Introduction
Serious adverse drug reactions (ADRs) represent an important problem for clinicians and for the drug development process. Since these are rare, case series for the analysis of risk are generally accrued across multiple institutions. Thus, a key step in this process is the development of standard phenotype definitions. To facilitate this, the International Severe Adverse Event Consortium (iSAEC) has initiated a phenotype standardization project to improve case ascertainment of different types of serious ADRs.1 For each ADR phenotype, a group of investigators with expertise in relevant disciplines (clinical and basic science; regulatory affairs) was convened to develop, in a pragmatic fashion, a standardized case definition with the goal of enhancing the recruitment and usability of data sets for future genomic analysis.
This manuscript describes the consensus phenotype definition for the relatively rare but potentially life-threatening ADR of drug-induced torsades de pointes (DITdP), the syndrome of polymorphic ventricular tachycardia (VT) associated with QT-interval prolongation and T-wave abnormalities. Torsades de pointes (TdP) may be self-limited arrhythmia or progress to ventricular fibrillation (VF) and cardiac arrest. It is most often caused by the use of QT-prolonging anti-arrhythmic drugs (e.g. quinidine, sotalol, dofetilide, and ibutilide),2,3 but can also occur with a wide variety of non-cardiovascular drugs including antibiotics/anti-infectives (erythromycin, clarithromycin, pentamidine), antipsychotics (thioridazine, haloperidol), and antihistamines (terfenadine, astemizole).2,3 A comprehensive list of drugs with a known or potential risk for TdP is maintained at www.QTdrugs.org. Approximately 1–5% of patients treated with QT-prolonging anti-arrhythmic drugs will develop TdP.2,3 The overall incidence of DITdP with ‘non-cardiovascular’ drugs, however, seems much smaller and has not been accurately determined due in part to the fact that most cases reported are not well characterized or are mainly derived from epidemiological and post-marketing surveillance studies4 and therefore have not been ascertained in a consistent fashion. Common clinical risk factors for DITdP are listed in Table 1.
Table 1.
Common clinical risk factors for drug-induced QT prolongation and torsades de pointes
| Female gender |
|---|
| Conditions predisposing to heightened QT prolongation and risk of arrhythmia |
| Heart disease |
| Congestive heart failure |
| Left ventricular hypertrophy |
| Hours following conversion of atrial fibrillation to sinus rhythm |
| Congenital long QT syndrome (may be clinically unrecognized) bradycardia and conduction disease |
| Digitalis use (possible) |
| Increased drug bioavailability |
| Altered function of specific cytochrome P450 (CYP450) isoforms (for liver metabolised drugs) |
| Genetic variants |
| Concomitant inhibitory drugs |
| Liver disease |
| Altered renal or liver function (for renally or hepatically excreted drugs) |
| Electrolyte imbalance |
| Hypokalaemia |
| Hypomagnesaemia |
| Hypocalcaemia (possible) |
The often sporadic and seemingly unpredictable nature of DITdP and the parallels between DITdP and arrhythmias seen in congenital long-QT syndrome (LQTS) suggests that some patients are genetically predisposed to the ADR. This is further supported by reports that LQTS disease-associated mutations are identified in a minority of DITdP cases, often with normal QT intervals after drug withdrawal.5–9 The overall prevalence of LQTS-associated mutations is ∼10% but this varies from 3 to 40% depending on the cohort studied. Other genomic biomarkers may, however, be involved. KCNE1 D85N, a more common rare variant of the LQTS-associated gene, has been associated with DITdP in white Caucasians.10 In addition the single nucleotide polymorphism (SNP), SCN5A S1103Y has been associated with the risk of acquired arrhythmia including DITdP in black Americans.11 More recently SNPs around the NOS1AP, a gene previously linked with QT-interval variation in the general population, have been associated with the risk of drug-induced ventricular arrhythmias in white Caucasian patients receiving amiodarone therapy.12
A vital first step in identifying these markers is a comprehensive genetic analysis of a large and diverse patient database in which reported DITdP cases are identified based on a standard set of phenotypic characteristics. Once these definitions are in place, cases can be amassed in a large, well-defined cohort that can then undergo a genome-based study to identify genetic markers. Studies to date have been hampered by the lack of a uniform case definition and by frequent failure of individual sites to collect possible covariates such as those listed in Table 1. The identification of genetic factors (acting in combination with known environmental and clinical factors) is important because (i) it may ultimately be feasible to pre-screen patients for genetic variability to further guide clinical practice and eventually reduce the overall incidence of DITdP and (ii) it will provide insights into the mechanisms of DITdP, which may ultimately provide lessons for future drug development.
We present here the minimum phenotypic characteristics of DITdP cases for inclusion in these studies, along with recommendations for the collection of clinical cofactors; consistent use of phenotype definitions such as this will enable thorough and global investigations into the clinical and genetic predictors of DITdP.
Consensus process
The consensus process used to develop the DiTdP phenotype was similar to that recently described for other iSAEC phenotypes.1,13,14 Briefly, iSAEC represents a collaboration among the Wellcome Trust, the US Food and Drug Administration (FDA), pharmaceutical companies, and academic institutions. The consortium organized a face-to-face meeting of experts in various phenotypes including DITdP. The members of this expert working group (EWG) comprised individuals from academic medicine and regulatory agencies with expertise in cardiology and clinical electrophysiology, pharmacoepidemiology, pharmacogenomics, and pharmacovigilance.
The task of the DITdP EWG was to define minimum phenotypic requirements that would allow accurate identification of patients with DITdP and to develop a corresponding algorithm to assist in the recruitment of such cases for the database.1 Expert working group participants also generated a set of recommendations for clinical cofactors to be included in the ascertainment process for accurate and comprehensive phenotyping. Participants were asked to consider these criteria in the context of both prospective and retrospective recruitment strategies where data may be largely extracted from medical notes. Following the meeting, the phenotypic criteria and algorithm were circulated to all participants for revision and approval.
Other methodological considerations
In determining phenotypic requirements, the group recognized that a competing research and regulatory question was the variability in the change in QT interval after a drug challenge; indeed, exaggerated QT prolongation [e.g. QTc corrected by Bazett's formula (QTcB) > 500 ms] during drug exposure or an increase in the QTc interval (ΔQT) of >60 ms are considered markers for increased scrutiny for the potential susceptibility to arrhythmias during drug development.13 The group elected to focus on the accrual of subjects with documented or suspected TdP rather than on the issue of variable QT responses in the absence of an arrhythmia. This represented a more important and clinically relevant endpoint than QT prolongation alone, which was viewed as an imperfect marker of potential risk.
Expert working group participants considered that by definition the DITdP phenotypes identified do introduce a bias, i.e. selection, for survivors. Therefore, the EWG agreed that post-mortem cases should be included in the database if enough information (e.g. ECG data including the QT interval prior to and upon the initiation of a suspected culprit drug, and supporting the presence of TdP) to establish a case as DITdP is available; however, it was also noted that all cases—prospective or retrospective—should be submitted for inclusion only after review by a physician (generally an arrhythmia specialist/electrophysiologist) familiar with the criteria outlined here.
The causality assessment was not formally considered by the participants in the DITdP group. Clearly, both drug- and non-drug-induced aetiologies for TdP are important. Attributing TdP to a drug is often complicated by the presence of other factors or possible causes, including underlying heart disease, comedications, comorbid conditions (such as hypokalaemia or bradycardia), and sudden death. The EWG took the position that even in the presence of such conditions, the development of TdP after administration of a suspect drug could still be a case of DITdP. Confirmation of drug causality requires an assessment of temporal relationships, withdrawal of drug exposure, exclusion of other causes, and a determination of potential previous reports of DITdP with the drug (or drugs from the same class) in question. Because of the potentially fatal nature of TdP, ‘rechallenge’ which is an important aspect of causality assessment is generally impractical.15 The temporal relationship is particularly important if TdP occurs after the initiation of drug therapy although delayed or ‘late proarrhythmia’ has been described with quinidine and non-cardiac drugs.16,17 A causal association can also be strengthened by the presence of QT prolongation during drug exposure, and at least partial resolution of QT prolongation, as well as THE cessation of TdP with drug withdrawal. The time course will, however, be dependent on the pharmacokinetic behaviour of the drug and its pharmacology including the interactions with multiple ion channels.18
For a genetic analysis, cases ascertained using the standard criteria outlined here could be compared with several control groups:
population controls matched for age, gender, BMI, and comorbidities;
patients in whom drug exposure does not result in marked lengthening of the QTc interval (e.g. <500 ms or ΔQTc < 60 ms relative to the baseline).
Because of the rarity of DITdP, population-based controls where genome-wide SNP data are freely available may be utilized in a similar fashion to other phenotypes even though there will not be a history of drug exposure and QT prolongation available.17
The EWG largely focused on the case definition and did not further consider the statistical design of such studies.
Description of phenotype
Characterization of torsades de pointes
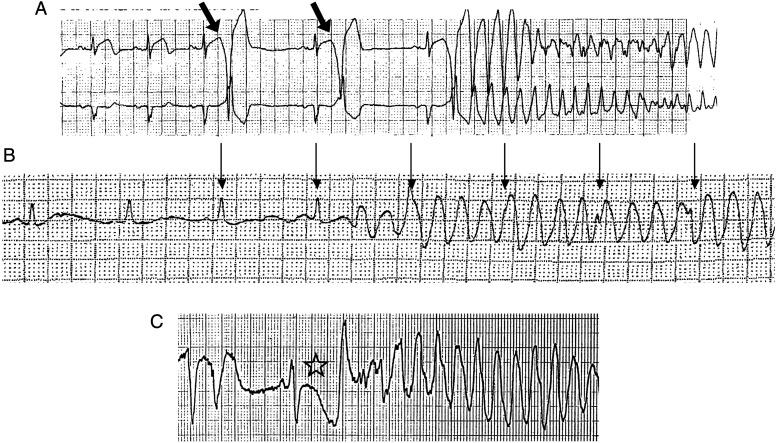
Torsades de pointes is characteristically preceded by a series of ‘short-long-short’ R–R interval cycles (‘pause dependent’ phenomenon) and is linked with QT-interval prolongation.19,20 Pause-dependent changes in T-wave morphology and late coupled ventricular ectopy are common associated features.21,22 It is often slower (160–240 b.p.m.) than coarse VF.19,20 Figure 1 shows three surface ECG recordings each with an arrhythmia that displays TdP-like characteristic ‘twisting of the points’, a beat-to-beat slow shift in the QRS electrical axis; however, only one of these is a true case of DITdP. The polymorphic morphology of TdP may not, however, always be evident, particularly when the arrhythmia is recorded in only one or two ECG leads or on implantable cardiovertor defibrillator (ICD) stored electrograms. A pause-dependent tachycardia associated with severe QT prolongation may therefore appear to be ‘monomorphic’ but in fact represents TdP.23 These diagnostic difficulties reinforce the need for documentation of the index arrhythmia event and for adjudication by expert physicians familiar with the nuances of the ECG diagnosis.
Figure 1.
Surface ECGs of polymorphic ventricular tachycardia and TdP. (A) A very short interval between the sinus beat and the subsequent ventricular ectopic (short coupling interval; arrows) and no QT-interval prolongation in the setting of an acute coronary syndrome (note elevated ST segment on the top lead). This is not TdP. (B) An apparent polymorphic tachycardia that is a recording artefact, identified by the continued regular rhythm ‘marching through’ the recording (arrows). (C) A case of TdP with ‘short-long-short’ cycle length changes at initiation, a long QT on the last sinus beat (star), and a long coupling interval.
Identifying suspected cases of drug-induced torsades de pointes for genetic evaluation
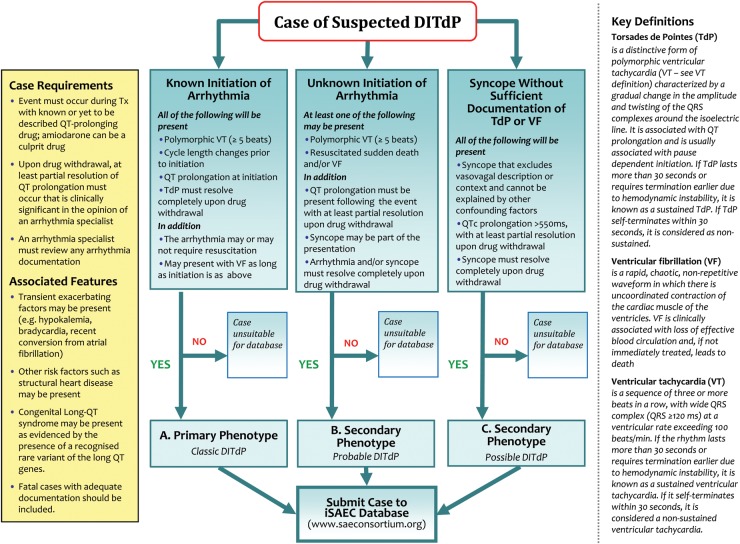
Based on the present understanding of the clinical features of DITdP and its risk factors, the EWG developed an algorithm to identify cases of DITdP (Figure 2). This algorithm identifies the minimum phenotypic requirements of candidate patients, whether cases originate from electronic medical records or from practising physicians and arrhythmia specialists. The minimum proposed requirements are the following:
The possible DITdP event must have occurred during treatment with a known QT-prolonging drug, including amiodarone, and/or a drug suspected of causing QT prolongation.
Upon drug withdrawal, arrhythmias abate and there is at least partial resolution of the QT prolongation that is clinically significant in the opinion of an arrhythmia specialist.
Arrhythmia documentation must be reviewed by an appropriately experienced physician/electrophysiologist.
Figure 2.
Algorithm to identify cases of drug-induced torsades de pointes.
Three clinical phenotypes for suspected DITdP (A, B, and C) were then defined (see below and Figure 2). In A, typical TdP was documented, while B and C are proposed as categories with increasingly incomplete documentation as illustrated. At all times, QT prolongation had to be present (as above) but at least partially resolve with drug withdrawal, in a time course consistent with the pharmacology of the drug. Arrhythmia or syncope should also similarly resolve. The Bazett formula for QTcB was retained because of its established role in clinical practice, although other correction methods, such as Fridericia's formula (QTcF) are often required for regulatory needs.
Phenotype A was defined as ‘classical DITdP’ where the pause-dependent onset of polymorphic VT (at least five beats) with associated QT prolongation is documented with or without resuscitation and/or subsequent VF and cardiac arrest.
Phenotype B was defined as ‘probable DITdP’ where polymorphic VT and/or VF have been documented but the onset of TDP has not been seen. QT prolongation must nonetheless have been documented.
Phenotype C was defined as ‘possible DITdP’ as ventricular arrhythmia has not been documented sufficiently but a history of unexplained syncope with no vagal or neurological features has been elicited with severe QT prolongation on the ECG (QTc >550 ms).
The presence of associated features, as described in Tables 2 and 3, is important and should be documented. This includes exposure to transient exacerbating factors such as hypokalaemia, bradycardia, and recent conversion from AF, and the presence of structural cardiac disease or the congenital long QT syndrome. The inclusion of cases with subsequent fatal outcomes would be acceptable provided other criteria were met. In cases with atrial fibrillation the QT interval will be measured manually at as stable heart rates as possible by averaging the QT and RR intervals of up to 10 cardiac cycles.
Table 2.
Data required for case submission
| Demographics |
| Age at event/date of birth |
| Sex |
| Ancestry (self-reported) |
| Event history |
| Date and time of event |
| Nature of event: type A, B, or C (see algorithm; ECG documentation required) |
| Medications |
| Drug therapy (any) and dosage |
| Culprit drug(s) |
| Date and time of culprit drug(s) administration |
| Outcome (survival/death) |
| ECG findings |
| Acute event |
| Arrhythmia documentation |
| QRS, QT, RR, QTcB, immediately prior to event |
| Documentation of at least partial resolution of QT prolongation with therapy and cessation of culpable drug exposure |
Table 3.
Further information to be requested
| Event history |
| Acute ischaemia (yes/no) |
| Acute reversion of AF (within 48 h of event) |
| Acute intracerebral event |
| Serum K, Mg, and Ca prior to and after the eventa |
| Acute therapy |
| Defibrillation |
| Temporary pacing |
| Electrolyte replacement |
| Beta-blockers |
| Other medications |
| Other |
| Long-term therapy |
| Permanent pacemaker |
| ICD |
| Other |
| ECG findings |
| After exposure to culpable drug fully removed (consistent with pharmacology of drug) |
| QRS, QT, RR, QTcB |
| Digital 24 h Holter monitor if available |
| Other findings: atrial fibrillation, heart block |
| Laboratory findings |
| Renal function parametersa |
| Liver function parametersa |
| Thyroid statusa |
| Past cardiac history |
| LV ejection fraction and hypertrophya |
| Type/extent of heart diseasea |
| Congenital long QT syndrome (mutation if known) |
| Presence of AF, last known AFa |
| Presence of paced rhythm |
| Past medical history |
| Diabetes |
| Hypothyroidism |
| Liver disease |
| Psychiatric disease |
| Family history |
| Congenital long QT syndrome |
| Sudden death age <age 45 |
| Sudden death >age 45 |
| Other heart disease |
| Patient characteristics |
| BMI |
aWith dates/times.
Case submission and clinical cofactors
The genetic basis of DITdP is under investigation by the iSAEC. However, we hope this guidance will also be of use to other researchers who may be working individually or within their own consortia—standardization across different research consortia will allow for future collaboration and individual patient data meta-analysis.
Submission of a case to the iSAEC database will need to meet criteria outlined above and in Figure 2, as well as ECGs documenting QT prolongation by drug, TdP, and any other associated features. For ECG documentation, a 12-lead ECG for QT measurements and TdP registration in more than a single lead are considered ideal, but single-lead tracings are acceptable if unambiguous.
Table 1 lists information required for submission as essential clinical data for confirming the phenotype, whereas data listed in Table 2 are considered desirable but not essential. A web-enabled data collection tool will be organized by the iSAEC to allow investigators to rapidly and efficiently provide this information. This will be part of 2012–14 work with the NIH/NCBI/EBI/PharmGKB to create a comprehensive, integrated drug safety-related database in the near future. The case will then be reviewed by an expert panel of clinicians led by the cochairs of the EWG to include or exclude the case according to the phenotype definitions. The iSAEC will act as the repository for data. DNA will be aggregated by a central academic coordinating centre. Once accepted, cases will be entered into genome-wide characterization in conjunction with the iSAEC. Rejection by the expert panel will be fed back with reasons to the reporting physician. Any identified rare genetic variation consistent with a diagnosis of the congenital long QT syndrome will be reported to the enrolling clinician if requested.
Concluding remarks
Using a consensus approach, a minimum set of phenotypic criteria to allow diagnostic standardization of the potentially fatal drug-induced arrhythmia known as TdP has been identified. The creation of consensus standardized criteria is necessary for facilitating accurate patient recruitment for pharmacogenetic studies, with the ultimate goal being to genetically identify those patients who are at an increased risk for developing DITdP. Such ‘personalized medicine’ may seem a distant goal, but it is only through a global and standardized effort can we make this a reality.
Funding
We also thank other members of the working group who contributed to the manuscript, including Sarah Mee, Aidan Power, Peter Shaw, and Miriam Sturkenboom. Finally, we are indebted to the International Serious Adverse Events consortium (Isaec) and its member companies (Abbott, Amgen, Astra-Zeneca, Daiichi- Sankyo, GSK, Merck, Novartis, Pfizer, Takeda, and Wellcome Trust) for their funding and support of this work.
Conflict of interest: E.R.B. receives research and fellowship funds from the International Serious Adverse Events Consortium, Biotronik and Boston Scientific. C.J. is a cofounder of Cellular Dynamics International, Inc. D.R. is a consultant to Merck, Sanofi, Dai-ichi-Sankyo, and Vitae Pharmaceutical, and receives royalities for a patent to predict drug-induced arrhythmia. A.A.G. is a consultant for Xention Pharma Ltd. M.F., S.B., and S.G. are employed full time by the US Food and Drug Administration and have no financial interests to disclose. The views expressed are those of the authors and are not necessarily those of the FDA. M.P., S.K., and A.Y. have no financial conflicts to disclose.
Acknowledgements
The authors wish to thank the following individuals from the US Food and Drug Administration for their valuable input in the development of this manuscript: Norman Stockbridge, MD, PhD, Director, Division of Cardiovascular and Renal Products; Suchitra Balakrishnan, MD, PhD; John Koerner, PhD; Karen Hicks, MD; Shari Targum, MD; Randall Brockman, MD; and Brian Lewis, MD.
References
- 1.Pirmohamed M, Aithal GP, Behr E, Daly A, Roden D. The phenotype standardization project: improving pharmacogenetic studies of serious adverse drug reactions. Clin Pharmacol Ther. 2011;89:784–785. doi: 10.1038/clpt.2011.30. [DOI] [PubMed] [Google Scholar]
- 2.Roden DM, Viswanathan PC. Genetics of acquired long QT syndrome. J Clin Invest. 2005;115:2025–2032. doi: 10.1172/JCI25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behr ER, Camm AJ. Acquired repolarization disorders. In: Elliott P, Kumar D, editors. Principles and Practice of Clinical Cardiovascular Genetics. New York: Oxford University Press; 2012. pp. 261–275. [Google Scholar]
- 4.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–1372. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donger C, Denjoy I, Berthet M, Neyroud N, Cruaud C, Bennaceur M, Chivoret G, Schwartz K, Coumel P, Guicheney P. KVLQT1 C-terminal missense mutation causes a forme fruste long-QT syndrome. Circulation. 1997;96:2778–2781. doi: 10.1161/01.cir.96.9.2778. [DOI] [PubMed] [Google Scholar]
- 6.Paulussen AD, Gilissen RA, Armstrong M, Doevendans PA, Verhasselt P, Smeets HJ, Schulze-Bahr E., Haverkamp W, Breithardt G, Cohen N, Aerssens J. Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J Mol Med. 2004;82:182–188. doi: 10.1007/s00109-003-0522-z. [DOI] [PubMed] [Google Scholar]
- 7.Yang P, Kanki H, Drolet B, Yang T, Wei J, Viswanathan PC, Hohnloser SH, Shimizu W, Schwartz PJ, Stanton M, Murray KT, Norris K, George AL, Jr, Roden DM. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943–1948. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- 8.Itoh H, Sakaguchi T, Ding WG, Watanabe E, Watanabe I, Nishio Y, Makiyama T, Ohno S, Akao M, Higashi Y, Zenda N, Kubota T, Mori C, Okajima K, Haruna T, Miyamoto A, Kawamura M, Ishida K, Nagaoka I, Oka Y, Nakazawa Y, Yao T, Jo H, Sugimoto Y, Ashihara T, Hayashi H, Ito M, Imoto K, Matsuura H, Horie M. Latent genetic backgrounds and molecular pathogenesis in drug-induced long-QT syndrome. Circ Arrhythm Electrophysiol. 2009;2:511–523. doi: 10.1161/CIRCEP.109.862649. [DOI] [PubMed] [Google Scholar]
- 9.Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long-QT syndrome: clinical impact. Circulation. 1999;99:529–533. doi: 10.1161/01.cir.99.4.529. [DOI] [PubMed] [Google Scholar]
- 10.Kaab S, Crawford DC, Sinner MF, Behr ER, Kannankeril PJ, Wilde AA, Bezzina CR, Schulze-Bahr E, Guicheney P, Bishopric NH, Myerburg RJ, Schott JJ, Pfeufer A, Beckmann BM, Martens E, Zhang T, Stallmeyer B, Zumhagen S, Denjoy I, Bardai A, van Gelder IC, Jamshidi Y, Dalageorgou C, Marshall V, Jeffery S, Shakir S, Camm AJ, Steinbeck G, Perz S, Lichtner P, Meitinger T, Peters A, Wichmann HE, Ingram C, Bradford Y, Carter S, Norris K, Ritchie MD, George AL, Jr, Roden DM. A large candidate gene survey identifies the KCNE1 D85N polymorphism as a possible modulator of drug-induced torsades de pointes. Circ Cardiovasc Genet. 2012;5:91–99. doi: 10.1161/CIRCGENETICS.111.960930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Splawski I, Timothy KW, Tateyama M, Clancy CE, Malhotra A, Beggs AH, Cappuccio FP, Sagnella GA, Kass RS, Keating MT. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 12.Jamshidi Y, Nolte IM, Dalageorgou C, Zheng D, Johnson T, Bastiaenen R, Ruddy S, Talbott D, Norris KJ, Snieder H, George AL, Marshall V, Shakir S, Kannankeril PJ, Munroe PB, Camm AJ, Jeffery S, Roden DM, Behr ER. Common variation in the NOS1AP gene is associated with drug-induced QT prolongation and ventricular arrhythmia. J Am Coll Cardiol. doi: 10.1016/j.jacc.2012.03.031. Advance Access published June 6, 2012, doi:10.1016/j.jacc.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, Wilke RA, Avigan M, Kaplowitz N, Bjornsson E, Daly AK. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
- 14.Pirmohamed M, Friedmann PS, Molokhia M, Loke YK, Smith C, Phillips E, La Grenade L, Carleton B, Papaluca-Amati M, Demoly P, Shear NH. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharmacol Ther. 2011;89:896–901. doi: 10.1038/clpt.2011.79. [DOI] [PubMed] [Google Scholar]
- 15.Malik M, Hnatkova K, Ford J, Madge D. Near-thorough QT study as part of a first-in-man study. J Clin Pharmacol. 2008;48:1146–1157. doi: 10.1177/0091270008323261. [DOI] [PubMed] [Google Scholar]
- 16.Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 2003;82:282–290. doi: 10.1097/01.md.0000085057.63483.9b. [DOI] [PubMed] [Google Scholar]
- 17.Oberg KC, O'Toole MF, Gallastegui JL, Bauman JL. ‘Late’ proarrhythmia due to quinidine. Am J Cardiol. 1994;74:192–194. doi: 10.1016/0002-9149(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 18.Ronaszeki A, Alings M, Egstrup K, Gaciong Z, Hranai M, Kiraly C, Sereg M, Figatowski W, Bondarov P, Johansson S, Frison L, Edvardsson N, Berggren A. Pharmacological cardioversion of atrial fibrillation—a double-blind, randomized, placebo-controlled, multicentre, dose-escalation study of AZD1305 given intravenously. Europace. 2011;13:1148–1156. doi: 10.1093/europace/eur120. [DOI] [PubMed] [Google Scholar]
- 19.Kay GN, Plumb VJ, Arciniegas JG, Henthorn RW, Waldo AL. Torsade de pointes: the long-short initiating sequence and other clinical features: observations in 32 patients. J Am Coll Cardiol. 1983;2:806–817. doi: 10.1016/s0735-1097(83)80226-5. [DOI] [PubMed] [Google Scholar]
- 20.Roden DM, Woosley RL, Primm RK. Incidence and clinical features of the quinidine-associated long QT syndrome: implications for patient care. Am Heart J. 1986;111:1088–1093. doi: 10.1016/0002-8703(86)90010-4. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhof P, Franz MR, Bardai A, Wilde AM. Giant T-U waves precede torsades de pointes in long QT syndrome: a systematic electrocardiographic analysis in patients with acquired and congenital QT prolongation. J Am Coll Cardiol. 2009;54:143–149. doi: 10.1016/j.jacc.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Birati EY, Belhassen B, Bardai A, Wilde AA, Viskin S. The site of origin of torsade de pointes. Heart. 2011;97:1650–1654. doi: 10.1136/hrt.2010.212381. [DOI] [PubMed] [Google Scholar]
- 23.Viskin S. Long QT syndromes and torsade de pointes. Lancet. 1999;354:1625–1633. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]