRNAs (P < 0.05) for the clustering analysis, we used three-way ANOVA for comparisons. Resulting P-values of above tests were corrected with adjusted Bonferroni method (FDR < 0.01, 1,000 permutations).
Construct modules of coexpressed genes
For LDM and PMM separately, modules of highly coexpressed genes were constructed using pair wise average-linkage cluster analysis as previously described [8,46]. We kept repeating this as an iterative process until the most significantly correlated pair was r < 0.8. To visualize the correlations between probes within the modules, we constructed colored heatmaps by plotting pair-wise correlation values of expression of all the probes within the modules. To calculate significance of overlap in gene content between modules and between different datasets, we performed Fisher’s exact tests.
Function enrichment analysis of genes
To elucidate the biological mechanisms associated with the genes that are correlated to the phenotypic traits, we performed functional enrichment analysis of Gene Ontology (GO) for genes using DAVID software [47].
Quantitative PCR (Q-PCR)
We selected six genes randomly to validation experiment using Q-PCR. Primer sequences used for the Q-PCR are shown in Additional file 9: Table S6. Porcine ACTB, TBP and TOP2B were simultaneously used as endogenous control genes [48]. Relative expression levels of objective mRNAs were calculated using the ΔΔCt method.
Competing interests
The authors have declared that no competing interests exist.
Authors’ contributions
MZL and XWL conceived and designed the experiment. JZ and MZL performed the data analysis and drafted the manuscript. AAJ, LZ, SRS and JYW collected the samples, statistical analysis and prepared nucleic acids. CWZ, JDM and LC performed gene expression microarray. All authors read and approved the final manuscript.
Supplementary Material
The differences of the (A) myofibre CSA and (B) myofibre ratio among samples. Data are means ± SD. The significance of differences among samples was determined by the three-way ANOVA; B, S and T refer to the breed, sex and tissue, respectively.
Serum parameters of the study population. Datas are means ± SD. Total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL), very-low density lipoprotein (VLDL), lactate dehydrogenase (LDH), apolipoprotein A-1 (Apo-A1), apolipoprotein B (Apo-B), adiponectin (Adipo), adiponectin receptor (AdipoR), C-peptide, cholecystokinin (CCK), gastrin receptor (GsaR), growth hormone (GH), highly sensitive C-reactive protein (hs-CRP), insulin, interleukin - 6 (IL-6), leptin (Lep), leptin receptor (LepR), orexin-B (OX-B), orexin receptor (OXR), plasminogen activator inhibitor-1 (PAI-1), tumor necrosis factor-α (TNF-α) and somatostatin (SS).
Complete gene list used analysis (annotated genes only).
Differential expressed genes among the (A) breeds, between the (B) sexes and the (C) anatomical locations.
Genes involved in tissue-specific DEGs. Datas are means ± SD, Student’s t-test; LDM and PMM refer to the longissimus doris muscle and psoas major muscle, respectively.
Validation of gene expression by Q-PCR. The data presented in Y-axis indicated the relative mRNA expression of both microarray and Q-PCR. Datas are means ± SD. The Pearson correlation coefficient (r) and the corresponding significance value (P) were shown above the columns.
Contents of genes in module generated in (A) longissimus doris muscle (LDM) and (B) psoas major muscle (PMM). For each module, the number genes and the names of all those genes are listed.
Overlap between genes in the modules identified in longissimus doris muscle (LDM) and psoas major muscle (PMM). P-values to determine the significance of the overlap between the modules were performed using a Fisher’s exact test: *1: P = 1.45 × 10-10; *2: P = 3.39 × 10-12; *3: P = 3.29 × 10-18; *4: P = 3.38 × 10-18; *5: P =1.09 × 10-2; *6: P = 7.24 × 10-15; *7: P = 9.52 × 10-5; *8: P = 1.62 × 10-4.
Primer sequences used for Q-PCR. *: ACTB (β actin), TBP (TATA box binding protein) and TOP2B (topoisomerase II β) are the endogenous control genes.
Contributor Information
Jie Zhang, Email: zhangjie813@163.com.
Chaowei Zhou, Email: zcwlzq666@163.com.
Jideng Ma, Email: jideng_ma@sina.com.
Lei Chen, Email: sicau.chen@gmail.com.
Anan Jiang, Email: lingdang317@163.com.
Li Zhu, Email: zhuli7508@163.com.
Surong Shuai, Email: srshuai@sohu.com.
Jinyong Wang, Email: kingyou@vip.sina.com.
Mingzhou Li, Email: mingzhou.li@163.com.
Xuewei Li, Email: xuewei.li@sicau.edu.cn.
Acknowledgments
This work was supported by grants from the Specialized Research Fund of Ministry of Agriculture of China (NYCYTX-009), the Project of Provincial Twelfth Five Years’ Animal Breeding of Sichuan Province (2011YZGG15) and the National Special Foundation for Transgenic Species of China (2011ZX08006-003) to X.L., the National High Technology Research and Development Program of China (863 Program) (2013AA102502) to M.L., the Chongqing Fund for Distinguished Young Scientists (CSTC2010BA1007) to J.W.
References
- Motta VF, de Lacerda CAM. Beneficial Effects of Exercise Training (Treadmill) on Body Mass and Skeletal Muscle Capillaries/Myocyte Ratio in C57BL/6 Mice Fed High-Fat Diet. Int J Morpho. 2012;30(1):205–210. doi: 10.4067/S0717-95022012000100037. [DOI] [Google Scholar]
- Matsakas A, Patel K. Skeletal muscle fibre plasticity in response to selected environmental and physiological stimuli. Histol Histopathol. 2009;24(5):611–629. doi: 10.14670/HH-24.611. [DOI] [PubMed] [Google Scholar]
- Choi Y, Kim B. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest Sci. 2009;122(2):105–118. [Google Scholar]
- Kim NK, Joh JH, Park HR, Kim OH, Park BY, Lee CS. Differential expression profiling of the proteomes and their mRNAs in porcine white and red skeletal muscles. Proteomics. 2004;4(11):3422–3428. doi: 10.1002/pmic.200400976. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron R. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Campbell WG, Gordon SE, Carlson CJ, Pattison JS, Hamilton MT, Booth FW. Differential global gene expression in red and white skeletal muscle. Am J Physiol Cell Physiol. 2001;280(4):C763–C768. doi: 10.1152/ajpcell.2001.280.4.C763. [DOI] [PubMed] [Google Scholar]
- Mo K, Razak Z, Rao P, Yu Z, Adachi H, Katsuno M, Sobue G, Lieberman AP, Westwood JT, Monks DA. Microarray analysis of gene expression by skeletal muscle of three mouse models of Kennedy disease/spinal bulbar muscular atrophy. PLoS One. 2010;5(9):e12922. doi: 10.1371/journal.pone.0012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs M, Rensen S, Bruin-Van Dijk E, Verdam F, Greve JW, Sanjabi B, Bruinenberg M, Wijmenga C, Van Haeften T, Buurman W. Co-expressed immune and metabolic genes in visceral and subcutaneous adipose tissue from severely obese individuals are associated with plasma HDL and glucose levels: a microarray study. BMC Med Genomics. 2010;3(1):34. doi: 10.1186/1755-8794-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MH, Schrage WG, McAllister RM, Garverick H, Jones A. Interaction of gender and exercise training: vasomotor reactivity of porcine skeletal muscle arteries. J Appl Physiol. 2001;90(1):216–227. doi: 10.1152/jappl.2001.90.1.216. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Welshons WV, Sturek M, Rush JWE, Turk JR, Taylor JA, Judy BM, Henderson KK, Ganjam V. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol. 2003;95(1):250–264. doi: 10.1152/japplphysiol.00061.2003. [DOI] [PubMed] [Google Scholar]
- Glenmark B, Nilsson M, Gao H, Gustafsson JÅ, Dahlman-Wright K, Westerblad H. Difference in skeletal muscle function in males vs. females: role of estrogen receptor-β. Am J Physiol Endocrinol Metab. 2004;287(6):E1125–E1131. doi: 10.1152/ajpendo.00098.2004. [DOI] [PubMed] [Google Scholar]
- Roth SM, Ferrell RE, Peters DG, Metter EJ, Hurley BF, Rogers MA. Influence of age, sex, and strength training on human muscle gene expression determined by microarray. Physiol Genomics. 2002;10(3):181–190. doi: 10.1152/physiolgenomics.00028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather RS, Shen M, Dai Y. Genetically modified pigs for medicine and agriculture. Biotechnol Genet Eng Rev. 2008;25(1):245–265. doi: 10.7313/upo9781904761679.011. [DOI] [PubMed] [Google Scholar]
- Rocha D, Plastow G. Commercial pigs: an untapped resource for human obesity research? Drug Discov Today. 2006;11(11–12):475–477. doi: 10.1016/j.drudis.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Andersson L. How selective sweeps in domestic animals provide new insight into biological mechanisms. J Int Medicine. 2011;271(1):1–14. doi: 10.1111/j.1365-2796.2011.02450.x. [DOI] [PubMed] [Google Scholar]
- Bai Q, McGillivray C, Da Costa N, Dornan S, Evans G, Stear M, Chang KC. Development of a porcine skeletal muscle cDNA microarray: analysis of differential transcript expression in phenotypically distinct muscles. BMC Genomics. 2003;4(1):8–15. doi: 10.1186/1471-2164-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu Z, Li H, Xiong Y, Zuo B. Differential transcriptional analysis between red and white skeletal muscle of Chinese Meishan pigs. Int J Biol Sci. 2010;6(4):350–360. doi: 10.7150/ijbs.6.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wu H, Luo Z, Xia Y, Guan J, Wang T, Gu Y, Chen L, Zhang K, Ma J. et al. An atlas of DNA methylomes in porcine adipose and muscle tissues. Nat Commun. 2012;3:850. doi: 10.1038/ncomms1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296(6):C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364(6437):501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Hanaoka K, Hayasaka M, Esuml E, Li S, Nonaka I. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364(6437):532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7(9):910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- Rivera-Ferre MG, Aguilera JF, Nieto R. Muscle fractional protein synthesis is higher in Iberian than in Landrace growing pigs fed adequate or lysine-deficient diets. J Nutr. 2005;135(3):469–478. doi: 10.1093/jn/135.3.469. [DOI] [PubMed] [Google Scholar]
- Senaeme C, Istasse L, Baldwin P, Gabriel A, Haan V, Bienfait JM. Muscle protein turnover in young bulls in relation to breed and hormonal status. Asian-Austral J Animal Sci. 1989;2(3):200–201. [Google Scholar]
- Vidal-Puig A, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem Biophys Res Commun. 1997;235(1):79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- Louis M, Van Beneden R, Dehoux M, Thissen JP, Francaux M. Creatine increases IGF-I and myogenic regulatory factor mRNA in C2C12 cells. FEBS Lett. 2004;557(1):243–247. doi: 10.1016/s0014-5793(03)01504-7. [DOI] [PubMed] [Google Scholar]
- Cagnazzo M, Te Pas MF, Priem J, De Wit AA, Pool MH, Davoli R, Russo V. Comparison of prenatal muscle tissue expression profiles of two pig breeds differing in muscle characteristics. J Anim Sci. 2006;84(1):1–10. doi: 10.2527/2006.8411. [DOI] [PubMed] [Google Scholar]
- Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, Collinet M, Bortoli S, Forest C, Benelli C. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes. 2008;57(9):2272–2279. doi: 10.2337/db08-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthny T, Kovarik M, Sispera L, Tilser I, Holecek M. Protein metabolism in slow- and fast-twitch skeletal muscle during turpentine-induced inflammation. Int J Exp Pathol. 2008;89(1):64–71. doi: 10.1111/j.1365-2613.2007.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair D, Garrel DR, Chaouki Zerouala A, Ferland LH. Activation of the ubiquitin pathway in rat skeletal muscle by catabolic doses of glucocorticoids. Am J Physiol. 1997;272(3):C1007–1016. doi: 10.1152/ajpcell.1997.272.3.C1007. [DOI] [PubMed] [Google Scholar]
- Cleveland BM, Evenhuis JP. Molecular characterization of atrogin-1/F-box protein-32 (FBXO32) and F-box protein-25 (FBXO25) in rainbow trout (Oncorhynchus mykiss): Expression across tissues in response to feed deprivation. Comp Biochem Physiol B Biochem Mol Biol. 2010;157(3):248–257. doi: 10.1016/j.cbpb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147(9):4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- Reid MB. Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1423–R1431. doi: 10.1152/ajpregu.00545.2004. [DOI] [PubMed] [Google Scholar]
- Agrawal P, Chen Y-T, Schilling B, Gibson BW, Hughes RE. Ubiquitin-specific Peptidase 9, X-linked (USP9X) Modulates Activity of Mammalian Target of Rapamycin (mTOR) J Biol Chem. 2012;287(25):21164–21175. doi: 10.1074/jbc.M111.328021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Yu Y, Wang N, Xu Y. Cloning and characterization of syap1, a down regulated gene in human hepatocellular carcinoma. Shi Yan Sheng Wu Xue Bao. 2001;34(4):319–322. [PubMed] [Google Scholar]
- Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science. 1997;278(5338):675–680. doi: 10.1126/science.278.5338.675. [DOI] [PubMed] [Google Scholar]
- Brocker C, Carpenter C, Nebert DW, Vasiliou V. Evolutionary divergence and functions of the human acyl-CoA thioesterase gene ( ACOT ) family. Hum Genomics. 2010;4(6):411–420. doi: 10.1186/1479-7364-4-6-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakme A, Huber A, Dolle P, Schreiber V. The macroPARP genes Parp-9 and Parp-14 are developmentally and differentially regulated in mouse tissues. Dev Dyn. 2008;237(1):209–215. doi: 10.1002/dvdy.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourim D, Lin JJ-C. Apolipoprotein A-1 expression is resistant to dimethyl sulfoxide inhibition of myogenic differentiation. Exp Cell Res. 1991;197(1):57–65. doi: 10.1016/0014-4827(91)90479-E. [DOI] [PubMed] [Google Scholar]
- Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Euro J Biochem. 1969;10(2):198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Ryu Y, Kim B. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71(2):351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473–494. doi: 10.1128/CMR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskowitz MS, Jones SM. GI Consult: Appendicitis. Emerg Med. 2004;36(10):10–15. [Google Scholar]
- Miller T, Al-Lozi M, Lopate G, Pestronk A. Myopathy with antibodies to the signal recognition particle: clinical and pathological features. J Neurol Neurosurg Psychiatry. 2002;73(4):420–428. doi: 10.1136/jnnp.73.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed A, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34(2):374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- Li M, Wu H, Wang T, Xia Y, Jin L, Jiang A, Zhu L, Chen L, Li R, Li X. Co-methylated Genes in Different Adipose Depots of Pig are Associated with Metabolic, Inflammatory and Immune Processes. Int J Biol Sci. 2012;8(6):831–837. doi: 10.7150/ijbs.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Erkens T, Van Poucke M, Vandesompele J, Goossens K, Van Zeveren A, Peelman L. Development of a new set of reference genes for normalization of real-time RT-PCR data of porcine backfat and longissimus dorsi muscle, and evaluation with PPARGC1A. BMC Biotechnol. 2006;6(1):41–48. doi: 10.1186/1472-6750-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The differences of the (A) myofibre CSA and (B) myofibre ratio among samples. Data are means ± SD. The significance of differences among samples was determined by the three-way ANOVA; B, S and T refer to the breed, sex and tissue, respectively.
Serum parameters of the study population. Datas are means ± SD. Total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL), very-low density lipoprotein (VLDL), lactate dehydrogenase (LDH), apolipoprotein A-1 (Apo-A1), apolipoprotein B (Apo-B), adiponectin (Adipo), adiponectin receptor (AdipoR), C-peptide, cholecystokinin (CCK), gastrin receptor (GsaR), growth hormone (GH), highly sensitive C-reactive protein (hs-CRP), insulin, interleukin - 6 (IL-6), leptin (Lep), leptin receptor (LepR), orexin-B (OX-B), orexin receptor (OXR), plasminogen activator inhibitor-1 (PAI-1), tumor necrosis factor-α (TNF-α) and somatostatin (SS).
Complete gene list used analysis (annotated genes only).
Differential expressed genes among the (A) breeds, between the (B) sexes and the (C) anatomical locations.
Genes involved in tissue-specific DEGs. Datas are means ± SD, Student’s t-test; LDM and PMM refer to the longissimus doris muscle and psoas major muscle, respectively.
Validation of gene expression by Q-PCR. The data presented in Y-axis indicated the relative mRNA expression of both microarray and Q-PCR. Datas are means ± SD. The Pearson correlation coefficient (r) and the corresponding significance value (P) were shown above the columns.
Contents of genes in module generated in (A) longissimus doris muscle (LDM) and (B) psoas major muscle (PMM). For each module, the number genes and the names of all those genes are listed.
Overlap between genes in the modules identified in longissimus doris muscle (LDM) and psoas major muscle (PMM). P-values to determine the significance of the overlap between the modules were performed using a Fisher’s exact test: *1: P = 1.45 × 10-10; *2: P = 3.39 × 10-12; *3: P = 3.29 × 10-18; *4: P = 3.38 × 10-18; *5: P =1.09 × 10-2; *6: P = 7.24 × 10-15; *7: P = 9.52 × 10-5; *8: P = 1.62 × 10-4.
Primer sequences used for Q-PCR. *: ACTB (β actin), TBP (TATA box binding protein) and TOP2B (topoisomerase II β) are the endogenous control genes.