Abstract
Aims
As part of the diagnosis related groups in Europe (EuroDRG) project, researchers from 11 countries (i.e. Austria, England, Estonia, Finland, France, Germany, Ireland, Netherlands, Poland, Spain, and Sweden) compared how their DRG systems deal with patients admitted to hospital for acute myocardial infarction (AMI). The study aims to assist cardiologists and national authorities to optimize their DRG systems.
Methods and results
National or regional databases were used to identify hospital cases with a primary diagnosis of AMI. Diagnosis-related group classification algorithms and indicators of resource consumption were compared for those DRGs that individually contained at least 1% of cases. Six standardized case vignettes were defined, and quasi prices according to national DRG-based hospital payment systems were ascertained. European DRG systems vary widely: they classify AMI patients according to different sets of variables into diverging numbers of DRGs (between 4 DRGs in Estonia and 16 DRGs in France). The most complex DRG is valued 11 times more resource intensive than an index case in Estonia but only 1.38 times more resource intensive than an index case in England. Comparisons of quasi prices for the case vignettes show that hypothetical payments for the index case amount to only €420 in Poland but to €7930 in Ireland.
Conclusions
Large variation exists in the classification of AMI patients across Europe. Cardiologists and national DRG authorities should consider how other countries' DRG systems classify AMI patients in order to identify potential scope for improvement and to ensure fair and appropriate reimbursement.
Keywords: Myocardial infarction, Diagnosis-related groups, Europe, Economics, Prospective payment system, Hospital
See page 1950 for the editorial comment on this article (doi:10.1093/eurheartj/eht062)
Introduction
DRGs are diagnosis-related groups of patients.1 They were originally developed in the 1970s by a group of researchers around Robert Fetter at Yale University in an attempt to define ‘hospital products’ and to enable the measurement of what hospital actually do.2,3 The basic idea of Fetter4 was to condense the confusingly large number of different (individual) patients treated by hospitals into a manageable number of (i) clinically meaningful and (ii) economically homogenous groups. Consequently, every DRG is characterized by certain clinical characteristics, e.g. certain diagnoses and/or procedures, and by a specific DRG weight, which is a measure of the average costs of treating patients falling into that DRG.5
Diagnosis-related groups enable assessments of hospital activity, which are often called performance assessments, and they allow comparisons of costs, which otherwise would not be possible.4 For example, hospital (or departmental) activity can be assessed by calculating the casemix, i.e. the sum of all DRG weights ‘produced’ by a hospital (or department) during a given period of time; and treatment costs can be compared for similar patients—those falling into the same DRG. In addition, Medicare in the USA soon came to realize the potential of DRGs (as definitions of hospital products) for payment purposes, and introduced the first DRG-based hospital payment system in 1983.2
Since then, DRGs have been adopted in most high-income countries around the world,6 albeit with different purposes.7 In some countries, e.g. Sweden and Finland, DRGs mainly serve as the basis for performance comparisons and benchmarking; in others, e.g. England, France, and Germany, DRGs are primarily used for hospital payment. Furthermore, considerable differences exist between countries in the combination of DRGs with other payment components, the methods used for the calculation of DRG weights,8 and the payment adjustments for structural characteristics of hospitals or for regional differences in costs.9 However, irrespective of the specific purpose of a DRG system, it is essential that the defined groups of patients are sufficiently homogenous in terms of treatment costs. Otherwise, performance comparisons on the basis of DRGs do not adequately control for differences between patients within the same groups; and reimbursement for a large number of patients is not appropriate; it can be either too high or too low.
Diagnosis-related groups are defined by patient classification systems (PCS)—i.e. DRG systems—which classify treatment cases into DRGs on the basis of classification variables such as diagnoses, procedures, and demographic characteristics. [Even though some systems do not define DRGs in the strict sense of the word (that is groups are not diagnosis related), this article uses the term DRGs to summarize all groups of patients defined by DRG systems or similar PCS.] To assure homogenous groups of patients, DRG systems need to consider the most important determinants of resource consumption as classification variables.
In many countries, professional medical associations, specialist experts or consultants formally participate in the process of selection, definition, and update of classification criteria via committees, expert hearings or consultations.10–12 Recently, Häkkinen et al.13 found that DRG-based hospital payment systems for acute myocardial infarction (AMI) patients could possibly be improved by incorporating additional disease-specific classification variables. Therefore, it is important that cardiologists are aware of how their respective patients are classified by their DRG systems in order to assess whether the classification variables adequately reflect differences in the complexity of treating different groups of patients using different techniques. Comparative analyses of how countries' DRG systems classify patients can help cardiologists to scrutinize national standards of classification against European equivalents in order to identify potential scope for improvement.14
This study performs a comprehensive assessment of DRG systems across 11 European countries and has three main objectives: (i) to assess classification variables and algorithms used to group patients with AMI into DRGs; (ii) to compare variations in DRG weights; and (iii) to determine DRGs and hospital quasi prices for six standardized case vignettes of AMI patients with different combinations of demographic, diagnostic, and treatment variables.
Materials
Definition of episode of care and acute myocardial infarction index case
Similar methods have been reported previously for other episodes of care (EoC).15–17 As part of the EuroDRG project, researchers from 11 European countries (i.e. Austria, England, Estonia, Finland, France, Germany, Ireland, the Netherlands, Poland, Spain, and Sweden) agreed upon a common definition for an AMI EoC. The definition was based on the International Classification of Diseases 10th edition (ICD-10) for diagnoses and ICD-9 Clinical Modification (ICD-9CM) for procedures and is presented in Table 1. Researchers from each country translated the definition into national codes for diagnoses and procedures considering available mappings from the Hospital Data Project (HDP) if applicable.18
Table 1.
Definition of episode of care and index case
| Definition | |
|---|---|
| Name | Acute myocardial infarction |
| Defined by | Primary diagnosis AND exclude bypass procedure |
| Primary diagnosis (ICD-10-WHO V2007) | I21 acute myocardial infarction OR |
| I22 subsequent myocardial infarction | |
| Procedure (ICD-9CM V2008) | 36.1 Bypass anastomosis for heart revascularization |
| Index case | |
| Age 70, AMI (NSTEMI) with no relevant complicationsa, no invasive diagnostic evaluation, no PCI, discharged alive, treated as inpatient for 6 days | |
aPatients with ‘no relevant complications’ may well have one or multiple secondary diagnoses. However, these diagnoses are not relevant for the grouping of patients into DRGs.
An AMI index case was defined to facilitate comparisons of relative resource intensity of DRGs within countries (see below). The index case is characterized by the most common patient and treatment characteristics of uncomplicated AMI cases in hospitals across the selected countries.19
Data sources
In each country, researchers identified national or regional hospital databases and obtained access to all information necessary for the purposes of this study. National researchers extracted the number of AMI cases and the corresponding DRGs from the databases for each country. Table 2 provides an overview to the databases and data years available. The number of identified AMI cases conforming to our definition ranged from ∼3400 cases in Estonia to ∼202 800 cases in Germany, while the rate per 100 000 inhabitants ranged from 109 in France to 366 in Sweden.
Table 2.
Number of identified acute myocardial infarction cases in data year and database by country
| Country | AMI cases |
Data year | Source of data | |
|---|---|---|---|---|
| Number | Rate/100 000 inhabitantsa | |||
| Austria | 16 545 | 184.6 | 2008 | Leistungsorientierte Krankenanstaltenfinanzierung (LKF) database of the Bundesministerium für Gesundheit (BMG) |
| England | 73 857 | 155.3 | 2007/08 | Hospital Epsiode Statistic (HES) |
| Estonia | 3409 | 230.0 | 2008 | Estonian Health Insurance Fund (EHIF) database |
| Finland | 12 007 | 248.7 | 2008 | Finnish Hospital Discharge Register and specialized hospitals owned by municipalities |
| France | 69 054 | 109.0 | 2008 | Programme de Médicalisation des Systèmes d'Information en Médecine, Chirurgie, Obstétrique (PMSI MCO) |
| Germany | 202 758 | 268.6 | 2008 | Fallpauschalenbezogene Krankenhausstatistik (DRG-statistic) of the Federal Statistical Office (Destatis) |
| Ireland | 6192 | 138.1 | 2008 | Hospital In-patient Enquirey (HIPE) data base of the Health Services Executive (HSE) |
| Netherlands | 31 341 | 145.3 | 2008 | Diagnose Behandeling Combinaties (DBC) Onderhoud database |
| Poland | 81 634 | 177.0 | 2009 | Register of episodes of care and reimbursements of the National Health Fund (NHF) |
| Spain (Catalonia) | 7721 | 121.7 | 2008 | Hospital Minimum Basic Data Set (CMBD) database of the Public Hospital Network of Catalonia (XHUP) |
| Sweden | 34 817 | 366.0 | 2008 | The National Patient register (NPR) of The Board of Health and Welfare |
aSource: Rate/100 000 inhabitants is from Eurostat.47 Eurostat data refer to all of Spain and to the UK. Our database contains data only from Catalonia and England.
Analysis of patient classification systems
National researchers performed detailed comparative analyses of classification variables and grouping algorithms of national DRG systems20–28 for the most frequent DRGs, i.e. those DRGs that individually contained at least 1% of all AMI cases in the relevant database. Grouping algorithms were mapped graphically to facilitate comparisons between systems.
To compare the national measure of DRG weight (i.e. cost weight, score, tariff) of individual DRGs within and across countries, a DRG weight index was calculated with the DRG containing the index case (compare Table 1) assuming a value of one. The index score of all other DRGs was calculated by dividing the national measure of DRG weight of each DRG by that of the index DRG.
Diagnosis-related groups and hospital quasi prices
For the comparison of variations in hospital price levels between countries, six standardized case vignettes of patients with different combinations of primary and secondary diagnoses, procedures, discharge status (dead or alive), and length of stay were defined in addition to the index case (Table 3). Case vignettes were selected specifically to illustrate differences in DRG systems across countries, i.e. only patient and treatment characteristics were specified that are relevant for the classification of patients and reimbursement of hospitals in at least one country but not in all other countries. Patient and treatment characteristics of case vignettes were varied in order to show the impact that different variables have on the classification of and reimbursement for different types of patients.
Table 3.
Description of case vignettes
| Case vignettesa | |
|---|---|
| Index case | NSTEMI, no relevant complicationsb, no invasive treatment, LOS 6 days |
| Patient 1 | STEMI, cardiogenic shock, diabetes, sequelae of stroke, no invasive treatment, death after 1 day |
| Patient 2 | NSTEMI, no relevant complicationsb, no invasive treatment, angiography for diagnostic evaluation, LOS 4 days |
| Patient 3 | STEMI, no relevant complicationsb, PCI with one BMS, LOS 5 days |
| Patient 4 | STEMI, no relevant complicationsb, PCI with multiple DES, LOS 15 days |
| Patient 5 | STEMI, left ventricular failure, diabetes, sequelae of stroke, haemorrhage complicating a procedure, PCI with multiple BMS, angiography, LOS 25 days |
| Patient 6 | Subsequent MI, VSD as complication of AMI, congestive heart failure, ischaemic cardiomyopathy, sequelae of stroke, PCI with multiple DES, angiography, death after 2 days |
aA complete specification of case vignettes is available as Supplementary material online, Table S1. All patients were specified to be 70 years old and to be treated as inpatients.
bPatients with ‘no relevant complications’ may well have one or multiple secondary diagnoses. However, these diagnoses are not relevant for the grouping of patients into DRGs.
BMS, bare metal stent; DES, drug-eluting stent; LOS, length of stay; NSTEMI, non-ST elevated myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevated myocardial infarction; VSD, ventricular septal defect.
Patient 1 receives only medical treatment, while Patients 2–6 receive invasive diagnostic and therapeutic interventions of increasing intensity. Patients 1 and 6 die shortly after admission, while all other patients are discharged alive after an increasingly long length of stay (from Patient 2 to Patient 5). The index case and Patients 1–3 were selected in order to illustrate more common combinations of patient and treatment characteristics. Patients 4–6 were selected to show how different DRG-based hospital payment systems take into account more complex cases, which can be particularly relevant for reimbursement.29
National researchers grouped the vignettes into DRGs and ascertained quasi prices for each case vignette using an approach similar to that of Koechlin et al.30 Quasi prices were calculated by converting national measures of DRG weight (i.e. cost weights, average tariffs, scores—taking account of outlier deduction/add-ons or additional payments where possible) into monetary values using national conversion rates. If necessary, prices were deflated to year 2008 national currency using national GDP deflators,31 and converted to Euros using average currency exchange rates for the year 2008.32
Results
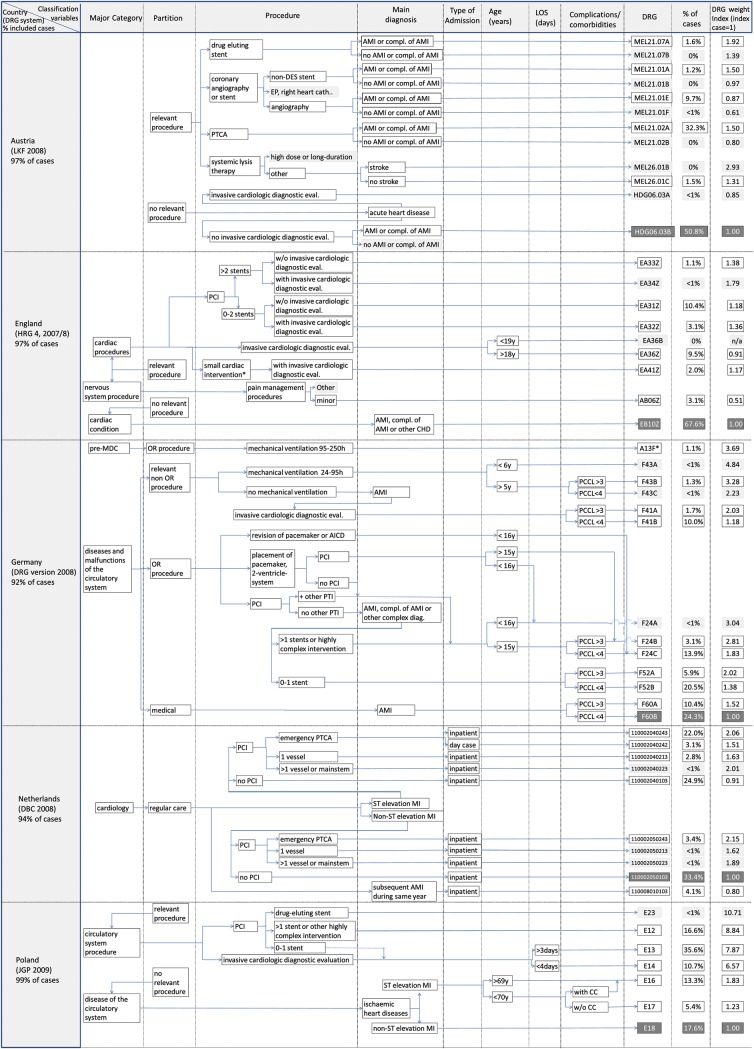
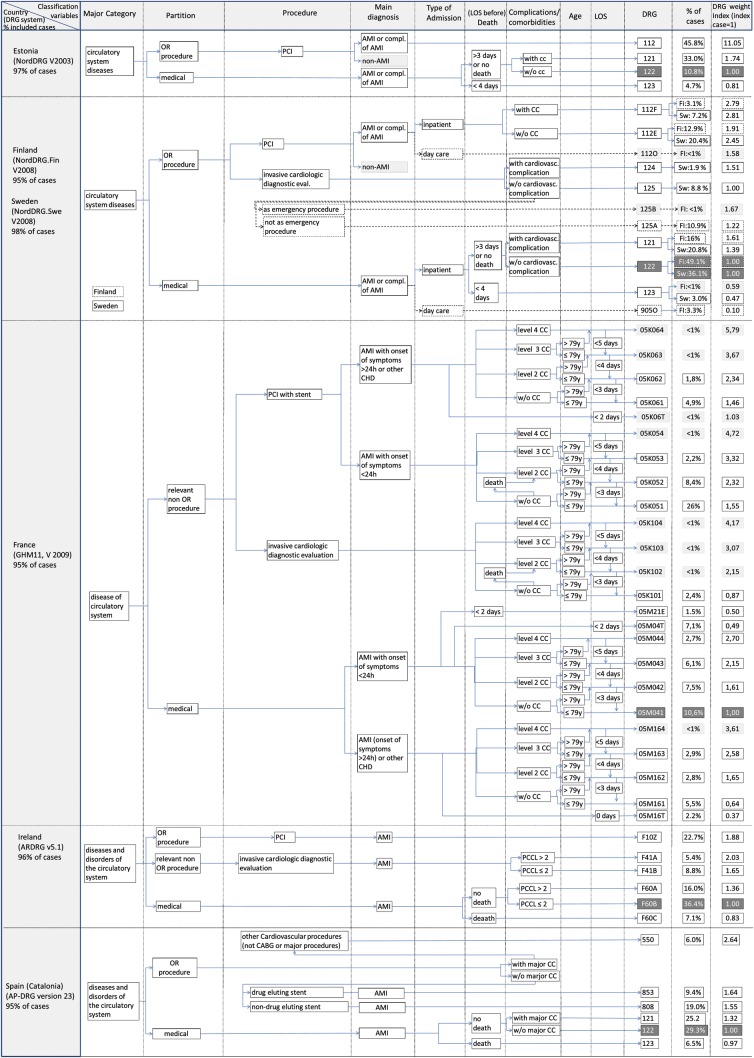
Figures 1 and 2 provide a graphic illustration of grouping algorithms and classification variables of DRG systems in 11 European countries. The figures show classification variables of those DRGs that individually represent at least 1% of AMI cases in each country (and certain DRGs that are necessary for understanding the grouping logic). On the left-hand side, the figures specify the version of the DRG system and the percentage of all identified AMI cases that are shown in the graphs. The arrows indicate the sequence in which different types of classification variables are considered in the grouping algorithm. The last column on the right shows the percentage of AMI cases covered by each DRG and the DRG weight index. Figures 1 and 2 differ slightly as countries included in Figure 2 use death or length of stay before death as a classification variable.
Figure 1.
Graphic illustration of grouping algorithms and classification variables of diagnosis-related group systems in five European countries.
Figure 2.
Graphic illustration of grouping algorithms and classification variables of diagnosis-related group systems in six European countries.
The Finnish and the Swedish algorithms are combined as both use versions of the NordDRG system which are very similar for AMI. Diagnosis-related group containing <1% of cases in the national database are shaded in light grey and are not considered in the following analysis. The index DRGs are highlighted in dark grey.
Patient classification of acute myocardial infarction cases in Europe
Overview: number of diagnosis-related groups, number of classification variables
Figures 1 and 2 demonstrate that there is great variation in DRG systems across Europe. The percentage of all AMI cases covered by the DRGs included in our analysis ranges from 92% in Germany to 99% in Poland. Similarly, the number of DRGs differs substantially across countries ranging from 4 DRGs in Estonia to 16 DRGs in France. The most populated DRG in Germany covers only ∼24% of cases, while in England ∼68% of all cases fall into a single DRG.
In addition, the number of classification variables differs: The Austrian system differentiates only between certain types of procedures, when classifying AMI patients. In contrast, the French system differentiates (i) primary diagnoses (concerning the time since onset of symptoms), (ii) procedures, (iii) level of complications or comorbidities (CC), age groups, (iv) with or without death during admission, and (v) length of stay.
Characteristics of classification variables
In all DRG systems, treatment characteristics, i.e. cardiologic procedures dominate the grouping algorithm. Most importantly, all systems identify patients treated with percutaneous coronary intervention (PCI). Some DRG systems, i.e. the English HRG system, the German (G)-DRG system, the Dutch DBC system, and the Polish JGP system, further, differentiate whether treatment involved more than one stent (Germany, Poland), more than two stents (England), or more than one vessel (Netherlands). Furthermore, some systems have specific DRGs for drug-eluting stents (DES), i.e. the Austrian LKF system, the Polish JGP system, and the AP-DRG system used in Spain. All DRG systems except for the Estonian version of NordDRGs and the AP-DRG system (Spain) use invasive cardiologic diagnostic evaluation as a classification variable. Finally, certain countries, e.g. England and Germany have further procedural classification variables, such as pain management procedures in England or the duration of mechanical ventilation in Germany.
Most DRG systems use a primary diagnosis of AMI as a classification variable after having considered certain procedures. Only the English HRG system and the Polish JGP system do not require a primary diagnosis of AMI for the classification of patients with cardiologic procedures. The Netherlands and Poland are the only countries distinguishing between patients with ST-segment elevation and those without.
The presence of relevant secondary diagnoses, i.e. CC, influences the classification of patients in most countries. Age plays a role in the classification process of some systems (i.e. England, Germany, Poland, and France). Death or death during the first days of admission is considered a classification variable in all DRG systems included in Figure 2. The length of stay is relevant for grouping cases into DRGs only in France and Finland, though it is considered for outlier deductions or additional payments in a number of other countries such as England, Germany, France, and Austria.9
Distribution of acute myocardial infarction cases and variation in relative resource intensity
In most countries, the majority of AMI cases is grouped into the highlighted index DRG (in Figures 1 and 2) containing the index case, i.e. patients without invasive procedures. In England, almost 68% of patients fall into the index DRG (EB10Z), while in all other countries, the index DRG accounts for ∼50% of AMI patients or less. However, in Poland, Estonia, and France, the most populated DRGs are for patients treated with PCI. In France, the most populated DRG containing 26% of cases has a DRG weight index score of 1.55, implying that hospitals receive a 55% higher reimbursement for these patients than for the index case. In Poland, the most populated DRG (∼36% of cases) has a DRG index score of ∼6.6. In Estonia, ∼46% of cases are grouped into a DRG with a DRG weight that is 11 times higher than that of the index DRG.
In Austria, England, and Ireland, the DRG with the highest weight has a DRG weight index score below or close to 2, indicating that these systems do not systematically account for cases that are more than twice as resource intensive as the index case. In Germany, Finland, Sweden, France, and Spain, the highest valued DRG has a DRG index score between 2.6 and 3.7.
Diagnosis-related groups for patients treated with PCI always have DRG weights higher than that of the index case. However, the size of this difference varies considerably. In England, the DRG for patients with PCI, having received 0–2 stents, and no subsequent angiography (HRG EA31Z) has a DRG weight index score of 1.18. In France and Germany, patients with PCI would be grouped into DRGs with a DRG weight index score of at least 1.38 in Germany (DRG F52B) and 1.46 in France (GHM 05K061). As mentioned above, in Estonia and Poland, PCI DRGs are associated with much higher DRG weights.
Interestingly, in Austria, England, and France, DRGs for patients who receive invasive diagnostic procedures (MEL21.01E in Austria, EA36Z in England, 05K101 in France) have a DRG weight below that of the index DRG, implying that hospitals would receive higher reimbursements if they did not perform these procedures. Furthermore, in England, a DRG for patients receiving certain pain management procedures has a DRG weight that is only about half the size of the index DRG.
In all countries taking into account secondary diagnoses in the classification of patients, DRGs for patients with CCs are always associated with much higher DRG weights than DRGs for similar patients without CCs.
Diagnosis-related groups and hospital quasi prices for case vignettes
Table 4 shows a comparison of DRGs and hospital quasi prices for the index case and the six case vignettes under the assumption that hospital payment would be exclusively based on DRGs. For each case vignette, the first column specifies the DRG into which a case vignette patient would be classified, while the second column shows the corresponding quasi price.30
Table 4.
Comparison of diagnosis-related groups and hospital (quasi) prices for acute myocardial infarction patients in Europe (in year 2008, €)
| Index case |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRG | (Quasi) Price (€) | DRG | (Quasi) Price (€) | DRG | (Quasi) Price (€) | DRG | (Quasi) Price (€) | DRG | (Quasi) Price (€) | DRG | (Quasi) Price (€) | DRG | (Quasi) Price (€) | |
| Austriaa | HDG06.03B | 2601 | HDG06.03Bj | 916 | MEL21.01E | 2258 | MEL21.02A | 5040 | MEL21.02A | 12 813 | MEL21.02Ak* | 11 220 € | MEL21.02Aa | 13 048 |
| Englandb | EB10Z | 4533 | EB10Z | 4533 | EA36Z | 4088 | EA31Z | 5825 | EA33Z* | 7626 | EA34Z* | 9622 € | EA34Z | 7988 |
| Estoniac | 122 | 960 | 123 | 774 | 122 | 960 | 112 | 10 606 | 112 | 10 606 | 112 | 10 606 € | 112 | 10 606 |
| Finlandd | 122 | 2189 | 123 | 1320 | 125A | 2681 | 112E | 4182 | 112E | 4182 | 112F | 6106 € | 112F | 6106 |
| Francee | 05M041 | 1837 | 05M21E | 912 | 05K101 | 1611 | 05K051 | 3662 | 05K051k | 7399 | 05K052k | 7119 € | 05K051 | 6975 |
| Germanyf | F60B | 2926 | F60Aa | 1180 | F41B | 3451 | F52B | 4045 | F24C | 8770 | F24Bk | 8490 € | F24Ba | 9187 |
| Irelandg | F60B | 7933 | F60C | 6576 | F41B | 13 100 | F10Z | 14 926 | F10Z | 14 926 | F10Zk | 17 894 € | F10Z | 14 926 |
| Netherlands | 110002050103 | 4493 | 110002040103 | 4111 | 110002050103 | 4493 | 110002040243 | 9260 | 110002040243 | 9260 | 110002040243 | 9260 € | 110002040243 | 9260 |
| Poland | E18 | 420 | E16 | 771 | E13 | 3306 | E13 | 3306 | E23 | 4133 | E12k | 4721 € | E23 | 4133 |
| Spainh | 122 | 4115 | 123 | 3973 | 122 | 4115 | 808 | 6363 | 853 | 6752 | 808 | 6363 € | 853 | 6752 |
| Swedeni | 122 | 2981 | 123 | 1397 | 125 | 2975 | 112E | 7304 | 112E | 7304 | 112F | 8362 € | 112F | 8362 |
aReported values are based on calculated scores. Actual hospital payment depends on decisions of states, which make the use of nationwide DRG scores in different ways. Patients 3–6 are grouped into MEL21.02A because the sum of the MEL score and procedural surcharge for the insertion of stents is higher for MEL21.02A than for MEL21.01A or MEL21.07A.
bBased on 2009–10 non-elective tariffs and HRG version 4.
cQuasi prices were calculated by multiplying cost weights with the national base rate. In actual payment, hospitals are paid through a mix of DRG-based payment and fee-for-service. The actual DRG-based payment is only 70% of the reported Quasi price. In Estonia outliers are identified on the basis of cost thresholds. Because costs were not specified for the case vignettes, outlier status for case vignettes could not be determined.
dActual hospital payment varies by type of hospital (i.e. university, central, and local hospitals) and hospital district. Provided figures based on cost accounting of Helsinki and Uusimaa Hospital district and thus do not represent any actual reimbursement systems. Outlier limits and associated outlier reimbursement differ between hospital districts.
eReported prices are for public sector hospitals since private hospital prices do not reflect full costs. Quasi prices for Patients 3–6 include the averages of maximum and minimum add-on tariffs for stents (between €1220 and 1550 for DES and between €550 and 1100 for BMS, up to a maximum of three stents per vessel, source: www.ameli.fr).
fCalculated using national DRG cost weights and the average of state-wide base rates in 2008 (€2803.05). Indicated (quasi) prices for Patients 4 and 6 include supplementary payments for drug-eluting stents (based on applicable supplementary payment tariffs for the year 2009 of €693.11/DES deflated to 2008).
gCalculated using AR-DRG V 5.1 and the national average inpatient ‘base price’ of €5219 for a relative value of 1 and the other casemix parameters (including low and high length of stay trim points) from the 2009 inpatient casemix model (used to estimate the 2010 casemix budgetary adjustments on the basis of 2008 activity and cost data). Information on type of stent (drug-eluting or non-drug-eluting) is not available in ICD-10-AM.
hAP-DRGs in Spain are used when patients receive care in non-resident autonomous communities (ACs). The prices shown are the rate that would be paid for these patients, calculated by multiplying national Spanish cost weights with the national base rate. The payment to hospitals for other patients depends on the hospital payment system in the AC. When using AP-DRGs in Spain, inliers/outliers are not determined.
iActual hospital payment depends on the county council, which decides how to pay hospitals. In Sweden, outliers are mostly identified on the basis of cost thresholds. However, because outlier payments differ between counties, relevant adjustments could not be determined.
jCases are considered short-stay outliers and presented (quasi) prices reflect outlier status.
kCases are considered long-stay outliers and presented (quasi) prices reflect outlier status.
Partially reflecting differences in terms of GDP per capita,31 the quasi price of the index case varies substantially across countries, ranging from €420 in Poland to €7930 in Ireland. However, countries that pay a higher price for one patient do not necessarily pay a higher price for all types of patients. For example, hospitals in England would receive much higher payments than hospitals in Germany for a patient with no comorbidities, treated with PCI and a single-bare metal stent (BMS) and who is discharged alive after 10 days (Patient 3). However, hospitals in Germany would receive much higher payments than hospitals in England for a 70-year-old patient with AMI and multiple comorbidities who is treated with PCI and multiple DES and who has a subsequent angiography but dies after 2 days in hospital (Patient 6). (see Supplementary material online, Figure S1 facilitates these kinds of comparisons by presenting the results of Table 4 using a quasi price index score, which compares hospital quasi prices within countries for each patient to the quasi price for the index case.)
In addition, Table 4 shows that countries award the highest payments for different kinds of patients. For example, in Austria, and Germany, hospitals would receive the highest payments for patients treated with multiple DES (Patients 4 and 6), with marginally higher payments for Patient 6 who has multiple comorbidities and who is evaluated with subsequent angiography. In contrast, in England, Ireland, and Poland, quasi prices are highest for Patient 5 who is treated with multiple BMS and has a very long length of stay (see Supplementary material online, Figure S1).
Interestingly, Austria, England, and Estonia pay a quasi price for Patient 2 (no secondary diagnoses, evaluated with angiography) that is below the quasi price for the index case, while Poland and Ireland pay quasi prices that are almost equivalent to those for Patient 3 who is treated with PCI and a BMS. Furthermore, in Estonia and Poland, quasi prices increase up to 11-fold for patients treated with PCI when compared with patients treated without PCI.
Discussion
This is the most comprehensive comparative analysis of grouping algorithms, classification variables, and (quasi) prices for AMI patients in different DRG systems in Europe. It shows great variation across countries: (i) in the number of DRGs used to classify AMI cases; (ii) in the characteristics of classification variables; (iii) in the degree of differentiation between DRG weights for complex and less complex cases; and (iv) in the quasi prices for different types of patients (case vignettes).
As DRGs are used to assess the activity (performance) of hospitals (including that of cardiologists) and to determine hospital payment,9,33 it is important that DRG systems consider the most appropriate classification variables and define as many groups as necessary to assure that performance comparisons and hospital payments are fair.34 However, at the same time, DRG systems must avoid providing unintended incentives that carry the danger of distorting decision-making towards more invasive treatment than clinically indicated.
Given the identified large variation between DRG systems for the classification of AMI patients, it is possible that some systems use classification variables that would improve the classification of patients also in other countries. Cardiologists can influence decisions about how to define classification variables in their roles as advisors to national authorities responsible for defining and updating the DRG systems of their countries.10–12 International comparisons can provide a useful new perspective when thinking about how to improve an existing DRG system. Yet, before drawing conclusions on the basis of this study's findings, limitations of our data and methodology need to be considered.
First, the data that were used to identify patients and to assess the relative importance of different DRGs in different countries, originated from routine inpatient databases in 11 countries, which have a number of drawbacks when compared with registries.35 For example, as highlighted by the HDP,18 there are differences in coding practices across countries, and the quality of data is not always comparable. Furthermore, coding practices may be influenced by the characteristics of the country-specific DRG system, which may provide incentives for increasing coding efforts or even manipulating coded data in order to increase reimbursement (‘upcoding’).9 In addition, differences exist in the coding systems used across countries. Most countries included in our study use a country modified version of ICD-10, while Spain uses ICD-9-CM codes. Yet, this should not be particularly problematic for our study as our definition of the AMI episode of care was based on the International Shortlist of Hospital Morbidity Tabulation (ISHMT), which also provides mappings from ICD-10 codes for AMI and their translation into ICD-9-CM.
Secondly, differences in hospital payment systems between countries complicate comparative analyses of payment levels (Table 4). On the one hand, different countries set DRG-based payment rates at different levels as they include different cost categories. For example, in Germany, fixed capital costs are not included in DRG-based payment rates, whereas in most other countries, DRG-based payment rates include capital costs.30 On the other hand, different systems of additional payments exist, e.g. England assigns additional (‘unbundled’) HRGs for intensive care unit treatment (‘critical care’),36 and Poland and Austria have additional per-diem-based payments for stays in intensive care units. Furthermore, the Netherlands and Finland have several DRGs per hospital stay, each leading to additional DRG-based payments. In Finland outlier limits and associated outlier reimbursement differ between hospital districts. Last but not least, DRG-based payments are adjusted in several countries to account for differences between hospitals or regions. Therefore, the absolute price levels should not be directly interpreted as reflecting more expensive care in one country compared with another. However, relative price levels within countries that were used for comparisons in Figure 1, Figure 2 and Supplementary material online, Figure S1 should be less affected by differences in payment systems as they were always compared with the in-country DRG index case.
Thirdly, as we limit part of our comparative analysis to DRGs that account for at least 1% of cases (Figure 1, Figure 2), we partially neglect how different systems deal with rare outliers, which may, however, be particularly relevant for reimbursement.
Finally, although there are European guidelines for the management of AMI patients,37,38 large disparities exist in clinical practice across countries. For example, >80% of AMI patients in Germany are treated with primary PCI, while this number is <25% in England.35 In addition, coded patient characteristics differ considerably: The percentage of ST-elevated MI (STEMI) ranges from 24% in Sweden to 77% in France, and there is a large variation in the presence of (coded) comorbidites.19 Therefore, it is possible that differences in DRG systems between countries reflect some of these disparities. However, there is no apparent relationship between differences in (coded) clinical practice and DRG system design. For example, PCI is used as a classification variable in all countries—even if the number of patients being treated with PCI is relatively low; the insertion of multiple stents is used as a classification variable in England, where the percentage of patients treated with multiple stents is only ∼1%; and the type of infarction (STEMI vs. non-STEMI) is considered only in the Polish and Dutch DRG systems, where the percentage of STEMI patients is neither particularly high nor particularly low.
In spite of these limitations, our study has major implications for cardiologists and national authorities involved in the redesigning of national DRG systems. First, awareness about classification algorithms and variables in other countries should encourage cardiologists to think about alternative and possibly better ways for classifying their patients into DRGs. For example, while eight countries differentiate between patients with certain CC and those without, three countries (Austria, England, and the Netherlands) do not make this distinction. Previous studies have found that comorbidities are associated with higher mortality and resource use in AMI patients.19,39–42 Diagnosis-related group systems should account for differences between patients, in order to assure that hospitals and cardiologists treating a greater share of more complex cases than others, are adequately paid for their greater efforts. Therefore, Austria, England, and the Netherlands could investigate whether homogeneity of patients within DRGs would be increased by introducing classification variables for comorbidities.
Secondly, concerning the reimbursement of hospitals, all DRG systems differentiate between patients treated with PCI and those without. This is in line with a comment by Widimsky et al.35 who compared reperfusion strategies across Europe and noted that PCI hospitals were, in general, adequately reimbursed, which is important as PCI is associated with higher costs of care for AMI patients.19,43 However, our results show that ‘adequately’ means very different things across Europe. For example, in England, where the percentage of patients treated with PCI is comparatively low,35 hospitals receive only 1.2 times higher reimbursement for patients treated with PCI (0–2 stents, without subsequent angiography, i.e. HRG EA32Z) than for those without PCI (see Figure 1). In contrast, hospitals in Germany and France would receive 1.4 and 1.5 times higher reimbursements, respectively, for PCI compared with no PCI; and in Estonia, hospitals receive 11 times higher reimbursements for PCI compared with no PCI. It is at least conceivable that the lower reimbursement for PCI in England is one of the reasons for the lower rate of PCI in England (15%), when compared with Estonia, Germany, or France (>40% in all of these countries, compare Figure 1 and Figure 2).
Thirdly, DRG-based hospital payment systems in Austria, France, Germany, Poland, and Spain differentiate between patients treated with DES and BMS, either by classifying cases into specific DES DRGs (Austria, Poland, and Spain) or by providing additional payments for DES (France and Germany). Current ESC guidelines on myocardial revascularization44 recommend that DES should be ‘considered by default in nearly all clinical conditions and lesion subsets’. However, a recent review by the Cochrane collaboration45 concluded that ‘the increased cost of drug-eluting stents and lack of evidence of their cost-effectiveness means that various health funding agencies are having to limit or regulate their use in relation to price premium.’ Therefore, both strategies, either providing incentives for the use of DES or avoiding these incentives, appear to be justified. However, being aware of different choices in different countries can inform policy debates within countries.
Finally, the aim of any DRG system is to give a concise measure of what hospitals do. This measure is useful only if DRGs describe a sufficiently homogenous group of patients.46 Therefore, quantitative research is needed to verify whether the most important determinants of cost are considered in different PCSs, and whether differences between systems reflect country-specific differences in treatment patterns. However, it is also important for cardiologists and other medical specialists to be aware of the significance of adequately designed DRG systems and to engage in optimizing these systems. Ultimately, this contributes to assuring adequate reimbursement for treated patients and fair comparisons of activity or performance on the basis of DRGs.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The work was funded through the seventh framework programme (FP7) of the European Commission under Grant Agreement Number 223300. Funding to pay the Open Access publication charges was available from general budget funds of the Centre for Health and Social Economics, National Institute for Health and Welfare, Helsinki, and the Department of Health Care Management, Technische Universität Berlin.
Conflict of interest: none declared.
Supplementary Material
Acknowledgement
The findings and results presented in this article were generated in the framework of the project ‘Diagnosis-Related Groups in Europe: Towards Efficiency and Quality (EuroDRG)’. We are grateful to all our partners who made this work possible and to Ms. Claudia Reiche for assistance in editing the Figures 1 and 2.
References
- 1.Fetter J, Shin Y, Freeman JL, Averill RF, Thompson JD. Case mix definition by diagnosis-related groups. Med Care. 1980;18:i–53. [PubMed] [Google Scholar]
- 2.Mayes R. The origins, development, and passage of Medicare's revolutionary prospective payment system. J Hist Med Allied Sci. 2007;62:21–55. doi: 10.1093/jhmas/jrj038. [DOI] [PubMed] [Google Scholar]
- 3.Goldfield N. The evolution of diagnosis-related groups (DRGs): from its beginnings in case-mix and resource use theory, to its implementation for payment and now for its current utilization for quality within and outside the hospital. Qual Manag Health Care. 2010;19:3–16. doi: 10.1097/QMH.0b013e3181ccbcc3. [DOI] [PubMed] [Google Scholar]
- 4.Fetter RB. Diagnosis related groups—understanding hospital performance. Interfaces. 1991;21:6–26. [Google Scholar]
- 5.Quentin W, Geissler A, Scheller-Kreinsen D, Busse R. Understanding DRGs and DRG-based hospital payment in Europe. In: Busse R, Geissler A, Quentin W, Wiley MM, editors. Diagnosis Related Groups in Europe: Moving Towards Transparency, Efficiency and Quality in Hospitals. Maidenhead: Open Univ. Press; 2011. pp. 23–36. [Google Scholar]
- 6.Paris V, Devaux M, Wei L. Health systems institutional characteristics: a survey of 29 OECD Countries. 2010. OECD Health working papers No. 50. Paris.
- 7.Geissler A, Quentin W, Scheller-Kreinsen D, Busse R. Introduction to DRGs in Europe: common objectives across different hospital systems. In: Busse R, Geissler A, Quentin W, Wiley MM, editors. Diagnosis Related Groups in Europe: Moving Towards Transparency, Efficiency and Quality in Hospitals? Maidenhead: Open Univ. Press; 2011. pp. 9–21. [Google Scholar]
- 8.Schreyögg J, Stargardt T, Tiemann O, Busse R. Methods to determine reimbursement rates for diagnosis related groups (DRG): a comparison of nine European countries. Health Care Manag Sci. 2006;9:215–223. doi: 10.1007/s10729-006-9040-1. [DOI] [PubMed] [Google Scholar]
- 9.Cots F, Chiarello P, Salvador X, Quentin W. DRG-based hospital payment: Intended and unintended consequences. In: Busse R, Geissler A, Quentin W, Wiley MM, editors. Diagnosis Related Groups in Europe: Moving Towards Transparency, Efficiency and Quality in Hospitals. Maidenhead: Open Univ. Press; 2011. pp. 75–92. [Google Scholar]
- 10.Casemix Service. HRG 4 Design Concepts. Leeds: The Information Centre, National Health Service (NHS; 2007. [Google Scholar]
- 11.InEK. Siegburg: Institut für das Entgeltsystem im Krankenhaus (InEK); 2011. Vorschlagsverfahren zur Einbindung des medizinischen, wissenschaftlichen und weiteren Sachverstandes bei der Weiterentwicklung des G-DRG-Systems für das Jahr 2012 [Procedure to Allow for the Systematic Incorporation of Medical, Scientific, and Other Expertise for the Development of the G-DRG System] [Google Scholar]
- 12.Patris A, Blum D, Girardier M. A change in the French patient classification system. CASEMIX Quat. 2001;3:128–138. [Google Scholar]
- 13.Häkkinen U, Chiarello P, Cots F, Peltola M, Rätto H the EuroDRG Group. Patient classification and hospital costs of care for acute myocardial infarction in nine European countries. Health Econ. 2012;21(Suppl. 2):19–29. doi: 10.1002/hec.2840. [DOI] [PubMed] [Google Scholar]
- 14.Reid B, Sutch S. Comparing diagnosis-related group systems to identify design improvements. Health Policy. 2008;87:82–91. doi: 10.1016/j.healthpol.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Quentin W, Scheller-Kreinsen D, Geissler A, Busse R. Appendectomy and diagnosis-related groups (DRGs): patient classification and hospital reimbursement in 11 European countries. Langenbeck's Arch Surg. 2012;397:317–326. doi: 10.1007/s00423-011-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheller-Kreinsen D, Quentin W, Geissler A, Busse R. Breast cancer surgery and diagnosis-related groups (DRGs): patient classification and hospital reimbursement in 11 European countries. Breast. doi: 10.1016/j.breast.2012.11.001. doi:10.1016/j.breast.2012.11.001. Published online ahead of print 7 December 2012. [DOI] [PubMed] [Google Scholar]
- 17.Peltola M, Quentin W. Diagnosis-related groups (DRGs) for stroke in Europe. Patient classification and hospital reimbursement in 11 countries. Cerebrovascular Dis. 2013 doi: 10.1159/000346092. doi:1010.1159/000346092. Publication forthcoming. [DOI] [PubMed] [Google Scholar]
- 18.HDP2. Prismant; 2008. Hospital Data Project Phase 2: final report. The need for metadata and data. [Google Scholar]
- 19.Quentin W, Scheller-Kreinsen D, Busse R. Technological innovation in DRG-based hospital payment systems across Europe. In: Busse R, Geissler A, Quentin W, Wiley MM, editors. Diagnosis Related Groups in Europe: Moving Towards Transparency, Efficiency and Quality in Hospitals. Maidenhead: Open Univ. Press; 2011. [Google Scholar]
- 20.BMGFJ. Leistungsorientierte Krankenanstaltenfinanzierung – LKF – Modell 2008. Wien: Bundesministerium für Gesundheit, Familie und Jugend; 2008. [Google Scholar]
- 21.NHS Information Centre for Health and Social Care. The Casemix Service. HRG4 Reference Cost Grouper – Guide to File Preparation. Leeds, Southport, London: NHS Information Centre for Health and Social Care; 2008. [Google Scholar]
- 22.ATIH. Manuel des GHM, version 11. Paris: Agence Technique de l'information sur l'hospitalisation (ATIH); 2009. [Google Scholar]
- 23.Nordic Centre for Classifications in Health Care. NordDRG Users' Manual. Uppsala: Nordic Centre for Classifications in Health Care; 2007. [Google Scholar]
- 24.Institut für das Entgeltsystem im Krankenhaus gGmbH (InEK) G-DRG German Diagnosis Related Groups Version 2008: Definitionshandbuch. Siegburg: InEK; 2007. [Google Scholar]
- 25.Commonwealth Department of Health and Ageing. Australian Refined Diagnosis Related Groups Version 6.0 Definitions Manuals. Canberra: Commonwealth Department of Health and Ageing; 2008. [Google Scholar]
- 26.DBC Onderhound. Diagnose Behandeling Combinaties. Amsterdam: DBC Onderhound; 2008. [Google Scholar]
- 27.The National Health Fund. Jednorodne Grupy Pacjentów -Technical material. Warsaw: The National Health Fund; 2008. [Google Scholar]
- 28.3M. Wallingford: 2005. All Patients Diagnosis Related Groups Definitions Manual version 23.0 3M Health Information Systems. [Google Scholar]
- 29.Antioch KM, Ellis RP, Gillett S, Borovnicar D, Marshall RP. Risk adjustment policy options for casemix funding: international lessons in financing reform. Eur J Health Econ. 2007;8:195–212. doi: 10.1007/s10198-006-0020-7. [DOI] [PubMed] [Google Scholar]
- 30.Koechlin F, Lorenzoni L, Schreyer P. Comparing price levels of hospital services across countries: results of pilot study. 2010. OECD Health Working Papers No. 53.
- 31.OECD. OECD Health Data 2011: Statistics and Indicators. Paris: Organisation for Economic Co-operation and Development (OECD); 2011. [Google Scholar]
- 32.ECB. Statistical Data Warehouse: National Currency Per Euro, Annual Bilateral Exchange Rates Time Series. Frankfurt: European Central Bank (ECB).; 2010. available from: URL http://sdw.ecb.europa.eu/browse.do?node=2018794. (9 January 2013) [Google Scholar]
- 33.Busse R, Geissler A, Quentin W, Wiley MM. Maidenhead: Open Univ. Press; 2011. Diagnosis related groups in Europe: moving towards transparency, efficiency and quality in hospitals. [Google Scholar]
- 34.Busse R, Quentin W. Moving towards transparency, efficiency and quality in hospitals: conclusions and recommendations. In: Busse R, Geissler A, Quentin W, Wiley MM, editors. Diagnosis Related Groups in Europe: Moving Towards Transparency, Efficiency and Quality in Hospitals. Maidenhead: Open Univ. Press; 2011. pp. 149–174. [Google Scholar]
- 35.Widimsky P, Wijns W, Fajadet J, de Belder M, Knot J, Aaberge L, Andrikopoulos G, Baz JA, Betriu A, Claeys M, Danchin N, Djambazov S, Erne P, Hartikainen J, Huber K, Kala P, Klinceva M, Kristensen SD, Ludman P, Ferre JM, Merkely B, Milicic D, Morais J, Noc M, Opolski G, Ostojic M, Radovanovic D, de Servi S, Stenestrand U, Studencan M, Tubaro M, Vasiljevic Z, Weidinger F, Witkowski A, Zeymer U. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31:943–957. doi: 10.1093/eurheartj/ehp492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casemix Service. Unbundled HRGs by Chapter in HRG4. Leeds: The Information Centre, National Health Service (NHS); 2007. [Google Scholar]
- 37.van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M, Vahanian A, Camm J, De CR, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Silber S, Aguirre FV, Al-Attar N, Alegria E, Andreotti F, Benzer W, Breithardt O, Danchin N, Di MC, Dudek D, Gulba D, Halvorsen S, Kaufmann P, Kornowski R, Lip GY, Rutten F. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 38.Hamm CW, Bassand J, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D, Bax JJ, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Windecker S, Achenbach S, Badimon L, Bertrand M, Bøtker HE, Collet J, Crea F, Danchin N, Falk E, Goudevenos J, Gulba D, Hambrecht R, Herrmann J, Kastrati A, Kjeldsen K, Kristensen SD, Lancellotti P, Mehilli J, Merkely B, Montalescot G, Neumann F, Neyses L, Perk J, Roffi M, Romeo F, Ruda M, Swahn E, Valgimigli M, Vrints CJ, Widimsky P. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 39.Lichtman JH, Spertus JA, Reid KJ, Radford MJ, Rumsfeld JS, Allen NB, Masoudi FA, Weintraub WS, Krumholz HM. Acute noncardiac conditions and in-hospital mortality in patients with acute myocardial infarction. Circulation. 2007;116:1925–1930. doi: 10.1161/CIRCULATIONAHA.107.722090. [DOI] [PubMed] [Google Scholar]
- 40.Evans E, Imanaka Y, Sekimoto M, Ishizaki T, Hayashida K, Fukuda H, Oh E. Risk adjusted resource utilization for AMI patients treated in Japanese hospitals. Health Econ. 2007;16:347–359. doi: 10.1002/hec.1177. [DOI] [PubMed] [Google Scholar]
- 41.Bramkamp M, Radovanovic D, Erne P, Szucs TD. Determinants of costs and the length of stay in acute coronary syndromes: a real life analysis of more than 10 000 patients. Cardiovasc Drugs Ther. 2007;21:389–398. doi: 10.1007/s10557-007-6044-0. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Z, Zhu B, Anderson J, Fu H, LeNarz L. Resource utilization and healthcare costs for acute coronary syndrome patients with and without diabetes mellitus. J Med Econ. 2010;13:748–759. doi: 10.3111/13696998.2010.535661. [DOI] [PubMed] [Google Scholar]
- 43.Kociol RD, Lopes RD, Clare R, Thomas L, Mehta RH, Kaul P, Pieper KS, Hochman JS, Weaver WD, Armstrong PW, Granger CB, Patel MR. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA. 2012;307:66–74. doi: 10.1001/jama.2011.1926. [DOI] [PubMed] [Google Scholar]
- 44.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 45.Greenhalgh J, Hockenhull J, Rao N, Dundar Y, Dickson RC, Bagust A. Drug-eluting stents versus bare metal stents for angina or acute coronary syndromes. Cochrane Database Syst Rev. 2010:CD004587. doi: 10.1002/14651858.CD004587.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Busse R, Schreyogg J, Smith PC. Hospital case payment systems in Europe. Health Care Manag Sci. 2006;9:211–213. doi: 10.1007/s10729-006-9039-7. [DOI] [PubMed] [Google Scholar]
- 47.Eurostat. Luxembourg: 2011. Eurostat statistical database: collection public health: health care activities: hospital patients 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.