Abstract
Aims
The chemokine receptor CXCR4 modulates endothelial progenitor cell migration, homing, and differentiation, and plays a key role in cardiovascular regeneration. Here we examined the effect of ex vivo acidic preconditioning (AP) on CXCR4 expression and on the regenerative potential of mouse bone marrow (BM) ckit+ cells.
Methods and results
Acidic preconditioning was achieved by exposing BM ckit+ cells to hypercarbic acidosis (pH 7.0) for 24 h; control cells were kept at pH 7.4. Acidic preconditioning enhanced CXCR4 and stromal cell-derived factor 1 (SDF-1) mRNA levels, as well as CXCR4 phosphorylation. Acidic preconditioning ability to modulate CXCR4 expression depended on cytosolic calcium [Ca2+]i mobilization and on nitric oxide (NO), as determined by [Ca2+]i buffering with BAPTA, and by treatment with the NO donor (DETA/NO) and the NO synthase inhibitor (L-NAME). Further, AP increased SDF-1-driven chemotaxis, transendothelial migration, and differentiation toward the endothelial lineage in vitro. In a mouse model of hindlimb ischaemia, control and AP ckit+ cells were transplanted into the ischaemic muscle; AP cells accelerated blood flow recovery, increased capillary, and arteriole number as well as the number of regenerating muscle fibres vs. control. These effects were abolished by treating AP cells with L-NAME.
Conclusion
Acidic preconditioning represents a novel strategy to enhance BM ckit+ cell therapeutic potential via NO-dependent increase in CXCR4 expression.
Keywords: Preconditioning, Chemokines, Cell therapy, Cell migration, Hindlimb ischaemia
Introduction
Cell therapy is a promising strategy for the treatment of a variety of cardiovascular ailments, including myocardial infarction and limb ischaemia. However, bone marrow (BM) cells from elderly patients and individuals with cardiovascular risk factors, including diabetes,1,2 hypercholesterolaemia,3 hypertension,3 and smoking,4 exhibit limited therapeutic potential. Therefore, there is a strong clinical need to develop cell enhancement strategies to improve the clinical benefit of BM cell transplantation. BM cells have been evaluated ex vivo, prior to being transplanted, and it has been shown that stromal cell-derived factor 1 (SDF-1) can direct cell migration,5–7 gauge BM cell quality, and predict therapeutic efficacy following transplantation. This is in agreement with the well-known role of SDF-1 and its receptor, CXCR4, in tissue repair. In response to ischaemia, SDF-1 is upregulated and acts as a potent chemoattractant to recruit circulating and resident CXCR4+ progenitor cells to the injury site.5,7–9 Further, ex vivo exposure to nitric oxide (NO) donors can increase BM cells regenerative properties10 and this positive effect has been related to enhanced CXCR4 expression.7,11–13 Preconditioning with brief episodes of acidosis is known to limit ischaemia/reperfusion injury in the heart,14,15 lung,16,17 and endothelium18,19; the mechanism(s) for this response have not been elucidated but may involve activation of prosurvival kinases Akt and ERK, and the overexpression of anti-apoptotic protein Bcl-XL. However, it is still unknown whether acidic preconditioning (AP) ex vivo enhances BM cells therapeutic potential. We have previously shown that acidosis modulates CXCR4 expression and that this effect is cell-type specific; endothelial cells kept at pH 7.0 exhibit a decrease in CXCR4 expression, whereas in other cell types CXCR4 levels are unchanged.20 Furthermore, Froyland et al.21 demonstrated pH-dependent up-regulation of CXCR4 mRNA in NT2-N neurons during hypoxia/reoxygenation.
The aim of the present work was to investigate the effect of AP on SDF-1/CXCR4 expression, on SDF-1/CXCR4-directed BM cell function in vitro, and regenerative potential in a mouse model of hindlimb ischaemia. We utilized BM ckit+ cells because transplantation of these cells in the infarcted heart leads to myocardial and vascular regeneration,22 therefore they represent an attractive population to develop cell therapy enhancement strategies.
Methods
For a detailed description of all methods, see Supplementary material online.
Animals
Swiss CD1 male mice, 4–8-week-old, were used for ckit+ cell isolation. All animal studies complied with the Guidelines of the Italian National Institutes of Health and with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee.
Cell isolation, culture, and treatments
See Supplementary material online, Methods.
Acidification protocol
Cells were seeded in 48 multi-well dishes (5 × 105 cells/well) and cultured in Stem Span serum-free medium (Stem Cell Technologies) containing the following recombinant human cytokines: 100 ng/mL stem cell factor, 20 ng/mL IL-3, 20 ng/mL IL-6, and 100 ng/mL Flt-3 ligand (R&D Systems). Immediately after seeding multi-well dishes were placed in airtight modular incubator chambers (Forma Scientific Inc) and infused for 20 min, with either 5%CO2/95% air or 20%CO2/80% air to achieve a buffer pH of 7.4 or 7.0, respectively, as previously described.23 After gas mixture, infusion chambers were sealed and placed at 37°C for the duration of the experiments, i.e. 24 h or longer, as indicated. After acidification, the dishes were removed from the incubator chambers and returned to 5%CO2/95% air to achieve a buffer pH of 7.4. Thereafter, the different assays were performed as described.
Analysis of proliferation and cell death
ckit+ cells cultured in the growth medium were counted daily from Day 1 to Day 5. Cell death was determined by FACS analysis following either Propidium Iodide (PI) staining, caspase-9, or caspase-3 staining (Oncogene Research Products).
Adhesion assay
ckit+ cell adhesion was analysed onto fibronectin-coated dishes and onto a monolayer of human umbilical vein endothelial cells (HUVECs). Assays were carried out in EBM-2 medium (Lonza).
Migration assay
Both SDF-1 (100 ng/mL, R&D Systems) directed chemotaxis and transendothelial migration across a HUVEC monolayer were evaluated as previously described.24
Differentiation assay
Cell differentiation assays were carried out in M199 medium (Sigma-Aldrich) supplemented with 20% FCS or 2% FCS ± SDF-1 (100 ng/mL).
mRNA extraction and qRT–PCR
RNA was extracted from ckit+ cells using Trizol reagent (Invitrogen) according to the manufacturer's instruction. The sequences of forward and reverse primers for each gene of interest were selected from NCBI database (program Primer3 version 0.4.0).
Flow cytometry
Purity of each cell preparation, i.e. ckit+ cell number, as well as the expression of CD34, Sca-1, Flk1/KDR, and CXCR4 by these cells, was assessed by FACS.
Nitric oxide production
Nitric oxide production was evaluated by 4,5-Diaminofluorescein (DAF-2 DA) (Alexis) added to the complete medium for 6 h and then analysed by FACS.
In vivo procedures and immunohistochemistry
See Supplementary material online, Methods.
Statistical analysis
Variables were analysed by two-side Student's t-test and two-way ANOVA. A value of P ≤ 0.05 was deemed statistically significant. Mean values are indicated ± SEM. The GraphPad Prism software (version 5.00 for Windows, GraphPad Software, San Diego, CA, USA) was used for computer analysis.
Results
Effect of acidification on ckit+ cell proliferation, death, and endothelial differentiation
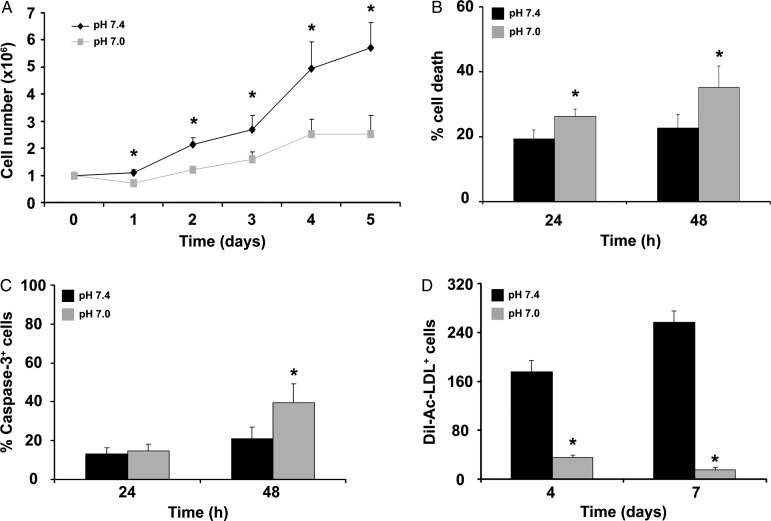
ckit+ cells were isolated from mice and cultured in growth medium, either at pH 7.4 or 7.0 for 5 days. In order to characterize AP as an ex vivo strategy to enhance BM cells regenerative properties, the effect of prolonged acidosis was first analysed. Acidification markedly inhibited the progressive increase in cell number observed under control conditions (Figure 1A), without a significant effect on cell cycle (see Supplementary material online, Figure S1). The percentage of ckit+ cells was >90% after isolation, decreased to ∼85% after 24 h and to ∼35% after 5 days in culture; interestingly, there was no effect of acidification on the percentage of cells expressing ckit, CD34, Sca-1, and KDR (see Supplementary material online, Figure S2). Additional experiments examined the effect of acidosis on cell death. By PI-staining and FACS analysis, acidification was found to enhance cell death both at 24 and 48 h (Figure 1B) and increase the number of caspase-3-positive cells at 48 h (Figure 1C); in contrast, it was found no significant increase in the number of caspase-9-positive cells (see Supplementary material online, Figure S3). Enhanced cell death at pH 7.0 may explain the apparent discrepancy between acidification ability to inhibit the progressive increase in cell number and the absence of an effect on cell cycle. Finally, the effect of acidification on ckit+ cell differentiation toward the endothelial lineage was examined. ckit+ cells were seeded onto fibronectin-coated dishes either at pH 7.4 or pH 7.0 in the presence of 20% FCS for 4 or 7 days. At both time points, a marked decrease in DiI-Ac-LDL positive cells was found at pH 7.0 vs. 7.4 (Figure 1D).
Figure 1.
Acidosis effect on ckit+ cell proliferation, death, and endothelial differentiation. (A) Acidosis inhibited the progressive increase in ckit+ cell number at pH 7.4 (n = 5). (B) Acidosis enhanced cell death, as assessed by FACS analysis of PI-stained cells (n = 9). (C) FACS analysis for caspase-3 (n = 3). (D) At 4 and 7 days, the number of DiI-Ac-LDL+ cells was lower at pH 7.0 vs. 7.4 (n = 3 in duplicate). Statistical significance: *P < 0.05 for pH 7.4 vs. 7.0.
Effect of acidic preconditioning on ckit+ cell proliferation and adhesion
The decrease in ckit+ cell functions after prolonged exposure to pH 7.0 prompted us to examine the effect of AP for 24 h on cell proliferation and adhesion at different time points after returning to pH 7.4. AP cells proliferated at a rate comparable to control cells that were kept at pH 7.4 throughout the 3-day course of the experiment (see Supplementary material online, Figure S4). Additional studies examined whether AP modulated ckit+ cell adhesion to fibronectin and TNF-α-activated endothelium at pH 7.4. Interestingly, AP enhanced ckit+ cell adhesion to both fibronectin-coated dishes (see Supplementary material online, Figure S5A) and activated HUVEC monolayer (see Supplementary material online, Figure S5B).
Effect of acidic preconditioning on CXCR4 and SDF-1 expression
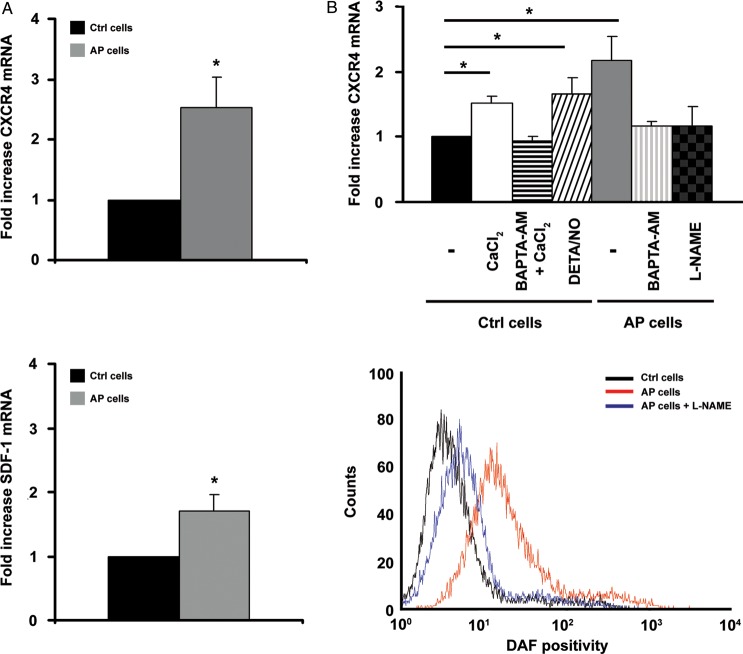
Since CXCR4 signalling plays a pivotal role in precursor cell migration and homing, we examined whether AP modulates CXCR4 and SDF-1 expression. ckit+ cells exposed to pH 7.0 exhibited a progressive increase in CXCR4 and SDF-1 mRNA levels; at the 5 h time point, neither the increase in CXCR4 nor in SDF-1 were statistically significant (data not shown), whereas at the 24 h time point CXCR4 and SDF-1 mRNA levels were 2.5 ± 0.4 and 1.7 ± 0.2 fold vs. control, respectively (Figure 2A). Interestingly, an increase in CXCR4 and SDF-1 mRNA was also observed in BM ckit+ cells from ApoE−/− and diabetic mice (see Supplementary material online, Figure S6). Further, human BM ckit+ cells exposed to AP exhibited a 1.3 ± 0.2-fold increase in CXCR4 mRNA (n = 5; P = 0.03). The percentage of CXCR4 positive cells was determined by FACS analysis. At the 24 h time point, ∼50% control cells and ∼60% AP cells expressed CXCR4 with a mean fluorescence intensity of ∼20 and ∼24 for control and AP cells, respectively; both achieved statistical significance at the 48 h time point (see Supplementary material online, Figure S7A). Further, we examined AP effect on CXCR4 phosphorylation; AP for 24 h enhanced CXCR4 phosphorylation, both under baseline conditions and upon exposure to SDF-1 (see Supplementary material online, Figure S7B). We next addressed the mechanisms that may be responsible for AP-mediated increase in CXCR4 expression. It has been previously reported that acidification increases [Ca2+]i,25 which is a key event in triggering NO production.26,27 Furthermore, increases in [Ca2+]i11 and NO7,13 have both been shown to enhance CXCR4 expression. Interestingly, we found that [Ca2+]i buffering with BAPTA-AM abolished the increase in CXCR4 induced by AP and also by raising bathing [Ca2+] from 0.2 to 0.5 mM at pH 7.4 (Figure 2B, upper panel). We next showed that NO played an important role in the upregulation of CXCR4 by acidosis. Nitric oxide donor DETA/NO enhanced CXCR4 expression in ckit+ cells kept at normal pH, whereas NOS inhibitor L-NAME abolished AP-mediated CXCR4 increase (Figure 2B, upper panel). In agreement with these results on NO and CXCR4 expression, ckit+ cells kept at pH 7.0 for 6 h exhibited an increase in DAF positivity which was prevented by L-NAME (Figure 2B, lower panel, and see Supplementary material online, Figure S8).
Figure 2.
Acidic preconditioning enhances CXCR4 and SDF-1 expression in c-kit+ cell. (A) Acidic preconditioning enhanced CXCR4 (upper panel; n = 6) and SDF-1 (lower panel; n = 10) mRNA levels, as assessed by qRT–PCR. (B) Upper panel shows that acidic preconditioning-mediated increase in CXCR4 mRNA was abolished by BAPTA-AM and L-NAME. Further, in control cells, CXCR4 expression increased upon raising bathing [Ca2+] from 0.2 to 0.5 mM or exposure to DETA/NO (n = 3; * P < 0.05 for treated vs. C cells). Lower panel shows a representative FACS analysis of DAF positivity; ckit+ cells exposed to acidic preconditioning exhibited an increase in NO production which was prevented by L-NAME.
As HIF-1α is a regulator of CXCR428,29 and its expression is enhanced by acidification20 and NO,30–32 we investigated whether AP effects on CXCR4 were paralleled by HIF-1α induction. To this end, we analysed HIF-1α expression in control cells following exposure to DETA/NO and in AP cells treated with L-NAME; both NO and acidification induced HIF-1α protein expression and L-NAME abolished this effect in AP cells (see Supplementary material online, Figure S9). Further, in control cells treated with L-NAME, there was a small decrease in HIF-1α expression, whereas in AP cells treated with DETA/NO there was an additional increase in HIF-1α expression (see Supplementary material online, Figure S9).
Effect of acidic preconditioning on SDF-1-directed ckit+ cell migration and differentiation toward the endothelial lineage
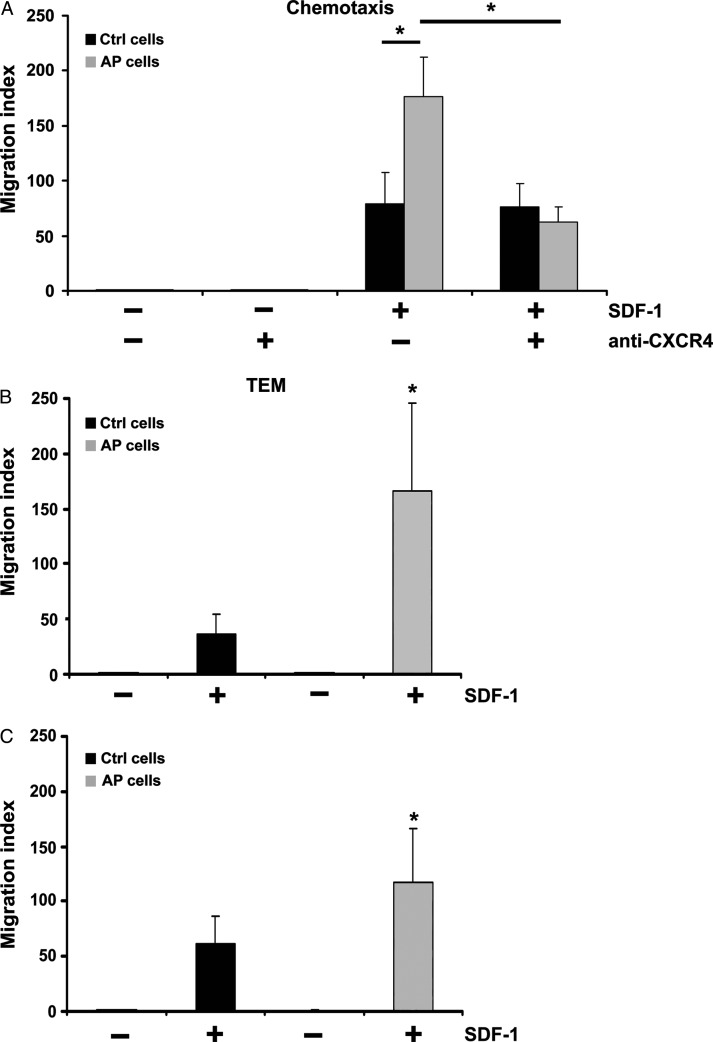
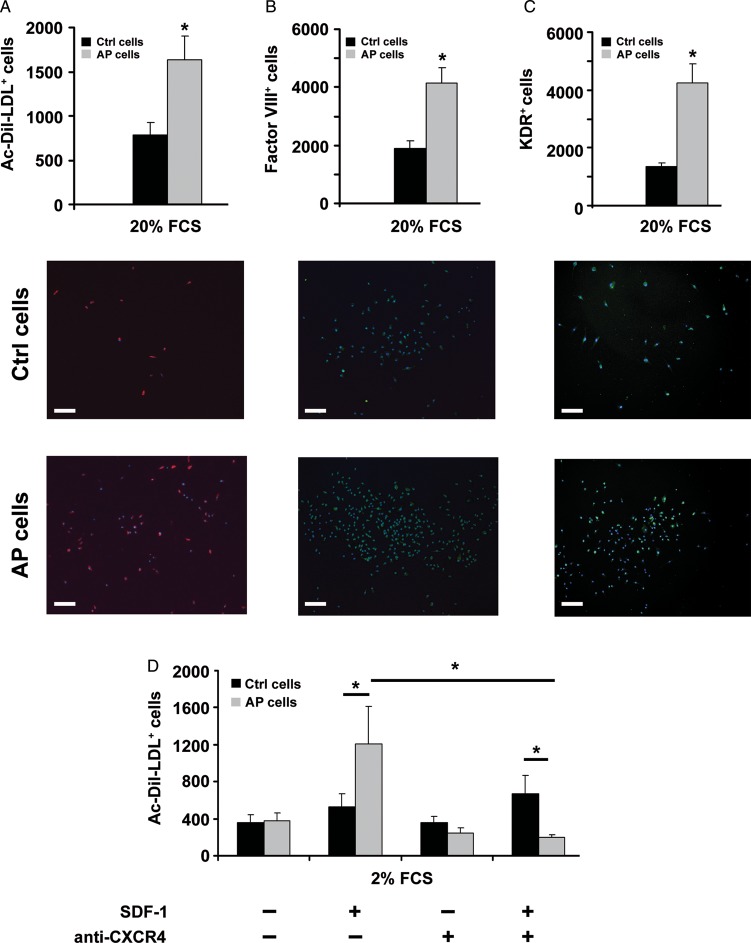
In subsequent in vitro experiments, we evaluated the functional significance of CXCR4 up-regulation induced by AP. ckit+ cells were kept for 24 h either at pH 7.4 or pH 7.0 and then analysed for their ability to migrate in response to SDF-1. Cell migration was evaluated, both in chemotaxis and in transendothelial migration assays at pH 7.4. AP cells exhibited a higher ability than control cells to migrate in response to SDF-1 and this effect was abolished upon treatment with an anti-CXCR4 antibody (Figure 3A). SDF-1-directed transendothelial migration assays were performed under two different pH conditions. The endothelial monolayer was constituted by HUVECs that were grown for 16 h, prior to the assay, at either pH 7.4 or at pH 7.0 (Figure 3B, upper and lower panel, respectively). Under both pH conditions, AP enhanced transendothelial migration. We then examined whether AP may modulate ckit+ cell differentiation toward the endothelial lineage. To that end, ckit+ cells were cultured for 24 h either at pH 7.4 or at pH 7.0 and then seeded onto fibronectin-coated dishes in the presence of 20% FCS for 7 days at pH 7.4. AP increased ckit+ cell adhesion with over 95% adherent cells expressing endothelial cell markers (i.e. Factor VIII and KDR) and displaying Ac-DiI-LDL uptake (Figure 4A–C).
Figure 3.
Acidic preconditioning enhances chemotaxis and transendothelial migration toward SDF-1. (A) Acidic preconditioning enhanced chemotaxis and this response was abolished by an anti-CXCR4 antibody (n = 3). (B) Acidic preconditioning enhanced ckit+ cell transendothelial migration, both through HUVEC grown at pH 7.4 (n = 5; upper panel) and through HUVEC grown at pH 7.0 (n = 3; lower panel). Statistical significance: * P < 0.05 for acidic preconditioning cells vs. C cells.
Figure 4.
Acidic preconditioning enhances ckit+ cell differentiation toward the endothelial lineage. In 20% FCS, acidic preconditioning enhanced: (A) DiI-Ac-LDL+ cell number (n = 10), (B) factor VIII+ cell number (n = 4), and (C) KDR+ cell number (n = 4). For all markers used, average positive cell number is shown in the upper panel and representative images in the lower panel; calibration bar = 50 µm. (D) In 2% FCS, DiI-Ac-LDL+ cell number was similar for cells treated with acidic preconditioning vs. control. SDF-1 addition enhanced DiI-Ac-LDL+ cell number only for cells treated with acidic preconditioning, whereas it had no effect on C cells; DiI-Ac-LDL uptake by acidic preconditioning cells was abolished by an anti-CXCR4 antibody (n = 6 in duplicate). Statistical significance: *P < 0.05 for acidic preconditioning cells vs. C cells.
Additionally, we examined the role of SDF-1 in AP cell differentiation. To address this issue, culture medium was supplemented with 100 ng/mL SDF-1 and FCS concentration was lowered from 20 to 2%. SDF-1 markedly enhanced the number of DiI-Ac-LDL-positive cells following AP, whereas it had no effect on cells cultured at pH 7.4. This effect of SDF-1 on DiI-Ac-LDL uptake was abolished by an anti-CXCR4 blocking antibody (Figure 4D).
Effect of acidic preconditioning on ckit+ cell therapeutic potential in a mouse model of hindlimb ischaemia
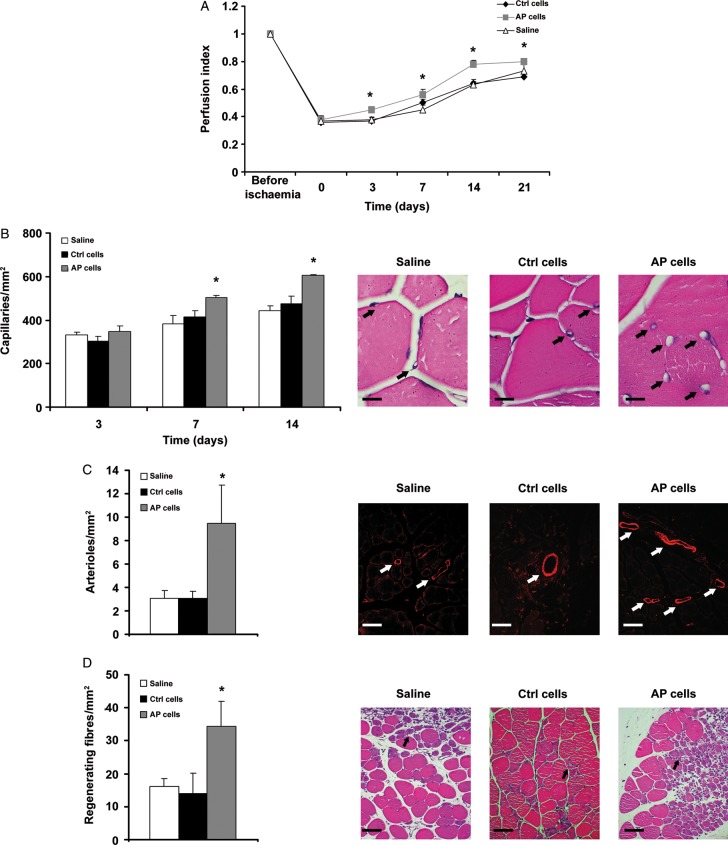
The experiments presented so far show that ckit+ cells exposed to AP in vitro exhibit an increase in CXCR4 expression and enhanced SDF-1-directed migration and differentiation toward the endothelial lineage. These results prompted us to evaluate the regenerative potential of AP-treated ckit+ cells in vivo, in a mouse model of hindlimb ischaemia. ckit+ cells were cultured for 24 h either at pH 7.4 or at pH 7.0, and then injected into the adductor muscle, immediately after femoral artery dissection. Hindlimb perfusion was evaluated by LDPI for 3 weeks after treatment. Interestingly, ckit+ cells exposed to AP significantly improved blood flow vs. control cells at each time point analysed after cell injection (Figure 5A). Further, in adductor muscles injected with AP cells, we found a significant increase in capillary number (Figure 5B) and arteriole density (Figure 5C), an increase in regenerating muscle fibres 7 days after cell injection (Figure 5D) and a decrease in the area of tissue damage (see Supplementary material online, Figure S10). Conversely, no significant difference was found between mice treated with ckit+ cells without exposure to AP vs. animals injected with saline. In light of the key role that NO plays in CXCR4 expression in response to AP, we next examined the therapeutic potential of ckit+ cells exposed to AP and treated with L-NAME. These cells failed to enhance limb perfusion (Figure 6A), did not increase capillary number, had no effect on muscle regeneration (Figure 6B and D), and markedly inhibited the increase in arterioles (Figure 6C). When control cells were exposed to L-NAME, their behaviour was similar to that of untreated control cells; it was found only a delay in the perfusion index at the 7-day time point, and a marginal decrease in capillary number at Day 14, whereas there was no effect on arterioles, capillaries at Day 7 and on regenerating muscle fibres (Figure 6).
Figure 5.
Acidic preconditioning enhances ckit+ cell therapeutic potential in a mouse model of hindlimb ischaemia. ckit+ cells were exposed to acidic preconditioning, then 5 × 105 cells were injected into the mouse adductor muscle at the time of femoral artery dissection. Control mice were injected with C cells or saline. (A) Hindlimb blood flow evaluated by LDPI (n = 8 at each time point). The progressive increase in blood flow was accelerated in acidic preconditioning cells-treated vs. C cells and saline-treated mice. (B) Acidic preconditioning cells increased capillary number vs. C cells or saline, at the 7- and 14-day time points (n = 6; calibration bar = 20 µm). (C) Acidic preconditioning cells increased arteriole number at the 14-day time point (n = 6). Arterioles stained with α-smooth muscle actin antibody; calibration bar = 30 µm. (D) Acidic preconditioning cells increased regenerating fibres at the 7-day time point (n = 6; calibration bar = 50 µm). For all assays performed, average positive cell number is shown in the left panel and representative images in the right. Statistical significance: *P < 0.05 for acidic preconditioning cells vs. C cells or saline.
Figure 6.
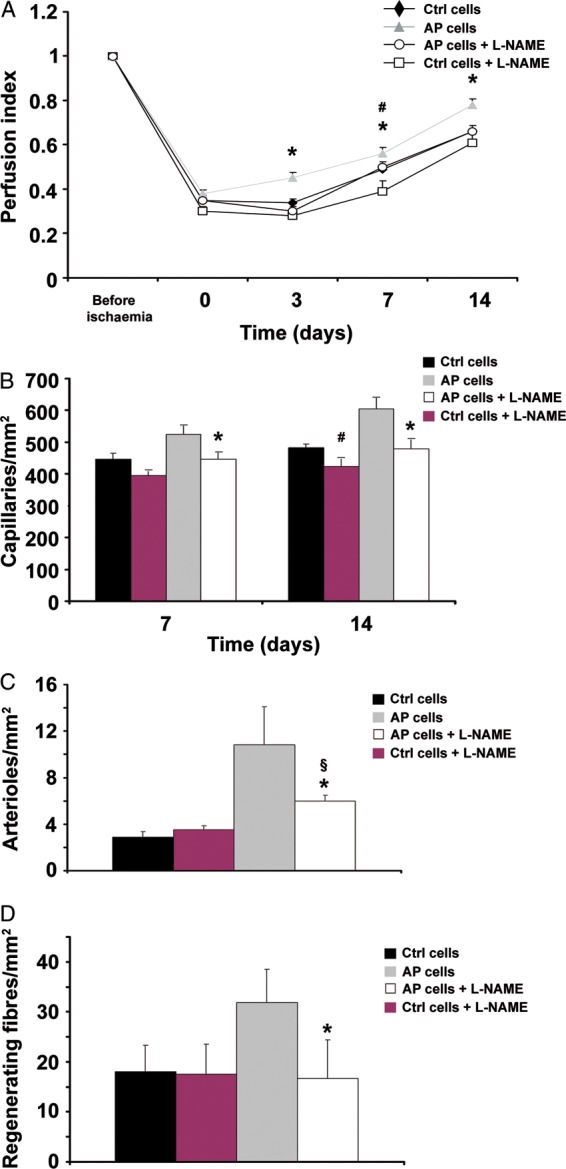
L-NAME abolishes acidic preconditioning ability to enhance ckit+ cell therapeutic potential in vivo. Treatment with L-NAME abolished acidic preconditioning cells ability to enhance limb perfusion (A), increase capillary number (B), and regenerating fibres at Day 7 (D); the increase in arterioles at Day 14 was markedly inhibited (C). Control cells, L-NAME treated and untreated, behaved similarly except for lower limb perfusion and capillary number in L-NAME-treated cells at Days 7 and 14, respectively (panel A, n = 7 in each group; panels B–D, n = 5 for each bar graph; *P < 0.05 L-NAME treated vs. untreated acidic preconditioning cells; §P < 0.05 for L-NAME-treated acidic preconditioning cells vs. control cells; #P < 0.05 for L-NAME-treated vs. untreated control cells).
Discussion
The present study shows that AP increased CXCR4 expression in BM c-kit+ cells. This effect was associated with enhanced SDF-1-directed cell migration and endothelial differentiation in vitro, and with a potentiated angiogenic and regenerative action in a mouse model of hindlimb ischaemia. Both in vitro and in vivo, AP effects were mediated by NO.
Prior studies have examined the effect of acidification in a variety of cell types. It has been reported that a marked and prolonged decrease in pH has a negative effect on cell survival and function.23,33 In contrast, at least in endothelial cells, a brief exposure to a mild acidotic milieu exerts a beneficial action on survival and, upon returning to a normal pH, also on cell function.33 These beneficial responses have been attributed to increased secretion of pro-survival angiogenic factors, i.e. fibroblast growth factor 2 and vascular endothelial growth factor,23 and to enhanced expression of the tyrosine kinase receptor AXL; upon binding to its ligand, the survival factor growth arrest-specific gene 6 product (Gas6), AXL exerts an antiapoptotic action.33 These in vitro studies are in agreement with in vivo results showing that preconditioning with brief episodes of acidosis limits ischaemia/reperfusion injury in the heart,14,15 lung,16,17 and endothelium.18,19
Our present work focused on CXCR4 because there is substantial evidence supporting SDF-1/CXCR4 key role in the response to cell therapy. In humans, impairment of CXCR4 signalling reduces the proangiogenic action of endothelial progenitor cells (EPC)9 and the response to autologous BM cell transplantation into the ischaemic heart.8 Another study has compared the functional activity of both CXCR4+ and CXCR4− human BM mononuclear cells and found that only CXCR4+ cells improved neovascularization in a murine model of hindlimb ischaemia.8 Further, in a rodent model of myocardial infarction, hypoxic preconditioning augmented cardiac progenitor cell therapeutic efficacy by inducing CXCR4 expression.12 We also found that ckit+ cells exposed to AP also exhibited a marked increase in SDF-1 expression; this is expected to have a positive action via both autocrine and paracrine mechanisms. Indeed, cell priming with SDF-1 prior to transplantation enhances their therapeutic potential34; further, direct SDF-1 injection into the ischaemic limb35 and heart36 induces a regenerative response and improves function. It is noteworthy that, in the present work, the increase in the CXCR4 protein induced by mild acidification for 24 h was relatively small. Nevertheless, AP for 24 h enhanced CXCR4 phosphorylation, both under baseline conditions and upon exposure to SDF-1. Further, a selective CXCR4 blocking antibody abolished both SDF-1-directed chemotaxis and differentiation toward the endothelial lineage. Taken together, these results link AP ability to modulate CXCR4 expression and activation, enhanced SDF-1 responsiveness in vitro, and improved therapeutic potential in vivo. The increase in CXCR4 expression in AP cells is expected to promote their migration toward ischaemic tissues which express high SDF-1 levels,5–7 and also facilitate CXCR4+ cell differentiation toward the endothelial lineage. Interestingly, AP enhanced CXCR4 expression also in BM ckit+ cells from humans and from hypercholesterolemic and diabetic mice.
Under our experimental conditions, NO is the key mediator linking acidification to CXCR4 expression. The NO donor DETA/NO mimicked AP ability to enhance CXCR4 expression; further, AP effects on CXCR4 expression and ckit+ cells regenerative potential in vivo were abolished by the NOS inhibitor L-NAME. These results are in agreement with prior studies showing that a mild decrease in pH enhances NO production in vivo37 and in vitro.38 We have previously shown that acidification raises [Ca2+]i,25 which is a key event in triggering NO production.26,27 Accordingly, the intracellular Ca2+ chelator BAPTA-AM prevented AP effect on CXCR4 expression. Further, CXCR4 expression is HIF-1α-dependent 28,29 and, under our experimental conditions, HIF-1α increased both in response to DETA-NO30–32 and to AP; the latter effect was prevented by L-NAME. Therefore, both [Ca2+]i and NO play a pivotal role in AP-mediated increase in CXCR4 expression.
Interestingly, here we found that control ckit+ cells, without AP exposure, failed to induce neovascularisation in vivo. It is noteworthy that the effect of EPC transplantation in animal models of hindlimb ischaemia is still controversial; most studies have shown an angiogenic response associated with an increase in blood flow,5,8,35 whereas others have failed to show any beneficial response.11,39,40 Further, numerous studies on this topic have utilized human cells obtained either from the peripheral circulation8,9,35 or cord blood34,41 rather than BM cells, and have transplanted such cells in immunocompromised mice; in contrast, we have transplanted mouse BM cells in the ischaemic limb of same strain mice. Finally, no prior study has utilized an enriched population of BM ckit+ cells for direct intramuscle injection in the mouse ischaemic hindlimb. These experimental differences may explain the discrepancy between our results with control cells and those prior studies which have shown a beneficial response to EPC transplantation in the mouse model of hindlimb ischaemia.
In conclusion, AP is a simple and unexpensive strategy to enhance BM ckit+ cell therapeutic potential. Further, unlike other cell potentiating interventions, such as viral-mediated transfer of angiogenic or antiapoptotic genes, it is expected that regulatory agencies would readily accept AP as part of the cell preparation protocol for clinical use. Once BM cells have undergone the selection process under Good Manufacturing Practice (GMP) conditions, they would be placed in a hypercarbic environment to achieve a buffer pH of 7.0 for 24 h, prior to transplantation into the patient.42 Therefore, AP represents a clinically applicable strategy to improve the therapeutic efficacy of BM cell transplantation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Author contributions
C.C.: designed, performed experiments, and contributed to write manuscript; R.M., S.S., M.R., C.C., V.A.: performed experiments; J.C.W., G.P., A.S., C.G., M.N.: contributed to design experiments; M.C.C.: designed experiments and wrote manuscript.
Funding
This work was supported by IDI-IRCCS-Ricerca Corrente 06-1.12 and by CCM-IRCCS-Ricerca Corrente 2010-BIO59. C.C. is a PhD student at the School of Experimental Medicine, University of Rome ‘La Sapienza’. Funding to pay the Open Access publication charges for this article was provided by Centro Cardiologico Monzino Spa Via Carlo Parea, Milano, Italy.
Conflict of interest: none declared.
Supplementary Material
References
- 1.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. doi:10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 2.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. doi:10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 3.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. doi:10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 4.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. doi:10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 5.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. doi:10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 6.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. doi:10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 7.Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, Savant-Bhonsale S, Chopp M. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25:2777–2785. doi: 10.1634/stemcells.2007-0169. doi:10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. doi:10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 9.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. doi:10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 10.Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. doi:10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Q, Shao H, Darwin ED, Li J, Yang B, Webster KA, Yu H. Extracellular calcium increases CXCR4 expression on bone marrow-derived cells and enhances pro-angiogenesis therapy. J Cell Mol Med. 2009;13:3764–3773. doi: 10.1111/j.1582-4934.2009.00691.x. doi:10.1111/j.1582-4934.2009.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. doi:10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spallotta F, Rosati J, Straino S, Nanni S, Grasselli A, Ambrosino V, Rotili D, Valente S, Farsetti A, Mai A, Capogrossi MC, Gaetano C, Illi B. Nitric oxide determines mesodermic differentiation of mouse embryonic stem cells by activating class IIa histone deacetylases: potential therapeutic implications in a mouse model of hindlimb ischemia. Stem Cells. 2010;28:431–442. doi: 10.1002/stem.300. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. doi:10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M, Asanuma H, Hirata A, Wakeno M, Takahama H, Sasaki H, Kim J, Takashima S, Tsukamoto O, Minamino T, Shinozaki Y, Tomoike H, Hori M, Kitakaze M. Prolonged transient acidosis during early reperfusion contributes to the cardioprotective effects of postconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H2004–H2008. doi: 10.1152/ajpheart.01051.2006. doi:10.1152/ajpheart.01051.2006. [DOI] [PubMed] [Google Scholar]
- 16.Chonghaile MN, Higgins BD, Costello J, Laffey JG. Hypercapnic acidosis attenuates lung injury induced by established bacterial pneumonia. Anesthesiology. 2008;109:837–848. doi: 10.1097/ALN.0b013e3181895fb7. doi:10.1097/ALN.0b013e3181895fb7. [DOI] [PubMed] [Google Scholar]
- 17.Costello J, Higgins B, Contreras M, Chonghaile MN, Hassett P, O'Toole D, Laffey JG. Hypercapnic acidosis attenuates shock and lung injury in early and prolonged systemic sepsis. Crit Care Med. 2009;37:2412–2420. doi: 10.1097/CCM.0b013e3181a385d3. doi:10.1097/CCM.0b013e3181a385d3. [DOI] [PubMed] [Google Scholar]
- 18.Flacke JP, Kumar S, Kostin S, Reusch HP, Ladilov Y. Acidic preconditioning protects endothelial cells against apoptosis through p38- and Akt-dependent Bcl-xL overexpression. Apoptosis. 2009;14:90–96. doi: 10.1007/s10495-008-0287-5. doi:10.1007/s10495-008-0287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Reusch HP, Ladilov Y. Acidic pre-conditioning suppresses apoptosis and increases expression of Bcl-xL in coronary endothelial cells under simulated ischaemia. J Cell Mol Med. 2008;12:1584–1592. doi: 10.1111/j.1582-4934.2007.00172.x. doi:10.1111/j.1582-4934.2007.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melchionna R, Romani M, Ambrosino V, D'Arcangelo D, Cencioni C, Porcelli D, Toietta G, Truffa S, Gaetano C, Mangoni A, Pozzoli O, Cappuzzello C, Capogrossi MC, Napolitano M. Role of HIF-1alpha in proton-mediated CXCR4 down-regulation in endothelial cells. Cardiovasc Res. 2010;86:293–301. doi: 10.1093/cvr/cvp393. doi:10.1093/cvr/cvp393. [DOI] [PubMed] [Google Scholar]
- 21.Froyland E, Skjaeret C, Wright MS, Dalen ML, Cvancarova M, Kasi C, Rootwelt T. Inflammatory receptors and pathways in human NT2-N neurons during hypoxia and reoxygenation. Impact of acidosis. Brain Res. 2008;1217:37–49. doi: 10.1016/j.brainres.2008.04.038. doi:10.1016/j.brainres.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. doi:10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 23.D'Arcangelo D, Facchiano F, Barlucchi LM, Melillo G, Illi B, Testolin L, Gaetano C, Capogrossi MC. Acidosis inhibits endothelial cell apoptosis and function and induces basic fibroblast growth factor and vascular endothelial growth factor expression. Circ Res. 2000;86:312–318. doi: 10.1161/01.res.86.3.312. [DOI] [PubMed] [Google Scholar]
- 24.Melchionna R, Porcelli D, Mangoni A, Carlini D, Liuzzo G, Spinetti G, Antonini A, Capogrossi MC, Napolitano M. Laminar shear stress inhibits CXCR4 expression on endothelial cells: functional consequences for atherogenesis. FASEB J. 2005;19:629–631. doi: 10.1096/fj.04-2219fje. [DOI] [PubMed] [Google Scholar]
- 25.Ziegelstein RC, Cheng L, Blank PS, Spurgeon HA, Lakatta EG, Hansford RG, Capogrossi MC. Modulation of calcium homeostasis in cultured rat aortic endothelial cells by intracellular acidification. Am J Physiol. 1993;265:H1424–H1433. doi: 10.1152/ajpheart.1993.265.4.H1424. [DOI] [PubMed] [Google Scholar]
- 26.Park YC, Jun CD, Kang HS, Kim HD, Kim HM, Chung HT. Role of intracellular calcium as a priming signal for the induction of nitric oxide synthesis in murine peritoneal macrophages. Immunology. 1996;87:296–302. doi: 10.1046/j.1365-2567.1996.456544.x. doi:10.1046/j.1365-2567.1996.456544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai H, Davis ME, Drummond GR, Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol. 2001;21:1571–1576. doi: 10.1161/hq1001.097028. doi:10.1161/hq1001.097028. [DOI] [PubMed] [Google Scholar]
- 28.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. doi:10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. doi:10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 30.Sandau KB, Fandrey J, Brune B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001;97:1009–1015. doi: 10.1182/blood.v97.4.1009. doi:10.1182/blood.V97.4.1009. [DOI] [PubMed] [Google Scholar]
- 31.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–2558. doi: 10.1074/jbc.M308197200. doi:10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 32.Nanni S, Benvenuti V, Grasselli A, Priolo C, Aiello A, Mattiussi S, Colussi C, Lirangi V, Illi B, D'Eletto M, Cianciulli AM, Gallucci M, De Carli P, Sentinelli S, Mottolese M, Carlini P, Strigari L, Finn S, Mueller E, Arcangeli G, Gaetano C, Capogrossi MC, Donnorso RP, Bacchetti S, Sacchi A, Pontecorvi A, Loda M, Farsetti A. Endothelial NOS, estrogen receptor beta, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest. 2009;119:1093–1108. doi: 10.1172/JCI35079. doi:10.1172/JCI35079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Arcangelo D, Gaetano C, Capogrossi MC. Acidification prevents endothelial cell apoptosis by Axl activation. Circ Res. 2002;91:e4–e12. doi: 10.1161/01.res.0000036753.50601.e9. doi:10.1161/01.RES.0000036753.50601.E9. [DOI] [PubMed] [Google Scholar]
- 34.Zemani F, Silvestre JS, Fauvel-Lafeve F, Bruel A, Vilar J, Bieche I, Laurendeau I, Galy-Fauroux I, Fischer AM, Boisson-Vidal C. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. doi:10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. doi:10.1161/01.CIR.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki T, Fukazawa R, Ogawa S, Kanno S, Nitta T, Ochi M, Shimizu K. Stromal cell-derived factor-1alpha improves infarcted heart function through angiogenesis in mice. Pediatr Int. 2007;49:966–971. doi: 10.1111/j.1442-200X.2007.02491.x. doi:10.1111/j.1442-200X.2007.02491.x. [DOI] [PubMed] [Google Scholar]
- 37.Gurevicius J, Salem MR, Metwally AA, Silver JM, Crystal GJ. Contribution of nitric oxide to coronary vasodilation during hypercapnic acidosis. Am J Physiol. 1995;268:H39–H47. doi: 10.1152/ajpheart.1995.268.1.H39. [DOI] [PubMed] [Google Scholar]
- 38.Bellocq A, Suberville S, Philippe C, Bertrand F, Perez J, Fouqueray B, Cherqui G, Baud L. Low environmental pH is responsible for the induction of nitric-oxide synthase in macrophages. Evidence for involvement of nuclear factor-kappaB activation. J Biol Chem. 1998;273:5086–5092. doi: 10.1074/jbc.273.9.5086. doi:10.1074/jbc.273.9.5086. [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, Langer R, Anderson DG. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci USA. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. doi:10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu JX, Huang XF, Lv WM, Ye CS, Peng XZ, Zhang H, Xiao LB, Wang SM. Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg. 2009;50:608–616. doi: 10.1016/j.jvs.2009.05.049. doi:10.1016/j.jvs.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 41.Pesce M, Orlandi A, Iachininoto MG, Straino S, Torella AR, Rizzuti V, Pompilio G, Bonanno G, Scambia G, Capogrossi MC. Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissues. Circ Res. 2003;93:e51–e62. doi: 10.1161/01.RES.0000090624.04507.45. doi:10.1161/01.RES.0000090624.04507.45. [DOI] [PubMed] [Google Scholar]
- 42.Gaipa G, Tilenni M, Straino S, Burba I, Zaccagnini G, Belotti D, Biagi E, Valentini M, Perseghin P, Parma M, Campli CD, Biondi A, Capogrossi MC, Pompilio G, Pesce M. GMP-based CD133(+) cells isolation maintains progenitor angiogenic properties and enhances standardization in cardiovascular cell therapy. J Cell Mol Med. 2010;14:1619–1634. doi: 10.1111/j.1582-4934.2009.00854.x. doi:10.1111/j.1582-4934.2009.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.