Abstract
Background
Lymphatics are important for their conduit functions of transporting antigen, immune cells, and inflammatory mediators to draining lymph nodes and to the general circulation. Lymphangiogenesis is involved in many pathologic processes; however, the roles for lymphatic responses in transplantation have not been thoroughly investigated.
Methods
Mice were made diabetic by a single high dose of streptozotocin and then received islet allografts. Animals were treated with three different lymphatic inhibitors. FTY720, an analog of sphingosine 1-phosphate, inhibited lymphocyte migration into afferent and efferent lymphatics. Sunitinib, a kinase inhibitor, blocked several receptors, including vascular endothelial growth factor receptor 3 (VEGFR3), the major growth factor receptor for lymphatic endothelial cells. Anti-VEGFR3 monoclonal antibody specifically inhibited VEGFR3. Diabetes was determined by daily monitoring of blood glucose levels. Inflammation within islet grafts was assessed by immunohistochemistry for insulin, T cells (CD3), and lymphatics (LYVE-1).
Results
After transplantation, lymphangiogenesis occurred in islet allografts and in draining lymph nodes. FTY720, sunitinib, and anti-VEGFR3 each inhibited lymphangiogenesis in the islets and significantly prolonged allograft survival. Immunofluorescent staining demonstrated that administration of each of the lymphatic inhibitors resulted in preservation of islets and β-cells along with a markedly reduced infiltration of T cells into the grafts.
Conclusion
Lymphangiogenesis occurs in islet allografts in response to inflammation and plays a key role in the islet inflammation in alloimmunity. Interfering with lymphatic function leads to inhibition of lymphangiogenesis and prolonged or indefinite allograft survival. These observations suggest new therapeutic targets for rejection and tolerance.
Keywords: Allograft, Lymphangiogenesis, Islet, Tolerance
The encounter of antigen-presenting cells (APCs) and T cells in secondary lymphoid organs is essential for the initiation of immune responses, so that interfering with leukocyte trafficking could modulate immunity. Lymphatics are important for their immunologic conduit functions of transporting antigen, immune cells, and inflammatory mediators from tissues to draining lymph nodes (LNs), and from secondary lymphoid organs to the general circulation (1–4). Lymphatics are also found within LN (5) and are important for intranodal leukocyte migration, along with modulating the expression and production of chemokines and other immune mediators (3, 4). Lymphatics undergo growth and remodeling during immunity (3, 4, 6); and lymphangiogenesis is present in many pathologic processes (1, 2, 7, 8). Human and rodent corneal transplantation induces new lymphatic vessels in the corneal graft bed. These vessels facilitate delivery of APCs to draining and systemic LNs to enhance immune responses (9, 10) and play an active role in corneal transplant rejection (10). In contrast, stimulation of lymphangiogenesis inhibits chronic skin inflammation (11). Therefore, the roles of lymphatics in regulating immune responses and how lymphatics are regulated are unclear. A functional lymphatic network is found in transplanted syngeneic islets (12). However, the significance of islet lymphatic vessels and lymphangiogenesis and their contribution to immunity are incompletely understood.
Vascular endothelial growth factors (VEGFs) C and D, and their receptor VEGFR3, are the most potent mediators of lymphatic growth (13–15). Lymphangiogenesis and lymphatic metastasis can be efficiently inhibited by VEGF C/D trap, neutralizing anti-VEGFR3 antibodies (13–15), or small molecule tyrosine kinase inhibitors that down-regulate VEGFR signaling, such as sunitinib (16). We took advantage of this knowledge and these reagents to explore the roles of lymphangiogenesis in islet allotransplantation. Lymphangio-genesis occurred both in islet allografts and the downstream draining LNs. The mechanistically diverse inhibitors of lymphatics such as FTY720, sunitinib, and anti-VEGFR3 monoclonal antibodies (mAbs) decreased graft or LN lymphangiogenesis, decreased T-cell infiltration into grafts, preserved islet architecture and function, and delayed or prevented rejection. These results show that lymphatics are not only simply conduits for cells and molecules but also active participants in inflammation and rejection, and targeting them is a powerful means to inhibit immunity.
RESULTS
Lymphangiogenesis Occurs in Rejecting Islet Allografts
To investigate whether lymphangiogenesis is involved in rejection of islet allografts, lymphatic vessels in rejecting islet grafts were examined by immunofluorescent staining. C57BL/6 mice were made diabetic by a single high dose of streptozotocin (STZ). Five days later, allogeneic BALB/c donor islets were implanted under the renal capsule, the recipients were not immunosuppressed, and the islets were analyzed at the height of rejection 14 days later. As shown in Figure 1, lymphatic capillaries were noted to be present in the connective tissue between the transplanted islets, implying that lymphangiogenesis might contribute to alloimmunity.
FIGURE 1.
Lymphangiogenesis occurs in rejecting islet allografts. C57BL/6 mice were rendered diabetic by a single high-dose injection of streptozotocin. Islets from BALB/c donors were implanted under the renal capsule. Rejecting islet grafts (14 days posttransplantation) were stained for β-cells (insulin) and lymphatic vessels (LYVE-1). 100× magnification.
Inhibition of Lymphatic Function Prevents Rejection of Islet Allografts
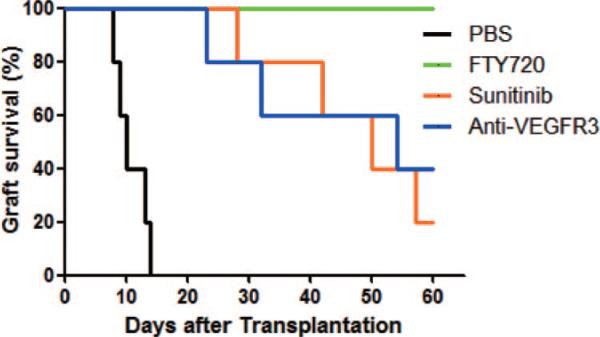
The importance of lymphatic vessel function and lymphangiogenesis was then evaluated after transplantation. Diabetic C57BL/6 recipients were transplanted with BALB/c islets and then treated with FTY720, sunitinib, or anti-VEGFR3 mAb for 2 weeks starting on the day of transplantation. The dose chosen for each compound was based on previous experience (17–19). As shown in Figure 2, control grafts were rejected 9 to 13 days after transplantation. In contrast, FTY720, sunitinib, and anti-VEGFR3 mAb each prolonged graft survival in all recipients. Grafts from all FTY720-treated, 20% of sunitinib-treated, and 40% of anti-VEGFR3 mAb-treated recipients survived for more than 60 days (Fig. 2). All grafts that survived for more than 60 days were surgically removed to prove that euglycemia resulted from the implanted and not regenerated islets. After graft removal, all recipients immediately became hyperglycemic (>300 mg/dL), proving that the grafts were functional. Because of the different structures and molecular mechanisms of action of each of these agents, the results demonstrate that the likely primary locus of their activity was on lymphatic cells and vessels and not on blood vascular endothelial cells, blood vessels, or leukocytes. These data demonstrated that inhibition of lymphatic function by diverse molecular inhibiters significantly delayed or prevented the rejection of fully allogeneic islets.
FIGURE 2.
Inhibition of lymphangiogenesis delays rejection of islet allografts. Diabetic C57BL/6 recipients transplanted with BALB/c islets were treated with FTY720, sunitinib, or anti-vascular endothelial growth factor receptor 3 (VEGFR3) monoclonal antibody (mAb), for 2 weeks starting on the day of transplantation, and groups of recipients were treated with FTY720 (1 mg/kg daily), sunitinib (40 mg/kg daily), or anti-VEGFR3 mAb (32 mg/kg three times/week) for 2 weeks starting on the day of transplantation. Graft survival (n=5 per group). P value versus controls: FTY720 less than 0.001, sunitinib, and anti-VEGFR3 less than 0.005. PBS, phosphate-buffered saline.
Inhibition of Lymphatic Function Prevents T-Cell Infiltration and Preserves Islets
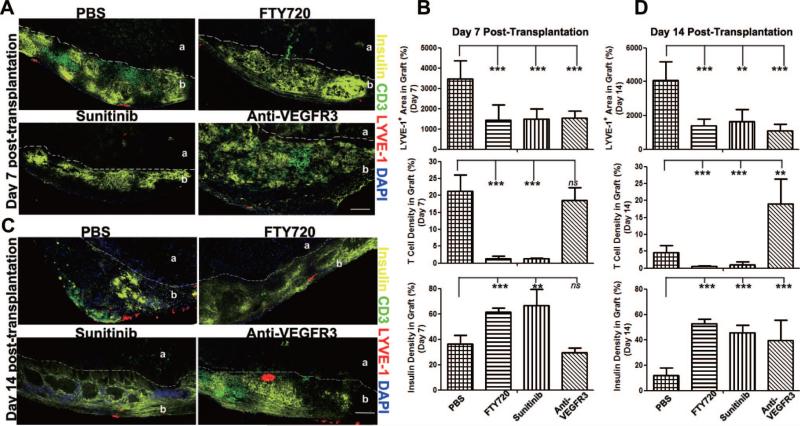
Islet survival, lymphangiogenesis, and T-cell infiltration at the graft site were evaluated after 7 to 14 days by quantitative immunofluorescent staining. By day 7 when all grafts were still functioning, lymphatic vessels appeared under the kidney capsule in proximity to the islets; and FTY720, sunitinib, and anti-VEGFR3 each inhibited lymphangiogenesis (Fig. 3A and B). Compared with controls, FTY720, and sunitinib but not anti-VEGFR3 mAb treated grafts had greater β-cell insulin preservation and less or no T-cell infiltration.
FIGURE 3.
Inhibition of lymphatic function prevents T-cell infiltration and preserves islets. (A) and (C) Immunofluores-cent analysis of β-cells (insulin), T cells (CD3), and lymphatic vessels (LYVE-1) in islet grafts 7 days (A) and 14 days (C) after transplantation. Dashed lines show boundary between kidney cortex (a) and graft (b). 100× magnification. Scale bars: 100 μm. (B) and (D) Quantitative analysis of insulin, CD3, and LYVE-1 staining of islet grafts 7 days (B) and 14 days (D) after transplantation. Two to three slides/mouse, two to three mice/group. Data are presented as mean±standard deviation. ns, not significant. **P less than or equal to 0.01; ***P less than or equal to 0.001.
By day 14, all grafts in control mice had been rejected, and there were few insulin+ β-cells. Likely due to complete graft rejection, there were only a few residual infiltrating T cells observed in these rejected graft beds (Fig. 3C and D). At this same time, all three compounds inhibited lymphangio-genesis in the grafts. FTY720 and sunitinib preserved insulin expression and prevented T-cell infiltration. Anti-VEGFR3 mAb also significantly preserved insulin expression but did not prevent T-cell infiltration. Overall, inhibition of lymphatic function most strongly correlated with inhibition of lymphangiogenesis, preservation of islets, and inhibition of T-cell infiltration.
Inhibition of Lymphatic Function Prevents Lymphangiogenesis in Draining LN
LNs play key roles in inflammation and immune responses. Immunofluorescent analysis of lymphatic vessels and high endothelial venules (HEVs) in draining renal LNs after transplantation showed that both lymphangiogenesis and HEV angiogenesis occurred in response to transplantation. Lymphangiogenesis was significantly inhibited by sunitinib and anti-VEGFR3 mAb and to a lesser extent by FTY720. Sunitinib also significantly inhibited renal LN angiogenesis, whereas neither FTY720 nor anti-VEGFR3 mAb caused such inhibition (Fig. 4A). Quantitative evaluation (Fig. 4B) confirmed these results. Thus, inhibition of lymphatic function by a variety of compounds preferentially inhibited draining LN lymphangiogenesis.
FIGURE 4.
Inhibition of lymphatic function prevents draining lymph node (LN) lymphangiogenesis. (A) Immunofluorescent analysis of T cells (CD3), lymphatic vessels (LYVE-1), and blood vessels (peripheral LN addressin [PNAd]) of draining renal LNs 7 days after transplantation. Normal, normal mesenteric LN. 100× magnification. Scale bars: 100 μm. (B) Quantitative analysis of LYVE-1 and PNAd in draining renal LNs 7 days after transplantation. Two to three sections/LN, two to three mice/group. Data are presented as mean±standard deviation. *P less than or equal to 0.05, **P less than or equal to 0.01, ***P less than or equal to 0.001 versus phosphate-buffered saline (PBS) treatment.
DISCUSSION
Afferent lymphatic vessels allow the transport of both recipient and donor APCs presenting allogeneic tissue antigens to the draining LN. Although lymphangiogenesis has received increasing attention and been believed to be important for enhancing alloantigen presentation after corneal transplantation, its role during inflammation remains controversial. For example, inhibition of lymphangiogenesis prolongs inflammation during chronic skin inflammation (11) and enhances the progression of inflammatory arthritis (20, 21). To investigate the role of lymphatic function in rejection, the diverse inhibitors FTY720, sunitinib, and anti-VEGFR3 mAbs were used in the model of islet allotransplantation. These experiments demonstrated that lymphangiogenesis occurred after transplantation and during allograft rejection in both the islet graft and draining LN, and targeting lymphangiogenesis limited islet destruction and prolonged allograft survival.
FTY720 is a partial agonist and antagonist for the sphingosine 1-phosphate (S1P) receptors S1P1, S1P3, S1P4, and S1P5 (22) and prevents egress of T cells from the LN to efferent lymphatics and general circulation (23, 24). FTY720 also inhibits entry of peripheral tissue T lymphocytes into afferent lymphatics (17). Consistent with previous reports (25), short-term FTY720 treatment prevented T-cell infiltration and resulted long-term islet allograft survival. There is agreement that one of the main modes of immunosuppressive action of FTY720 is by blocking T lymphocyte egress from the thymus and LNs (23, 24, 26, 27), and thus, FTY720 inhibited T-cell infiltration into the islets. In addition, we observed that FTY720 inhibited lymphangiogenesis in the islet graft. To our knowledge, this is the first description of FTY720 inhibiting lymphangiogenesis induced by allogeneic transplantation and suggests additional mechanisms for the immunosuppressive actions of this drug.
Anti-VEGFR3 mAb is a specific antilymphangiogenic reagent for lymphatic vascular endothelium (28). It is interesting to note the more prominent T-cell infiltration in groups treated with anti-VEGFR3 mAb in comparison with other treatments. This is likely because anti-VEGFR3 mAb did not directly target T cells, although some have suggested that T cells may express VEGFR3 (29). The observation that T-cell infiltration was not diminished also suggests that specific inhibition of only one highly restricted aspect of the lymphatic endothelial cell response might inhibit afferent lymphatic function, thereby preventing the egress of T cells out of the inflamed islets (11, 20).
Sunitinib is a potent antiangiogenic and antilymphangiogenic kinase inhibitor that targets multiple tyrosine kinase receptors, including VEGFR, PDGFR, c-kit, and FLT3 (14, 30, 31). By targeting VEGFRs, it prevented islet and LN lymphangiogenesis. Although sunitinib inhibited T-cell infiltration, it does not have inhibitory effects on dendritic cell function and does not alter peptide-induced CD8+ T-cell responses in vivo (32). Thus, sunitinib, which is clinically approved for treatment of malignancies (33–35), may have potential application in inflammatory disease therapies, because it might not have the side effects of broad-spectrum immunosuppressives. Although sunitinib inhibited LN blood vascular angiogenesis in our study, it did not interfere with islet graft survival and function, both of which require angiogenesis (36, 37). Thus, sunitinib might possess advantages for treatment of alloreactivity and thus be an appropriate intervention for rejection prophylaxis or treatment. In our study, sunitinib was more effective than anti-VEGFR3 mAb for inhibiting T-cell infiltration and islet destruction, suggesting that sunitinib may possess some advantages for treatment of transplant rejection. Further investigations are needed to elucidate the effect of sunitinib in autoimmunity and alloimmunity.
To our knowledge, this is the first study to demonstrate that lymphangiogenesis occurred during islet allograft rejection and that antilymphangiogenic treatment prevented allogeneic islet graft rejection. These findings indicate that lymphangiogenesis is intimately involved with inflammatory responses, and the lymphatic system plays a crucial role in alloimmunity, providing a novel therapeutic approach in islet allotransplantation. This is the first attempt to use sunitinib in islet allotransplantation, and our data suggest several advantages for its potential in clinical use.
MATERIALS AND METHODS
Mice
BALB/c and C57BL/6 were purchased from The Jackson Laboratories (Bar Harbor, ME). All mice were housed in a pathogen-free animal facility. All experimental protocols were approved by the Institutional Animal Care and Utilization Committee.
Antibodies
Purified rat antiperipheral LN addressin (MECA79), Armenian hamster anti-CD3ε (145-2C11), and rat anti-CD31 (390) were from BD Biosciences-Pharmingen (San Jose, CA). Guinea pig anti-swan insulin was from Dako Cytomation Inc. (Carpinteria, CA). Purified rabbit anti-LYVE-1 was from Fitzgerald Industries International Inc. (Concord, MA). Cy5-conjugated goat anti-rabbit IgG, fluorescein isothiocyanate-conjugated goat anti-hamster IgG, fluorescein isothiocyanate-conjugated goat anti-rat IgM, and Cy3-conjugated goat anti-guinea pig IgG were from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA).
Diabetes Induction
To induce diabetes, male C57BL/6 mice (8- to 10-week old, 20–25 g) were given a single intraperitoneal injection of STZ (Sigma-Aldrich, St. Louis, MO) at a dose of 180 mg/kg. Animals were considered diabetic when tail vein blood glucose levels were more than 300 mg/dL for 2 consecutive days, as determined by glucometer (Bayer, Mishawaka, IN).
Islet Isolation and Transplantation
Male BALB/c mice were killed; the common bile duct was exposed and injected with 3 mL cold Hanks’ buffer containing 1.5 mg/mL of collagenase-P (Roche Diagnostics, Indianapolis, IN); the pancreas was excised; and digestion was allowed to continue at 37°C for 15 min. The digested pancreas was disrupted by trituration, and the suspension was washed twice with Roswell Park Memorial Institute 1640 containing 10% fetal bovine serum. Pancreatic islet separation was performed by centrifugation on a discontinuous Ficoll (Sigma) gradient of 11%, 21%, 23%, and 25%. Islets were picked from the second layer, and 400 islets were implanted beneath the renal capsule of STZ-induced diabetic C57BL/6 male mice (37).
Agent Administration
FTY720 was a gift from Dr. V. Brinkmann (Novartis Pharma, Basel, Switzerland). Rat anti-VEGFR3 mAb (mF4-31C1) was a gift from Dr. B. Pytowsky (ImClone Systems, Eli Lilly and Company, New York, NY) (25). Sunitinib (sunitinib malate, SU-11248-L) was a gift from Dr. James Christensen (Pfizer Inc., Groton, CT). FTY720 (1 mg/kg) and sunitinib (40 mg/kg) were administered by oral gavage (once daily), and phosphate-buffered saline (PBS) and anti-VEGFR3 mAb (32 mg/kg) were administered by intraperitoneal injection (three times per week), for 2 weeks, starting on the day of transplantation.
Immunofluorescent Staining and Quantitative Image Analysis
Eight- to 10-μm frozen sections of LNs and grafts in situ in the kidneys were fixed with acetone. After incubation with blocking buffer (PBS with 5% donkey serum), sections were incubated with primary antibodies in PBS with 1% donkey serum for 1 hr, and followed by secondary antibodies in PBS with 1% donkey serum for 40 min at room temperature, and sections were mounted with Vectashield with DAPI (Vector Laboratories Inc., Berlingame, CA). Images were acquired with a Leica DMRA2 fluorescent microscope (Leica Microsystems, Wetzlar GmbH, Germany) and Openlab software (Improvision Inc., Waltham, MA). Quantitative analysis was performed with NIH Image J software (NIH, Bethesda, MD).
Two to three tissue sections from grafts or draining LN of each animal were randomly chosen for NIH Image J software analysis. For islet grafts, in each chosen section, the whole graft area at 100× magnification was selected. The graft areas were manually delineated according to DAPI, CD3, and insulin staining. The areas covered by CD3 or insulin staining inside the graft boundaries were measured as T-cell infiltration and insulin preservation, respectively. The density of lymphatic vessels was measured and expressed as pixels. The area covered by T cells and insulin within the islet graft were quantified and expressed as a percentage of the whole islet graft area. In draining LNs, lymphatic vessels and HEV were measured as LYVE-1 and peripheral LN addressin positively staining areas, respectively, and expressed as a percentage of the whole LN area.
Statistical Analysis
Each histologic parameter was measured in a blinded fashion and expressed as the mean±standard deviation. The differences were assessed using Student's t test. Survival curves were constructed with Kaplan-Meier estimates, and survival rates were analyzed by the generalized Wilcoxon's test. A two-tailed P values less than 0.05 was considered statistically significant.
Acknowledgments
This work was supported by grants from the Emerald Foundation, JDRF S-2007-236 and 1-2008-90, NIH AI72039, and AI41428 (J.S.B.); and the American Society of Transplant Surgeons-Genentech Laboratories Scientist Scholarship (N.Y.).
Footnotes
The authors declare no conflict of interests.
N.Y. participated in research design, performance of the research, data analysis, and writing of the manuscript; N.Z. participated in research design, performance of the research, and data analysis; J.X. and Q.S. participated in data analysis; Y.D. participated in data analysis and review of the article; and J.S.B. participated in research design, data analysis, and writing of the manuscript.
REFERENCES
- 1.Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007;25:4298. doi: 10.1200/JCO.2006.07.1092. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 3.Angeli V, Ginhoux F, Llodrà J, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol. 2006;4:217. doi: 10.1089/lrb.2006.4406. [DOI] [PubMed] [Google Scholar]
- 5.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 7.El-Chemaly S, Levine SJ, Moss J. Lymphatics in lung disease. Ann N Y Acad Sci. 2008;1131:195. doi: 10.1196/annals.1413.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliver G, Alitalo K. The lymphatic vasculature: Recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- 9.Hamrah P, Chen L, Zhang Q, et al. Novel expression of vascular endothelial growth factor receptor (VEGFR)-3 and VEGF-C on corneal dendritic cells. Am J Pathol. 2003;163:57. doi: 10.1016/S0002-9440(10)63630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich T, Bock F, Yuen D, et al. Cutting edge: Lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huggenberger R, Ullmann S, Proulx ST, et al. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med. 2010;207:2255. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Källskog O, Kampf C, Andersson A, et al. Lymphatic vessels in pancreatic islets implanted under the renal capsule of rats. Am J Transplant. 2006;6:680. doi: 10.1111/j.1600-6143.2006.01234.x. [DOI] [PubMed] [Google Scholar]
- 13.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 14.Pepper MS, Skobe M. Lymphatic endothelium: Morphological, molecular and functional properties. J Cell Biol. 2003;163:209. doi: 10.1083/jcb.200308082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 16.O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 17.Ledgerwood LG, Lal G, Zhang N, et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 18.Ebos JM, Lee CR, Christensen JG, et al. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 20.Guo R, Zhou Q, Proulx ST, et al. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009;60:2666. doi: 10.1002/art.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 23.Henning G, Ohl L, Junt T, et al. CC chemokine receptor 7-dependent and -independent pathways for lymphocyte homing: Modulation by FTY720. J Exp Med. 2001;194:1875. doi: 10.1084/jem.194.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 25.Fu F, Hu S, Deleo J, et al. Long-term islet graft survival in streptozotocin- and autoimmune-induced diabetes models by immunosuppressive and potential insulinotropic agent FTY720. Transplantation. 2002;73:1425. doi: 10.1097/00007890-200205150-00011. [DOI] [PubMed] [Google Scholar]
- 26.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 27.Pappu R, Schwab SR, Cornelissen I, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 28.Pytowski B, Goldman J, Persaud K, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 29.Leclers D, Durand K, Cook-Moreau J, et al. VEGFR-3, VEGF-C and VEGF-D mRNA quantification by RT-PCR in different human cell types. Anticancer Res. 2006;26:1885. [PubMed] [Google Scholar]
- 30.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327. [PubMed] [Google Scholar]
- 31.Faivre S, Demetri G, Sargent W, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 32.Hipp MM, Hilf N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 33.Defuentes G, Bladé JS, Berets O. Tyrosine kinase inhibitors for chronic myelogenous leukemia. N Engl J Med. 2007;357:1556. doi: 10.1056/NEJMc072356. [DOI] [PubMed] [Google Scholar]
- 34.George S. Sunitinib, a multitargeted tyrosine kinase inhibitor, in the management of gastrointestinal stromal tumor. Curr Oncol Rep. 2007;9:323. doi: 10.1007/s11912-007-0040-1. [DOI] [PubMed] [Google Scholar]
- 35.Grimaldi AM, Guida T, D'Attino R, et al. Sunitinib: Bridging present and future cancer treatment. Ann Oncol. 2007;18(suppl 6):vi31. doi: 10.1093/annonc/mdm221. [DOI] [PubMed] [Google Scholar]
- 36.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749. doi: 10.1007/s00125-002-0827-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhang N, Richter A, Suriawinata J, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]