Abstract
Background
Because cancer patients survive longer, the impact of cardiotoxicity associated with the use of cancer treatments escalates. The present study investigates whether early alterations of myocardial strain and blood biomarkers predict incident cardiotoxicity in patients with breast cancer during treatment with anthracyclines, taxanes, and trastuzumab.
Methods and Results
Eighty-one women with newly diagnosed human epidermal growth factor receptor 2–positive breast cancer, treated with anthracyclines followed by taxanes and trastuzumab were enrolled to be evaluated every 3 months during their cancer therapy (total of 15 months) using echocardiograms and blood samples. Left ventricular ejection fraction, peak systolic longitudinal, radial, and circumferential myocardial strain were calculated. Ultrasensitive troponin I, N-terminal pro–B-type natriuretic peptide, and the interleukin family member (ST2) were also measured. Left ventricular ejection fraction decreased (64 ± 5% to 59 ± 6%; P<0.0001) over 15 months. Twenty-six patients (32%, [22%–43%]) developed cardiotoxicity as defined by the Cardiac Review and Evaluation Committee Reviewing Trastuzumab; of these patients, 5 (6%, [2%–14%]) had symptoms of heart failure. Peak systolic longitudinal myocardial strain and ultrasensitive troponin I measured at the completion of anthracyclines treatment predicted the subsequent development of cardiotoxicity; no significant associations were observed for left ventricular ejection fraction, N-terminal pro–B-type natriuretic peptide, and ST2. Longitudinal strain was <19% in all patients who later developed heart failure.
Conclusions
In patients with breast cancer treated with anthracyclines, taxanes, and trastuzumab, systolic longitudinal myocardial strain and ultrasensitive troponin I measured at the completion of anthracyclines therapy are useful in the prediction of subsequent cardiotoxicity and may help guide treatment to avoid cardiac side-effects.
Keywords: chemotherapy, echocardiography, biomarkers, left ventricular function, heart failure, trastuzumab
Because early diagnosis and therapies of breast cancer have improved, >2.2 million women in the United States are now breast cancer survivors. This increase in survival, however, raises the likelihood that patients will experience side-effects of anticancer therapies, notably cardiotoxicity.
The cardiotoxicity of anthracyclines is well recognized. Trastuzumab increases the cardiotoxicity of anthracyclines treatment, with left ventricular (LV) dysfunction noted in as many as a third of the patients and an incidence of congestive heart failure of 2% to 5% in patients treated with both therapies.1 Although the decrease in LV ejection fraction (LVEF) appears to be responding to treatment in a majority of patients,2 its long-term prognosis is unknown.
LVEF, which is widely used to monitor cardiac systolic function after chemotherapy, fails to detect subtle alterations in LV function. Once the LVEF has decreased in patients treated with anthracyclines, it may be too late to reverse the course of the rdiomyopathy.3 More sensitive and specific markers of chemotherapy-induced cardiac dysfunction or myocardial injury may allow for earlier and better adaptations of oncologic and cardiac treatments.
Evidence in support of the fact that recently developed echo-cardiographic indices and biomarkers may be useful in the detection of early cardiac injury is accumulating. Decreases in myocardial strain and strain rate have been described after anthracyclines-based chemotherapies.4–8 In a preliminary study, we reported that in a small cohort of patients treated with anthracyclines, taxanes, and trastuzumab, measurements of peak longitudinal systolic strain at 3 months of follow-up predicted a decrease in LVEF 3 months later.8 The longer-term predictive value of these measures in a larger cohort, and the optimum measurement time point remain to be determined.
Troponin measurements have been used to measure myocardial injury and predict incident LV dysfunction in patients receiving high doses of anthracyclines,9 but their role in patients receiving low to moderate doses of anthracyclines is not established.10 Importantly, those studies reporting predictive values of noninvasive measurements included patients with multiple treatments and at different stages during their therapies. This heterogeneity has not allowed for the differentiation of the role of the various therapies on myocardial function, and has limited the clinical use of the measurements.
The primary objective of this prospective study was to assess whether LVEF, myocardial strain, and biomarkers of cardiac injury (troponin), wall stress (N-terminal pro–B-type natriuretic peptide [NT-proBNP]), and remodeling (NT-proBNP; ST2) obtained early in the course of the treatment in a homogeneous cohort of women with newly diagnosed human epidermal growth factor receptor 2–positive breast cancer treated with anthracy-clines followed by taxanes and trastuzumab could predict subsequent cardiotoxicity occurring throughout the full course of the treatment. Another objective of the study was to investigate the time course of these imaging and blood markers throughout the treatment. For the models predicting cardiotoxicity, we a priori selected to study the value of the parameters obtained at the completion of the anthracyclines treatment, before taxanes and trastuzumab were introduced, as anthracyclines have been shown to induce severe cardiotoxicity than either taxanes or trastuzumab.
Methods
Patients of at least 18 years of age newly diagnosed with human epidermal growth factor receptor 2-overexpressing breast cancer and scheduled to receive adjuvant therapy including anthracyclines, taxanes, and trastuzumab were eligible. Patients who had a baseline LVEF <50% were excluded.
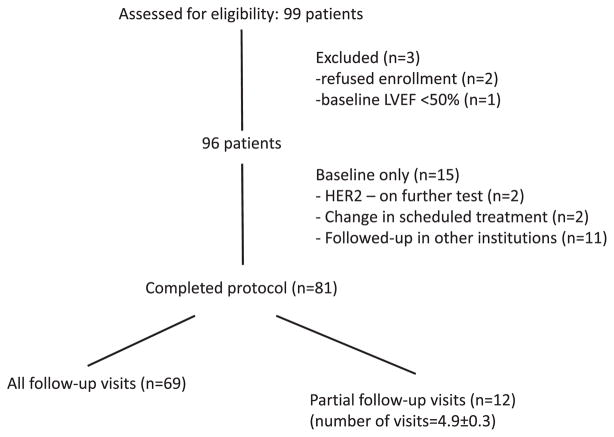
The study was approved by the Internal Review Board of the 4 participating institutions. After a signed informed consent, patients were studied before chemotherapy, at the completion of anthracyclines therapy (19 ± 9 days after the last anthracyclines cycle), and every 3 months subsequently until the end of the trastuzumab treatment (12 months in duration), for a total of 6 studies over 15 months (Figure 1). Each study visit included a detailed questionnaire including the presence of cardiac symptoms, an echocardiogram, and blood sampling.
Figure 1.
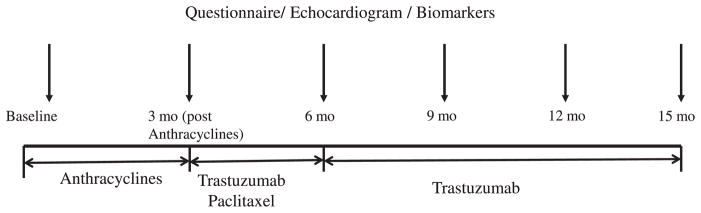
Time course of the study protocol. Mo indicates months.
The primary end point was the occurrence of cardiotoxicity as defined by the Cardiac Review and Evaluation Committee of trastuzumab-associated cardiotoxicity (CREC), that is, either a cardiomyopathy with decreased LVEF, a reduction of LVEF ≥5% to <55% with symptoms of heart failure (diagnosed by a cardiologist at the site), or an asymptomatic reduction of LVEF ≥10% to <55%.11
Echocardiograms
Transthoracic echocardiograms were acquired using the Vivid 7 or E9 (GE Healthcare, Milwaukee, WI). The same ultrasound machine was used to acquire all echocardiograms in each patient. All echocardiograms were analyzed by 2 readers (M. Scherrer-Crosbie for the LVEF; H. Sawaya for all other measurements). The readers were blinded to each other’s measurements and to the patient visit number. LVEF was calculated from the apical 4- and 2-chamber views using a modified Simpson biplane method. The intraobserver variability of the LVEF reported as the mean error±SD of 10 measurements was 1 ± 5% in absolute values (1 ± 8% in percentages) and the interobserver variability −2 ± 5% in absolute values (−4 ± 8% in percentages). Peak systolic strain was measured using speckle tracking (Echopac; GE Medical, Milwaukee, WI), at a frame rate of 80 to 100 fps. Peak systolic radial and circumferential strain was calculated by averaging the peak systolic strain values in all 6 segments of the parasternal short-axis view at midpapillary level. Peak systolic longitudinal strain was calculated by averaging the values of peak systolic strain in the basal and midventricular segments of the 4- and 2-chamber views.8 The intra- and interobserver variabilities of the strain in the laboratory were reported in a previous study.8
Biomarkers
Troponin I was determined using a research-phase highly sensitive assay based on LOCI technology and run on a Dimension Vista 1500 System (Siemens Healthcare Diagnostics, Deerfield, IL). This ultra-sensitive troponin I (usTnI) assay has a range of 0.5 to 20,000 pg/mL and a 10% coefficient of variation of 3 pg/mL. All values of troponin I >30 pg/mL (95th percentile of values obtained before treatment) were considered elevated.
NT-proBNP was measured on the Dimension Vista 500 Intelligent Laboratory System (Siemens Healthcare Diagnostics). The limit of detection of NT-proBNP assay is 0.8 pg/mL. The highest value of NT-proBNP reported in healthy subjects aged <75 years is 125 pg/mL, thus all values of NT-proBNP >125 pg/mL were considered elevated.
ST2 was measured using a research use only assay (Presage ST2, Critical Diagnostics, San Diego, CA) on an automated enzyme linked–immunosorbent assay platform. This assay has been reported to have an interrun coefficient of variation of 2.0%, with values <35 pg/mL considered normal.12
Statistics
The study was designed as a prospective multicenter study. The primary aim was to test the association between echocardiographic markers of systolic function and biomarkers (predictors) and subsequent occurrence of cardiotoxicity (outcome). Based on our previous study in which the sensitivity of deformation measurements in the prediction of LV dysfunction in mice was 95%,4 and assuming a rate of cardiotoxicity of 25% in patients,13 80 patients were needed for a confidence interval of the deformation indices sensitivity of (80%–100%).
To investigate the time course of echocardiographic parameters and biomarkers, LVEF, longitudinal strain and log-transformed bio-markers (see below) were compared at baseline, postanthracyclines, 6, 9, 12, and 15 months by using a 1-way ANOVA for repeated measures for the patients who completed all follow-up visits. If the effect of time was significant, changes in the parameters compared with baseline were explored using contrast analysis. The normality of the distribution of the parameters studied was checked using the Shapiro-Wilk test. None of the blood biomarkers levels followed a normal distribution. The nonnormal data were log-transformed.
Baseline characteristics of patients who developed or did not develop cardiotoxicity were compared using χ2 tests. Possible predictors of cardiotoxicity were determined before data collection and were tested using univariate logistic regression as continuous variables for the echocardiographic parameters and as continuous variables and with the predetermined elevation thresholds for the biomarkers. For the echocardiographic parameter that was predictive as a continuous variable (longitudinal strain), a receiver operating characteristic curve was obtained and the optimal strain value with the greatest total of sensitivity and specificity in the prediction of cardiotoxicity was selected. The primary covariates that were considered as possible predictors of cardiotoxicity were the absolute levels of LVEF, strain, and biomarkers at the end of the anthracycline treatment. We also described an alternative method to analyze LVEF and strain (changes from baseline) because this approach has been reported in the literature. A multiple nominal logistic regression model was then applied to the univariable predictors (longitudinal myocardial strain and usTnI). The effects of age and the presence of hypertension were controlled for in this model. P<0.05 were defined as significant.
Statistical analyses were performed using JMP statistical package (SAS Institute Inc, Cary, NC). Data are expressed as mean±SD or median with interquartile range.
Results
Population
The enrollment and completion of the study are summarized in Figure 2. Eighty-one patients completed the study. Of these, 12 had partial follow-up (average of 4.9 ± 0.3 visits), but did not differ from the rest of the cohort. Patients were treated with either doxorubicin (cumulative dose of 240 mg/m2) or epirubicin (cumulative dose of 300 mg/m2) for 3 months, followed by weekly paclitaxel (80 mg/m2) and trastuzumab (2 mg/kg) for 3 months, and trastuzumab only (6 mg/kg) every 3 weeks for 9 more months. Their baseline clinical characteristics are presented in Table 1.
Figure 2.
Consort diagram of the study protocol. LVEF indicates left ventricular ejection fraction; HER2, human epidermal growth factor receptor 2.
Table 1.
Baseline Clinical Characteristics of Patients Treated With Anthracyclines, Taxanes, and Trastuzumab Who Developed or Did Not Develop Cardiotoxicity
| Variable | Entire Cohort (n=81) | Cardiotoxicity (n=26) | No Cardiotoxicity (n=55) | P Value |
|---|---|---|---|---|
| Age, y | 50 ± 10 | 49 ± 10 | 50 ± 10 | 0.78 |
| Dose of anthracyclines | ||||
| Doxorubicin 240 mg/m2 | 71 (88%) | 22 (85%) | 49 (89%) | 0.57 |
| Epirubicin 300 mg/m2 | 10 (12%) | 4 (15%) | 6 (11%) | |
| Radiotherapy | 49 (60%) | 13 (50%) | 36 (65%) | 0.22 |
| Side of breast cancer | ||||
| Right | 34 (42%) | 11 (42%) | 23 (42%) | 0.61 |
| Left | 41 (51%) | 12 (46%) | 29 (53%) | |
| Both | 6 (7%) | 3 (11%) | 3 (5%) | |
| CV risk factors | ||||
| HTN | 26 (32%) | 8 (31%) | 18 (33%) | 0.75 |
| DM | 1 (1%) | 1 (4%) | 0 (0%) | 0.32 |
| Hyperlipidemia | 18 (22%) | 6 (23%) | 12 (22%) | 0.90 |
| Smoking | 6 (7%) | 2 (8%) | 4 (7%) | 0.95 |
| CV treatment | ||||
| ACE inhibitor | 14 (17%) | 6 (23%) | 8 (14%) | 0.36 |
| β-blockers | 9 (11%) | 4 (15%) | 5 (9%) | 0.45 |
| BMI, kg/m2 | 26 ± 5 | 26 ± 9 | 25 ± 6 | 0.77 |
| SBP, mm Hg | 124 ± 19 | 123 ± 17 | 124 ± 20 | 0.77 |
| DBP, mm Hg | 73 ± 10 | 74 ± 11 | 73 ± 10 | 0.93 |
| Heart rate, beats per minute | 71 ± 11 | 71 ± 10 | 70 ± 11 | 0.75 |
| LVEF | 64 ± 5 | 64 ± 4 | 64 ± 6 | 0.82 |
CV indicates cardiovascular; HTN, hypertension; DM, diabetes mellitus; ACE, angiotensin-converting enzyme; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; and LVEF, left ventricular ejection fraction.
Effect of Chemotherapy and Trastuzumab on LVEF
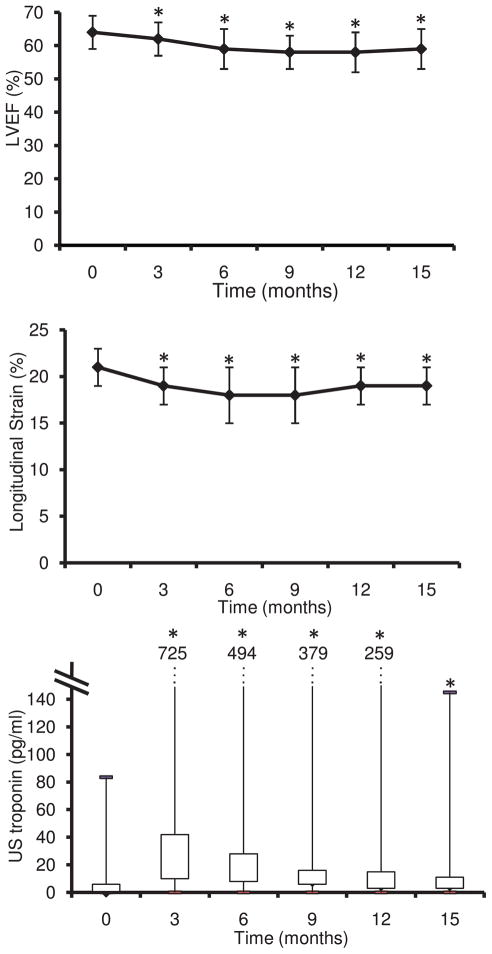
The mean LVEF decreased during treatment (from 64 ± 5 to 59 ± 6%; Table 2; Figure 3A; P<0.0001 over the duration of the study). Twenty-six patients (32% of the entire cohort) developed CREC-defined cardiotoxicity. There was no difference in the baseline clinical characteristics of the women who developed cardiotoxicity compared with the ones who did not (Table 1). In women developing cardiotoxicity, the decrease in LVEF was detected most frequently 3 months after the end of the anthracyclines therapy (11 patients), less commonly early (n=3), 6 months (n=5), 9 months (n=5), and 12 months (n=2) after the end of anthracyclines.
Table 2.
Temporal Changes in Echocardiographic Parameters During Treatment by Anthracyclines, Taxanes, and Trastuzumab
| Before Treatment | Postanthracyclines (3 Mo) | 6 Mo | 9 Mo | 12 Mo | End of Treatment | |
|---|---|---|---|---|---|---|
| LVEF, % | 64 ± 5 | 62 ± 5* | 59 ± 5† | 58 ± 5† | 58 ± 6† | 59 ± 6† |
| Longitudinal strain, % | 21 ± 2 | 19 ± 2† | 18 ± 3† | 18 ± 3† | 19 ± 2† | 19 ± 2† |
| Radial strain, % | 53 ± 15 | 50 ± 17* | 43 ± 16‡ | 37 ± 16‡ | 34 ± 16† | 41 ± 17‡ |
| Circumferential strain, % | 18 ± 4 | 16 ± 4‡ | 15 ± 3‡ | 15 ± 3‡ | 15 ± 3‡ | 16 ± 3§ |
LVEF indicates left ventricular ejection fraction.
The analysis was performed using an ANOVA for repeated measurements on 69 patients with complete follow-up.
P<0.03;
P<0.0001;
P<0.005;
P<0.001 vs before treatment.
Figure 3.
Time course of the left ventricular ejection fraction (LVEF; top), the longitudinal strain (middle), and the ultrasensitive (US) troponin (bottom) in 69 patients with breast cancer treated by anthracyclines followed by taxanes and trastuzumab. *P<0.0001 vs before treatment.
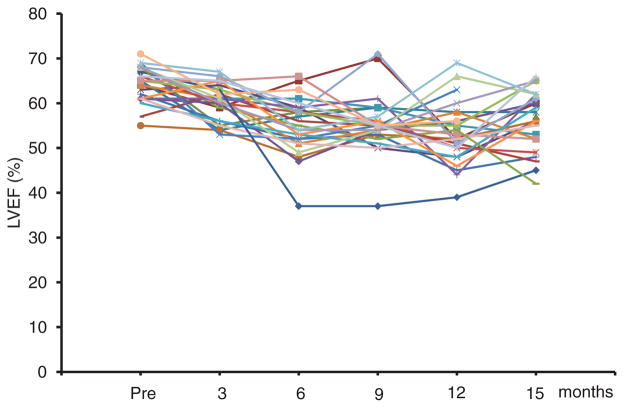
The mean decrease in LVEF in patients with CREC-defined cardiotoxicity was partially reversible (49 ± 4% at the nadir to 56 ± 7% at the end of the follow-up; P<0.0001). The LVEF of 9 patients (11% of the entire cohort) remained <55% until the end of the follow-up. Twelve patients (15% of the entire cohort) decreased their LVEF to <50%, 5 of whom remained <50% at the end of the follow-up. The individual time course of the LVEF in patients with cardiotoxicity is shown in Figure 4. After the diagnosis of cardiotoxicity, trastuzumab was discontinued in 3 patients, and 1 of these patients was treated with angiotensin-converting enzyme inhibitors and β-blockers. No intervention was performed in the other patients.
Figure 4.
Individual time course of left ventricular ejection fraction (LVEF) in 26 patients diagnosed with cardiotoxicity.
Of note, 6 of 14 patients treated before cancer therapy with angiotensin-converting enzyme inhibitors (43%) and 4 of 9 patients treated before cancer therapy with β-blockers (44%), all for a diagnosis of hypertension, developed cardiotoxicity (P=0.39 and P=0.43 versus no angiotensin-converting enzyme inhibitors or no β-blockers).
Effect of Chemotherapy and Trastuzumab on Myocardial Strain and Biomarkers
Peak systolic longitudinal myocardial strain decreased during the study period (from 21 ± 2% to 19 ± 2%; Table 2; Figure 3B; P<0.0001 over the duration of the study). In women who developed cardiotoxicity, the mean longitudinal strain decreased to a nadir of 15 ± 3%. Both radial and circumferential components of the strain also decreased (P<0.005 for radial and P<0.02 for circumferential strain over the duration of the study).
usTnI concentrations increased during the follow-up period (from a median of 1.3 pg/mL to 23 pg/mL; P<0.0001; Table 3; Figure 3C) and were highest at the completion of the anthracyclines treatment. In women who developed cardiotoxicity, the usTnI concentrations at completion of anthracyclines were 32 pg/mL (10–56 pg/mL), whereas they were 17 pg/mL (5–35 pg/mL) in women who did not (P=0.18). NT-proBNP levels and ST2 levels did not change significantly throughout follow-up (Table 3).
Table 3.
Temporal Changes in Biomarkers During Treatment by Anthracyclines, Taxanes, and Trastuzumab
| Before Treatment | Postanthracyclines (3 Mo) | 6 Mo | 9 Mo | 12 Mo | End of Treatment | |
|---|---|---|---|---|---|---|
| UsTnI, pg/mL | 1.3 (0.7–6) | 23 (10–42)* | 14 (8–28)* | 9 (6–16)* | 6 (3–15)* | 6 (3–11)* |
| NT-proBNP, pg/mL | 71 (37–139) | 75 (34–117) | 59 (32–100) | 62 (39–109) | 61 (32–113) | 75 (38–148) |
| ST2, pg/mL | 26 (23–35) | 27 (23–42) | 26 (21–32) | 27 (21–33) | 25 (22–32) | 25 (22–31) |
UsTnI indicates ultrasensitive troponin I; NT-proBNP, N-terminal pro–B-type natriuretic peptide; and ST2, ST2 protein.
The parameters were log-transformed and the analysis was performed using an ANOVA for repeated measurements on 69 patients with complete follow-up.
P<0.0001 vs before treatment.
Predictive Value of Strain and Biomarkers in the Development of Cardiotoxicity
The predictive value of parameters measured at the completion of anthracyclines therapy and of the changes of these parameters compared with baseline was studied. For these analysis, the 3 patients who had already developed cardiotoxicity at the completion of anthracyclines were excluded, thus the analysis was done on 23 cases of cardiotoxicity.
Predictive Value of LVEF
LVEF measured at the completion of the anthracyclines treatment was not predictive of later cardiotoxicity (P=0.075). Similarly, the changes in LVEF between baseline and the completion of anthracyclines treatment was not predictive of later cardiotoxicity, whether they were analyzed as a continuous variable or as a discrete change of 8% or 10% (clinically used)14 (P=0.081, 0.23, 0.34, respectively). A decrease of LVEF >8% was detected in only 15% of the patients developing cardiotoxicity subsequently, a decrease of 10% was detected in only one of these patients.
Predictive Value of Myocardial Strain
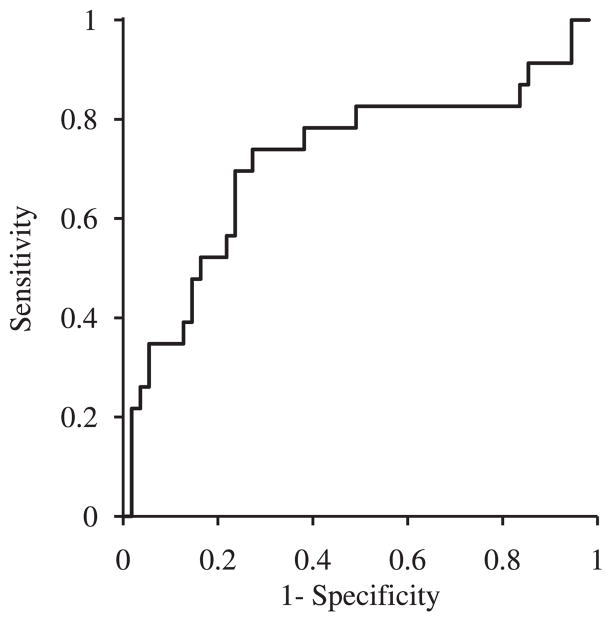
In contrast, peak systolic longitudinal myocardial strain measured at completion of the anthracyclines treatment was predictive of the later development of cardiotoxicity (P=0.0003). Based on the receiver operating characteristic curve (Figure 5), a value <19% at the completion of the anthracyclines treatment was selected to detect patients at high risk of developing cardiotoxicity.8 Longitudinal strain <19% was measured in 74% of the patients developing cardiotoxicity subsequently (sensitivity; Table 4). Fifty-three percent of patients with strain <19% developed cardiotoxicity during follow-up (positive predictive value; Table 4). In contrast, 13% of the patients with longitudinal strain ≥19% at completion of the anthracyclines treatment developed cardiotoxicity. A decrease of longitudinal strain of 10% between baseline and the end of anthracyclines treatment was also predictive of subsequent cardiotoxicity (p=0.011). Of note, longitudinal strain measured at the completion of the anthracyclines treatment predicted decreases of LVEF to <50% (P<0.0001). Neither radial nor circumferential strain were predictive of subsequent cardiotoxicity (P=0.25 and P=0.67, respectively).
Figure 5.
Receiver operating characteristic curve for peak systolic longitudinal myocardial strain measured at the completion of the anthracyclines treatment in the prediction of cardiotoxicity.
Table 4.
Sensitivity, Specificity, PPV, and NPV of the Predictors of Cardiotoxicity
| Predictors (Measured At the Completion of Anthracyclines) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Long strain <19% | 17/23 (74%) (0.51–0.90) | 40/55 (73%) (0.59–0.84) | 17/32 (53%) | 40/46 (87%) |
| usTnI >30 pg/mL | 11/23 (48%) (0.27–0.69) | 40/55 (73%) (0.59–0.84) | 11/26 (44%) | 40/52 (77%) |
| Long strain <19% and usTnI>30 pg/mL | 8/23 (35%) (0.16–0.57) | 51/55 (93%) (0.82–0.98) | 8/12 (67%) | 51/66 (77%) |
| Long strain <19% or usTnI>30 pg/mL | 20/23 (87%) (0.66–0.97) | 29/55 (53%) (0.39–0.66) | 20/46 (43%) | 29/32 (91%) |
PPV indicates positive predictive value; NPV, negative predictive value; and usTnI, ultrasensitive troponin I.
Long strain is peak systolic longitudinal myocardial strain.
The 95% exact CIs are provided in brackets.
Predictive Value of Biomarkers
None of the biomarkers were predictive of cardiotoxicity when measured as continuous variables. However, elevated usTnI concentrations (≥30 pg/mL) at the completion of the anthracyclines treatment were predictive of subsequent cardiotoxicity (P=0.04; Table 4). Neither elevated NT-proBNP nor ST2 were predictive of later cardiotoxicity (P=0.39 and P=0.78, respectively).
Multivariate Analysis
When longitudinal strain and usTnI were included in a multivariate analysis adjusted for age and the presence of hypertension, longitudinal strain <19% remained the only independent predictor of cardiotoxicity (P=0.0003).
Clinical Symptoms
Five patients developed symptoms of heart failure during the course of the study (6%). One patient presented at the completion of the anthracyclines treatment, 1 patient 3 months, 2 patients 6 months, and 1 patient 12 months after treatment by anthracyclines.
Cardiotoxicity was noted in all symptomatic patients. Peak longitudinal strain <19% soon after anthracyclines therapy was present in all symptomatic patients. usTnI was ≥30 pg/mL soon after anthracyclines treatment in 2 patients who later developed symptoms of heart failure (both 6 months later). usTnI measured 3, 6, and 9 months after anthracyclines did not detect any symptomatic patients.
Discussion
In this prospective study of women with breast cancer treated with anthracyclines followed by taxanes and trastuzumab, peak systolic longitudinal myocardial strain and usTnI measured at the completion of anthracyclines therapy were predictive of the development of cardiotoxicity, as defined by the CREC during the subsequent treatment course (12 months after the completion of anthracyclines). A significant decrease of LVEF (8% or more)14 was detected at the completion of anthracyclines treatment in only 15% of the patients developing cardiotoxicity during follow-up. In contrast, changes in more sensitive markers of myocardial injury or dysfunction such as troponin or strain were detected at completion of the anthracyclines in 78% of patients developing subsequent cardiotoxicity. Furthermore, longitudinal strain <19% was present in all those patients who later developed symptoms of heart failure.
The present study was designed to investigate a homogeneous population of chemotherapy-treated patients. The women enrolled were representative of a population diagnosed with first-time breast cancer, in terms of their age, cardiovascular risk factors, cardiovascular and cancer treatments.15 The applicability of the results to patients treated with anthracyclines, taxanes, and trastuzumab was confirmed by the fact that the incidence of CREC-defined cardiotoxicity and symptomatic heart failure were similar to those observed in larger trials.13 It is noteworthy that the LVEF decreases during the treatment period but remains within normal limits when averaged in the whole group, a finding that has also been reported in large studies.16
Anthracyclines-induced cardiotoxicity is mainly mediated through the generation of reactive oxygen species and is accompanied by increased cardiomyocyte calcium overload and apoptosis, an irreversible process.17 In contrast, human epidermal growth factor receptor 2-inhibitors disrupt myofibrillar structure but do not appear to cause extensive cardiomyocyte death.18 In the present study, the changes of troponin and strain detected after the anthracyclines treatment and before any other treatment underline the crucial role of anthracyclines in the development of cardiotoxicity. Hare et al7 did not observe a decrease in strain in patients who had already received anthracyclines and were treated with trastuzumab only, suggesting that the additional effect of trastuzumab on cardiac function may be limited.
The long-term natural history of cardiotoxicity in the association of anthracyclines and trastuzumab is not yet known. In an intermediate study following women treated with 1 year of trastuzumab for 3 additional years, cardiac events were detected during the initial year,19 thereby underlining the importance of the follow-up period throughout the trastuzumab treatment chosen in the present study.
Several studies reporting decreases in myocardial deformation parameters in patients previously treated with anthracyclines did not, however, investigate the predictive value of these indices.5,6 The predictive value of a decreased global longitudinal and radial strain was reported recently in 43 highly symptomatic patients (24% of symptomatic heart failure), however, the precise timing of the measurement and of the follow-up were not clarified.20 The present study demonstrates the value of a measurement of longitudinal strain obtained at the completion of the anthracyclines treatment in the prediction of cardiotoxicity during the subsequent taxanes and trastuzumab treatment. No predictive value of radial strain was found, possibly due to the variability of the measurement.
In the present study, NT-proBNP concentrations did not change and did not predict cardiotoxicity. Similarly, ST2, a novel marker that is associated with the occurrence of remodeling and heart failure,21 was unchanged. Of note, the baseline level of ST2 observed in patients before any cardiotoxic treatment was high compared with the reported median in a healthy population,22 suggesting that levels may be altered in these patients. Thus, the negative results of NT-proBNP and ST2 may possibly be attributed to the multiple comorbidities found in cancer patients capable of modifying these biomarkers levels.23
Cardinale et al have reported that the measurement of troponin I predicted the development of later cardiac events in patients treated with high doses of anthracyclines9 or chmotherapies and trastuzumab.24 The present study confirms the value of measuring troponin in patients with breast cancer treated with anthracyclines, taxanes, and trastuzumab and clarifies its optimal timing. Although ultrasensitive troponin was not an independent predictor of later cardiotoxicity, its measurement combined with the measurement of longitudinal strain increased the sensitivity of the biomarkers from 74% to 87%, allowing a negative predictive value of 91%. Thus, measuring both strain and troponin may be of value in predicting the absence of toxicity of the cancer treatment.
In the present study, few patients had symptoms of heart failure, precluding any analysis of the value of echocardiography and biomarkers in the prediction of symptomatic heart failure. Peak longitudinal myocardial strain, however, was not only predictive of CREC-defined cardiotoxicity but also of decreases of LVEF to <50%. The clinical relevance of such a decrease in LVEF is high; in a study of 4257 participants from the Framingham study, the presence of an asymptomatic LVEF between 40% and 50% was accompanied with a 3.9 risk in heart failure and 1.9 risk in mortality compared with participants with LVEF >50%.25 Accordingly, the American Heart Association/American College of Cardiology guidelines recommend the use of cardioprotective drugs in asymptomatic patients with abnormal LVEF.
There are limitations to the present study. The apical strain values were not analyzed as in majority of patients the apex was not well visualized at all time points, mainly due to breast surgery, postsurgical changes, and the presence of expanders or breast implants. The 3-chamber apical view was not acquired in the protocol design. Both the absence of the apex and of the 3-chamber view limits our ability to analyze every segment of the myocardium. Another limitation of the study is that 12 patients did not complete all the follow-up studies (average of 4.9 ± 0.3 studies in these patients), decreasing our ability to define transient episodes of cardiotoxicity. Other limitations include the relatively small sample size (23 to 26 cases of cardiotoxicity), and the fact that sensitivity and specificity are likely overestimated because they were estimated from the same data used to define optimal cut points. Finally, the study is testing whether assessment of LVEF at one time could be predictive of a change of LVEF at a later time. Investigating the value of early LVEF in the prediction of stronger end points, such as symptomatic heart failure or cardiac mortality would be of great interest. Because the incidence of these events is low, however, such a study would require a much greater number of patients and a longer follow-up. Other sensitive indices of LV function, such as strain rate or mitral annular systolic displacement26 may also be tested in further studies.
In conclusion, the peak systolic longitudinal myocardial strain and ultrasensitive troponin measured at the completion of treatment with anthracyclines in women with breast cancer treated by anthracyclines, taxanes, and trastuzumab predict the occurrence of subsequent cardiotoxicity and may help to guide the clinician on the subsequent treatment plan in terms of therapy adjustment, closer follow-up of cardiac function, and appropriateness of cardiovascular therapy.
CLINICAL PERSPECTIVE.
Because cancer patients survive longer, the impact of cardiotoxicity associated with the use of cancer treatments on cardiac morbidity and mortality is increasing. Left ventricular ejection fraction is the recognized method to monitor the cardiotoxic effects of cancer treatments; however, it may not detect subtle myocardial injury. The present study investigated whether early alterations of myocardial strain and blood biomarkers could predict incident cardiotoxicity in a cohort of 81 patients with breast cancer during treatment with anthracyclines followed by taxanes and trastuzumab. Peak systolic longitudinal myocardial strain and ultrasensitive troponin I measured after completion of the anthracyclines were predictive of cardiotoxicity (defined by a decrease of left ventricular ejection fraction with or without symptoms of heart failure) occurring later during the treatment. In patients with breast cancer treated with anthracyclines, taxanes and trastuzumab, systolic longitudinal myocardial strain and ultrasensitive troponin I measured at the completion of anthracyclines therapy are useful in the prediction of subsequent cardiotoxicity and may help guide treatment to avoid cardiac side-effects.
Acknowledgments
We thank Laurie Farrell, RN, from the Cardiology Division for her help in the blood sampling and processing, Steven Isakoff, MD, Alisha Polewarczyk, BA, Jennifer Acuna, International Health Policy and Management, BA, from the Gillette Center for Breast Cancer of Massachusetts General Hospital for their contribution in recruiting patients, Stephanie Fuoco, RN, from the Sir Mortimer B. Davis-Jewish General Hospital/McGill University for coordinating patient enrollment, Mary Lou Gantzer, PhD, from Siemens HealthCare Diagnostics for measurement of ultrasensitive troponin I and N-terminal pro–B-type natriuretic peptide, James Snider, PhD, from Critical Diagnostics for measurement of ST2 and Arthur E Weyman, MD, from the Cardiac Ultrasound Laboratory at the Massachusetts General Hospital for his careful review of the paper.
Sources of Funding
This work was supported by an investigator-initiated grant from the Susan G. Komen for the Cure Foundation (KG 080818), a Claflin Distinguished Scholar Award and a Clinical Innovation Award from MGH, Boston, MA (all to Dr Scherrer-Crosbie), and by the Kynett Focus Junior Faculty Investigator Award, Philadelphia, PA (to Dr Ky).
Footnotes
Disclosures
Dr Januzzi receives consultancy fees and research funding from Critical Diagnostics and Roche. Dr Plana receives honoraria and research funding from General Electrics.
References
- 1.Guglin M, Cutro R, Mishkin JD. Trastuzumab-induced cardiomyopathy. J Card Fail. 2008;14:437–444. doi: 10.1016/j.cardfail.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 3.Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracy-cline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol. 2002;13:699–709. doi: 10.1093/annonc/mdf132. [DOI] [PubMed] [Google Scholar]
- 4.Neilan TG, Jassal DS, Perez-Sanz TM, Raher MJ, Pradhan AD, Buys ES, Ichinose F, Bayne DB, Halpern EF, Weyman AE, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. Eur Heart J. 2006;27:1868–1875. doi: 10.1093/eurheartj/ehl013. [DOI] [PubMed] [Google Scholar]
- 5.Ganame J, Claus P, Eyskens B, Uyttebroeck A, Renard M, D’hooge J, Gewillig M, Bijnens B, Sutherland GR, Mertens L. Acute cardiac functional and morphological changes after Anthracycline infusions in children. Am J Cardiol. 2007;99:974–977. doi: 10.1016/j.amjcard.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 6.Jurcut R, Wildiers H, Ganame J, D’hooge J, De Backer J, Denys H, Paridaens R, Rademakers F, Voigt JU. Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. J Am Soc Echocardiogr. 2008;21:1283–1289. doi: 10.1016/j.echo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009;158:294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 10.Morris PG, Chen C, Steingart R, Fleisher M, Lin N, Moy B, Come S, Sugarman S, Abbruzzi A, Lehman R, Patil S, Dickler M, McArthur HL, Winer E, Norton L, Hudis CA, Dang CT. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res. 2011;17:3490–3499. doi: 10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 11.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, Murphy M, Stewart SJ, Keefe D. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 12.Dieplinger B, Januzzi JL, Jr, Steinmair M, Gabriel C, Poelz W, Haltmayer M, Mueller T. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma–the Presage ST2 assay. Clin Chim Acta. 2009;409:33–40. doi: 10.1016/j.cca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Tan-Chiu E, Yothers G, Romond E, Geyer CE, Jr, Ewer M, Keefe D, -non RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 14.Otterstad JE. Measuring left ventricular volume and ejection fraction with the biplane Simpson’s method. Heart. 2002;88:559–560. doi: 10.1136/heart.88.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, Ageev FT, Hitre E, Groetz J, Iwata H, Knap M, Gnant M, Muehlbauer S, Spence A, Gelber RD, Piccart-Gebhart MJ. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–3865. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 16.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 18.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM. Suter Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 19.Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, Climent MA, Rechberger E, Liu WT, Toi M, Coombes RC, Dodwell D, Pagani O, Madrid J, Hall M, Chen SC, Focan C, Muschol M, van Veldhuisen DJ, Piccart-Gebhart MJ. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28:3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 20.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, Tian G, Kirkpatrick ID, Singal PK, Krahn M, Grenier D, Jassal DS. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 21.Pascual-Figal DA, Manzano-Fernández S, Boronat M, Casas T, Garrido IP, Bonaque JC, Pastor-Perez F, Valdés M, Januzzi JL. Soluble ST2, high- sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: J, Car complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13:718–725. doi: 10.1093/eurjhf/hfr047. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Snider JV, Grenache DG. Establishment of reference intervals for soluble ST2 from a United States population. Clin Chim Acta. 2010;411:1825–1826. doi: 10.1016/j.cca.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Baggish AL, van Kimmenade RR, Januzzi JL., Jr The differential diagnosis of an elevated amino-terminal pro-B-type natriuretic peptide level. Am J Cardiol. 2008;101(3A):43–48. doi: 10.1016/j.amjcard.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nolè F, Veglia F, Cipolla CM. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 25.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 26.Qin JX, Shiota T, Tsujino H, Saracino G, White RD, Greenberg NL, Kwan J, Popovic ZB, Agler DA, Stewart WJ, Thomas JD. Mitral annular motion as a surrogate for left ventricular ejection fraction: real-time three-dimensional echocardiography and magnetic resonance imaging studies. Eur J Echocardiogr. 2004;5:407–415. doi: 10.1016/j.euje.2004.03.002. [DOI] [PubMed] [Google Scholar]