IVIG is essential therapy for a rapidly expanding number of debilitating and life-threatening conditions. The evidence-based use of IVIG from an American perspective was reviewed in 2006 by the American Academy of Allergy, Asthma and Immunology (AAAAI) [1], and has been similarly considered elsewhere [2–4]. The number of clinical indications supported by at least some experimental evidence, however, is increasing. Future evidence-based lists of diseases advantageously modified by IVIG are likely to continue increasing in scope and may eventually include subsets of patients affected by common debilitating illnesses such as Alzheimer’s disease [5, 6]. Given the number of indications for therapeutic immunoglobulin and the diversity of related clinical fields, careful consideration of the usefulness of IVIG in each condition is warranted. Examples for considering the evidence supporting the use of IVIG do exist across distinct clinical disciplines [1, 7–9]. Detailed assessment of the value of IVIG within specific clinical areas is critical, since IVIG is a finite resource derived mostly from dedicated plasma donations and its production and distribution are dependent on an increasingly complex set of variables. Intermittent shortages as well as reimbursement-related difficulties in access to IVIG have occurred and are likely to increase if demand continues to rise [10]. In this light a comprehensive evaluation and demand-based model has been developed in the UK and is currently active [11]. Furthermore, IVIG is expensive and its administration can be labor intensive. While costs can be justified [12, 13], the increasing use of IVIG can place specific burdens on the healthcare system.
The use of IVIG in many diseases is the standard of care; in others, its use is supported by reasonable evidence and is often without effective therapeutic alternatives. The latter includes a few life-threatening diagnoses, which invariably lead to premature death without treatment.
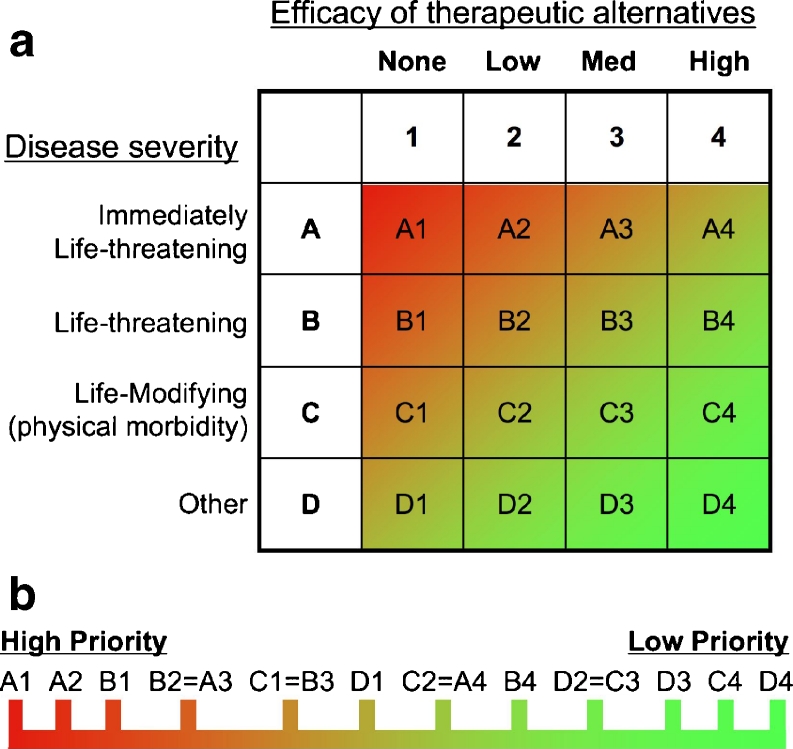
Given the multifaceted current and future demands for IVIG, specific prioritization of evidence-based disease indications may be necessary. This may be particularly relevant if new indications for IVIG result in substantial numbers of new patients who could potentially benefit from IVIG, which could place critical stress upon existing supply and demand. A survey conducted by the Immune Deficiency Foundation found 27 % of American hospital pharmacists to already have locally-defined protocols for prioritizing IVIG indications [14]. Given that there are no specific guidelines in place for this process, there is likely to be tremendous variability amongst institutions and practices. To reduce inconsistency and promote best practice, we propose an IVIG prioritization algorithm for strong evidence-based indications (Fig. 1). Specifically, we propose two additional axes be added for the clinical indications for which there is evidence to support use. This would, therefore, be on top of the “first axis” which represents utilization defined by strong evidence-based medicine. Consequently, the evidence-supported medical conditions would be additionally considered and ranked according to the severity of the disease as well as the availability of effective therapeutic alternatives (Fig. 1a) as “second” and “third” axes. The highest priority indication according to this algorithm would be evidence-supported use that is an immediately life-threatening disease that does not have reasonable treatment alternatives (Fig. 1b). Such an indication would score an “A” on the disease severity scale for being “immediately life-threatening” and a “1” on the therapeutic alternatives axis due to their being no efficacious alternatives to IVIG therapy. Although not without controversy, an example of an “A1” indication might be toxic epidermal necrolysis in specific subsets of patients [15, 16].
Fig. 1.
Algorithm for the prioritization of evidence-based indications for IVIG. a Indications for IVIG based upon experimental evidence can be considered according to the severity of the disease (y-axis) and the efficacy of therapeutic alternatives to IVIG (x-axis). Each is based upon a 4-point scale leading to 16 potential ratings for an indication and/or sub-categories of specific indications. b The prioritization of specific indications can then be determined using a linear scale, which ranks the individual severity and efficacy of alternatives. The prioritization of the ratings within each of the individual cells from the severity and alternatives grid is displayed linearly from left (high priority) to right (low priority). Indications having the same rating should be considered to be of equal priority
In order to illustrate this algorithm, the authors each independently considered a list of 8 primary immunodeficiency and 9 other diagnoses and provided their opinion regarding a disease severity score and efficacy of therapeutic alternatives score (Table I). These considerations were applied as the “second” and “third” axes independently of the “first” axis of evidence-based usage. None of the authors were aware of the others scores for these additional two axes. As an exercise it demonstrates that there were a few areas of consensus, but more commonly minor differences in opinion. In a minority there were substantive differences.
Table I.
Author ratings of primary immunodeficiency and other indications for IVIG
| Diseasea | Disease Severity Ratingb (A, B, C, D) | Efficacy of Alternativesc rating (1, 2, 3, 4) | Supporting evidence and benefit recommendationd | Evidence supports algorithm usee |
|---|---|---|---|---|
| Primary immunodeficiencies | ||||
| XLA | ABA | 221 | IIb-B, DBf | yes |
| XHM | ABA | 221 | IIb-B, DB | yes |
| CVID | BCA | 222 | IIb-B, DB | yes |
| SCID | AAA | 141 | IIb-B, DB | yes |
| XLP | BBB | 232 | IIb-B, DB | yes |
| SAD | BCC | 343 | IIb-B, PB | yes |
| IgGSD | CCC | 344 | IIb-B, PB | yes |
| IgA deficiency | CDD | 434 | IV-D, UB | no |
| Other conditions | ||||
| Toxic Epidermal Necrolysis | ABA | 141 | IIa-B, PB | yes |
| ITP | BBB | 343 | Ia-A, DB | yes |
| Autoimmune hemolytic anemia | BCC | 343 | III-D, MPB | maybe |
| Pemphigus vulgaris | CBC | 243 | III-C, MPB | maybe |
| Kawasaki disease | ABA | 121 | Ia-A, DB | yes |
| Recurrent spontaneous abortion | DCD | 133 | Ia-A, UB | no |
| autism | DDD | 241 | III-C, UB | no |
| CIDP | CCC | 322 | Ia-A, DB | yes |
| PANDAS | CCC | 341 | IIb-B, MPB | maybe |
Each author scored the disease indications listed according to the paradigm in Fig. 1 without knowing the scores provided by the other authors. The scores listed do not correspond to any particular author order. Consensus scores among the authors are shown in boldface
a XLA X-linked agammaglobulinemia, XHM X-linked hyper IgM syndrome, CVID common variable immunodeficiency, SCID severe combined immunodeficiency, XLP X-linked lymphoproliferative disease, SAD specific antibody deficiency, IgGSD IgG subclass deficiency, ITP idiopathic thrombocytopenic purpura, CIDP chronic immune demyelinating polyneuropathy, PANDAS pediatric autoimmune neuropsychiatric disorders associated with Streptococcal infection
bSeverity corresponds to the y-axis in Fig. 1a, where A represents immediately life-threatening, B represents life-threatening, C represents Life-modifying and D represents other. The three letters listed correspond to the score of each of the three authors
cEfficacy corresponds to the x-axis in Fig. 1a where the score corresponds to the perceived efficacy of therapeutic alternatives to IVG, 1 = none, 2 = low, 3 = medium, 4 = high. The three numbers are the scores for each of the diseases provided by the individual authors
dAs per the text, this algorithm should only be utilized when evidence supports the provision of therapy for the particular condition. The level of evidence and strength of recommendation from the 2006 IVIG [1] evidence review are listed. The roman numerals (and lowercase letter where appropriate) denote the evidence category and the hyphenated letter represents the strength of recommendation (see the 2006 document for additional explanation). The evidence-based recommendation provided in the 2006 evidence review is also listed using the following abbreviations: DB definitely beneficial, PB probably beneficial, MPB might provide benefit, UB unlikely beneficial
eThe application of the algorithm should be reserved for those in which IVIG is recommended based upon the existing evidence, which of course is subject to change with time. For the purposes of the present algorithm this is divided into three categories: yes – where the supporting evidence is perceived as definitely or probably beneficial; no – where the supporting evidence is perceived as unlikely to be beneficial; and maybe – where the supporting evidence is perceived as “might provide benefit”
fIt is important to note that in some cases stronger evidence is available now as compared to 2006 and the reader is referred to subsequent revisions of the 2006 document, alternative documents of similar nature, or the direct evidence
In order to illustrate the role and interplay of the “first” axis of strong evidence supporting use, the supporting evidence rating and evidence-based recommendation from the 2006 AAAAI IVIG evidence review is provided in Table I (fourth column). While those recommendations are now several years old and in need of update, they are provided as a frame of reference for the “first” axis. This demonstrates that even within indications supported by similar levels of evidence, there is typically a range of generally aligned scores on the “second” and “third” axes of the algorithm. For example both SCID and CVID have the same level of evidence and evidence-based recommendation for IVIG usage, but the disease severity and effectiveness of therapeutic alternatives rankings are discordant (the difference in the latter for SCID amongst the authors was likely that one author perceived hematopoietic stem cell transplantation as an effective “alternative”, while the other two authors did not perceive this as an alternative to the need for therapeutic immunoglobulin). Another example of the utility of the algorithm and use of these additional axes would be in the comparison of the PID diagnoses such as XLA to ITP. The use of IVIG in ITP is supported by the highest level of evidence and the AAAAI recommendation for use is that of “definitely beneficial”. The evidence for use of IVIG in XLA is also perceived as “definitely beneficial” in the AAAAI document but is supported by a lower level of evidence. The comparison of these two indications by the authors using algorithm, however, demonstrates distinct differences in prioritization in the opposite direction of the grade of evidence. Thus the quality of evidence alone does not necessarily provide the full dynamic range of utility of therapeutic immunoglobulin in specific clinical indications. It is hoped that the application of the algorithm as “second” and “third” axes can provide this additional granularity and resolution that could prove helpful in meeting increased demands for a limited supply of IVIG.
Overall this proposal and exercise demonstrates that were such an algorithm to be applied at any level (institutional, regional, or national) there would be a need for consensus building (which was not part of the exercise in the Table). Ideally this would include involvement of individuals with academic and clinical expertise appropriate to the different disease indications under consideration.
As described above, an assumption inherent in this algorithm is that IVIG uses are based upon valid experimental data. Quality of evidence does constitute a separate axis in the algorithm, but the authors have chosen not to treat this “first” axis as continuous. In particular, the use of a continuous “first” axis might introduce an unfair bias against certain indications for which IVIG is effective, but not supported by the highest levels of evidence (as emphasized above). In aggregate, primary immunodeficiency diseases represent an original indication for immunoglobulin therapy and will never be amenable to placebo-controlled randomized trials of IVIG because its application represents standard of care without current therapeutic alternatives to study. To be effective, however, the prioritization algorithm would need to be supplemented by rigorous evidence-based use of IVIG within the specific disease categories for particular indications. Thus, the use of the algorithm is only recommended for diseases in which the evidence-based recommendation for use is graded as definitely, or probably beneficial (based upon AAAAI recommendations as provided in the rightmost column of Table I). The algorithm may be applicable for diseases in which the evidence is perceived as “may provide benefit”, but is not applicable for diseases in which evidence is graded as “unlikely to provide benefit”, as IVIG should not be used to treat those diseases (even though a few of these were included in Table I to demonstrate how they would fare on these additional “axes”). How to integrate the prioritization of the diseases for which IVIG “may provide benefit” into those where it is perceived as more likely to provide benefit is complex and should rely upon meaningful dialogue among key academic entities.
In coming years, it is likely that physicians whose patients now rely on IVIG will need to be proactive and consider discussion of prioritization in order to enable preparedness should strain be placed on the supply relative to demand for IVIG. The patients whose lives can be definitively most improved and/or preserved by IVIG should be ensured ready access. Community-wide consideration of this challenge is most likely to result in a just and rational outcome.
Contributor Information
Jordan S. Orange, Phone: +1-832-8241319, FAX: +1-832-8251260, Email: orange@bcm.edu
Hans D. Ochs, Email: Allgau@uw.edu
Charlotte Cunningham-Rundles, Email: Charlotte.Cunningham-Rundles@mssm.edu.
References
- 1.Orange JS, Hossny EM, Weiler CR, Ballow M, Berger M, Bonilla FA, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117:S525–53. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Constantine MM, Thomas W, Whitman L, Kahwash E, Dolan S, Smith S, et al. Intravenous immunoglobulin utilization in the Canadian Atlantic provinces: a report of the Atlantic Collaborative Intravenous Immune Globulin Utilization Working Group. Transfusion. 2007;47:2072–80. doi: 10.1111/j.1537-2995.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- 3.Provan D, Chapel HM, Sewell WA, O’Shaughnessy D. Prescribing intravenous immunoglobulin: summary of Department of Health guidelines. BMJ. 2008;337:a1831. doi: 10.1136/bmj.a1831. [DOI] [PubMed] [Google Scholar]
- 4.Delforge M, Farber CM, Spath P, Kaveri S, Witte T, Misbah SA, et al. Recommended indications for the administration of polyclonal immunoglobulin preparations. Acta Clin Belg. 2011;66:346–60. doi: 10.2143/ACB.66.5.2062586. [DOI] [PubMed] [Google Scholar]
- 5.Dodel R, Neff F, Noelker C, Pul R, Du Y, Bacher M, et al. Intravenous immunoglobulins as a treatment for Alzheimer’s disease: rationale and current evidence. Drugs. 2010;70:513–28. doi: 10.2165/11533070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Solomon B. Intravenous immunoglobulin and Alzheimer’s disease immunotherapy. Curr Opin Mol Ther. 2007;9:79–85. [PubMed] [Google Scholar]
- 7.Enk A. Guidelines on the use of high-dose intravenous immunoglobulin in dermatology. Eur J Dermatol. 2009;19:90–8. doi: 10.1684/ejd.2008.0580. [DOI] [PubMed] [Google Scholar]
- 8.Liumbruno GM, Bennardello F, Lattanzio A, Piccoli P, Rossettias G. Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7:216–34. doi: 10.2450/2009.0094-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patwa HS, Chaudhry V, Katzberg H, Rae-Grant AD, So YT. Evidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2012;78:1009–15. doi: 10.1212/WNL.0b013e31824de293. [DOI] [PubMed] [Google Scholar]
- 10.Bayry J, Kazatchkine MD, Kaveri SV. Shortage of human intravenous immunoglobulin—reasons and possible solutions. Nat Clin Pract. 2007;3:120–1. doi: 10.1038/ncpneuro0429. [DOI] [PubMed] [Google Scholar]
- 11.National Demand Mangament Programme for Immunoglobulin. http://www.ivig.nhs.uk/index.html Link active as of 1/11/13.
- 12.Modell V, Gee B, Lewis DB, Orange JS, Roifman CM, Routes JM, et al. Global study of primary immunodeficiency diseases (PI)–diagnosis, treatment, and economic impact: an updated report from the Jeffrey Modell Foundation. Immunol Res. 2011;51:61–70. doi: 10.1007/s12026-011-8241-y. [DOI] [PubMed] [Google Scholar]
- 13.Simoens S. Pharmacoeconomics of immunoglobulins in primary immunodeficiency. Expert Rev Pharmacoecon Outcomes Res. 2009;9:375–86. doi: 10.1586/erp.09.37. [DOI] [PubMed] [Google Scholar]
- 14.2007. Hospital usage of intravenous immunoglobulin: A 2006 survey of pharmacy directors. http://primaryimmune.org/idf-survey-research-center/idf-surveys?aid=1369&sa=1 Link active as of 1/11/13.
- 15.Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol. 2012;167:424–32. doi: 10.1111/j.1365-2133.2012.10965.x. [DOI] [PubMed] [Google Scholar]
- 16.Stella M, Clemente A, Bollero D, Risso D, Dalmasso P. Toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS): experience with high-dose intravenous immunoglobulins and topical conservative approach. A retrospective analysis. Burns. 2007;33:452–9. doi: 10.1016/j.burns.2006.08.014. [DOI] [PubMed] [Google Scholar]