Abstract
Bisphenol A (BPA) is an estrogenic environmental toxin widely used in the production of plastics and ubiquitous human exposure to this chemical has been proposed to be a potential risk to public health. Animal studies suggest that in utero and early postnatal exposure to this compound may produce a broad range of adverse effects, including impaired brain development, sexual differentiation, behavior, and immune function, which could extend to future generations. Molecular mechanisms that underlie the long-lasting effects of BPA continue to be elucidated, and likely involve disruption of epigenetic programming of gene expression during development. Several studies have provided evidence that maternal exposure to BPA results in postnatal changes in DNA methylation status and altered expression of specific genes in offspring. However, further studies are needed to extend these initial findings to other genes in different tissues, and to examine the correlations between BPA-induced epigenetic alterations, changes in gene expression, and various phenotypic outcomes. It will be also important to explore whether the epigenetic effects of BPA are related to its estrogenic activity, and to determine which downstream effector proteins could mediate changes in DNA methylation. In this review, we will highlight research indicating a consequence of prenatal BPA exposure for brain, behavior, and immune outcomes and discuss evidence for the role of epigenetic pathways in shaping these developmental effects. Based on this evidence, we will suggest future directions in the study of BPA-induced epigenetic effects and discuss the transgenerational implications of exposure to endocrine disrupting chemicals.
Keywords: bisphenol A, DNA methylation, gene expression, epigenome, estrogen receptor, brain development, behavior, immune, endocrine disruptor, transgenerational
The pervasive effects of early-life environments are becoming increasingly evident in studies of social, nutritional, and physiological experiences. However, the environment is also saturated with chemicals that have a significant potential to interfere with normal biological functions and cause adverse health effects. Clearly, in the life span of an individual, prenatal development represents the most vulnerable period for any exogenous interference, since a series of complex and precisely timed events need to take place during this period in order to ensure normal tissue and organ development (Bernal and Jirtle, 2010). Many environmental toxins are able to cross the placenta, and produce developmental defects in maternally exposed offspring (Patisaul and Adewale, 2009). In addition, evidence suggests that prenatal environmental exposures can significantly increase the risk of developing chronic diseases later in life, including cancer, infertility, diabetes, cardiovascular disease, obesity and psychiatric and behavioral conditions such as schizophrenia and mood disorders (Bernal and Jirtle, 2010; Jirtle and Skinner, 2007).
Endocrine disrupting compounds (EDCs) are chemicals in the environment that mimic or inhibit the actions of endogenous hormones, and have the potential to alter the structure and function(s) of the endocrine system (Patisaul and Adewale, 2009). EDCs have been largely shown to produce adverse effects in animals, with some supporting evidence in humans, and include the components of plastics [e.g. bisphenol A and phthalates (Fisher, 2004; Richter et al., 2007)], fungicides [e.g. vinclozolin (Kavlock and Cummings, 2005)], pesticides [e.g. methoxychlor and dichlorodiphenyltrichloroethane (Cohn et al., 2007; Cummings, 1997; Mills and Yang, 2006)], pharmaceuticals [e.g. diethylstilbestrol (Veurink et al., 2005)], and various naturally occurring phytoestrogens [e.g. genistein (Kwack et al., 2009; Patisaul and Adewale, 2009)]. These compounds mainly act as estrogens, anti-estrogens, and anti-androgens, and affect normal reproductive function and the organization of neuroendocrine circuits that coordinate sex-specific physiology and behavior (Gore, 2008; McLachlan, 2001). However, many of the adverse effects of developmental exposure to an EDC, including various cancers of the reproductive tract, emerge well beyond the period of exposure (Patisaul and Adewale, 2009). The effects of EDCs may also extend beyond the reproductive and neuroendocrine system to include multiple adult organs and tissues (such as kidneys and spleen), and may even be transmitted across several generations (Anway and Skinner, 2006).
Though hormone signaling is normally reversible and induces dynamic changes in cellular function, during development steroid hormones can induce permanent effects on gene activity that enable cellular and tissue differentiation, and prime genes to respond to secondary hormonal cues later in life. This so-called hormonal imprinting or gene programming likely involve epigenetic mechanisms, such as DNA methylation, which can be passed from one cell generation to another and persist into adulthood (McLachlan, 2001). Therefore, it has been proposed that EDCs may interfere with epigenetic programming resulting in adverse developmental effects. Diethylstilbestrol (DES) was the first estrogenic xenobiotic to be shown to demethylate the DNA sequence of an estrogen-responsive gene during development, associated with persistent abnormal gene expression and tumorigenesis in the adult animals (Li et al., 1997). There is now increasing evidence that EDCs are able to modify the epigenome (Alworth et al., 2002; Anway et al., 2005; Dolinoy et al., 2007), providing further indication that epigenetic mechanisms may underlie the long-lasting effects of these compounds. However, recent studies also suggest that at least some of the epigenetic affects of EDCs may not be mediated by effects on hormonal signaling (Anway et al., 2005), but could be potentially exerted through hormone-independent pathways.
In this review, we will discuss emerging evidence for the behavioral, neuroendocrine, and immune consequences of developmental exposure to bisphenol A (BPA). BPA is an endocrine disruptor that has recently been the subject of intense public and scientific scrutiny due to increasing evidence from animal studies that this compound poses a potential health risk, especially if exposure occurs prenatally and in early postnatal life. First, we will review the animal laboratory data and, where available, human epidemiological studies implying broad adverse effects of developmental BPA exposure, particularly effects on brain, behavior, and immune function as well as the cellular/molecular pathways thought to be relevant to BPA-associated effects. We will then discuss why the epigenome represents a plausible target for the action of environmental agents, including EDCs and BPA, during development followed by evidence for the molecular effects of BPA that support this epigenetic perspective. Finally, we will highlight potential future directions of epigenetic research in the study of BPA and discuss the implications of these molecular changes for the transgenerational inheritance of disease susceptibility.
Bisphenol A: A Ubiquitous Toxic Exposure
BPA is one of the world’s highest-production volume chemicals, and is mainly used in the manufacture of polycarbonate plastics and epoxy resins. Polycarbonate plastics are widely used for food and drink packaging, while resins are utilized as protective coatings on metal products including food cans, bottle tops and water supply pipes. BPA is also found in polymers that are used in dental materials (Chapin et al., 2008). The ubiquitous and extensive use of BPA-containing products results in a high human exposure worldwide (Vandenberg et al., 2010). Recent studies suggest that more than 90% of the U.S. population has detectable levels of BPA in urine samples (Calafat et al., 2005; Calafat et al., 2008). It is thought that human exposure mainly occurs through diet, as polymers containing BPA can be hydrolyzed under high temperature and acidic or basic conditions, leading to BPA leaching into food and drink containers (Welshons et al., 2006). However, recent evidence also indicates that exposure may occur through dermal contact with thermal paper, used widely in cash register receipts (Biedermann et al., 2010). Importantly, the concentration of urine BPA was found highest in children, followed by adolescents, while the lowest levels were found in the adults. Generally, females had higher urinary BPA concentrations than males (Calafat et al., 2008). BPA has also been detected in urine and serum of pregnant women, as well as in placental tissue, umbilical cord blood, amniotic fluid, and the urine from newborn infants (Schonfelder et al., 2002; Vandenberg et al., 2010). In addition, levels of BPA were found to be 5-fold higher in amniotic fluid at 15–18 weeks of gestation as compared to maternal serum (Ikezuki et al., 2002). These data imply that BPA is able to cross the placenta, and may accumulate in the embryo/fetal compartment after repeated maternal exposure, most likely due to inability of fetus to efficiently metabolize BPA (Taylor et al., 2008).
Evidence from laboratory studies suggest that BPA may act as a toxicant for developing tissues and has stimulated debate regarding the impact of human exposure to this compound. Prenatal exposure of rodent fetuses to BPA, even at doses below those currently considered safe for humans (see below), has been shown to have significant developmental effects. The observed adverse effects are diverse, and include altered development of the male and female reproductive tracts, altered development and tissue organization of the mammary gland, increased prostate gland volume, disruption of sexual differentiation in the brain, accelerated growth and puberty, higher incidence of breast and prostate cancer, increased body weight, altered reproductive function and sexual behavior, and immune dysregulation (Chapin et al., 2008; Clayton et al., 2010; Richter et al., 2007). The widespread consumption of BPA-containing products has raised concerns amongst scientists and regulatory agencies that human exposure to BPA may occur at levels shown to have adverse effects in rodent models. BPA has been officially declared to be a “toxic substance” in Canada (Canada Gazette, October 13, 2010, Vol. 144, No. 21), while the newest FDA update on BPA use states that there is “some concern about the potential effects of BPA on the brain, behavior, and prostate gland in fetuses, infants, and young children” (www.fda.gov, January, 2010). In the following section, we will summarize animal studies that indicate a possible effect of BPA on brain development, behavior, and immune function in the offspring of BPA-treated mothers, which have contributed to these policy and health concerns.
Neurobiological and Behavioral Consequences of Prenatal BPA Exposure
The prenatal and early postnatal period of development is a time of rapid change in brain architecture which shapes a broad range of neuroendocrine and behavioral characteristics. Animal studies have been used to explore the consequences of in vivo exposure to BPA during fetal/postnatal development and indicate multiple neurobiological and behavioral outcomes linked to BPA exposure. Here, we provide a summary of these findings in studies of the offspring of BPA-treated mothers. This summary includes information regarding the dose, route of administration, and rodent strain/species – key variables to consider when interpreting the magnitude of the effect [see (Richter et al., 2007) for a more in depth discussion of the implications of these experimental parameters for in vivo BPA studies] and possible relevance of these data for our understanding of human health risk. It is important to note that the U.S. Environmental Protection Agency (EPA) calculated the reference dose for BPA to be 50 μg/kg/day, which is “an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime” (www.epa.gov). The EPA reference dose for BPA was calculated based on the lowest-observed-adverse-effect level (LOAEL, 50 mg/kg/day) derived from high-dose toxicological studies and by applying a 1000-fold safety factor (vom Saal and Welshons, 2006). In the following section describing the consequences of BPA exposure, it is evident that BPA can cause adverse effects in animals at doses several orders of magnitude lower than not only LOAEL but also the reference dose, suggesting a need for the “safe” human exposure level for BPA to be re-evaluated (vom Saal and Hughes, 2005). In this review, all animal studies that have utilized BPA doses lower than the current reference dose for humans (50μg/kg/day) will be considered “low dose” studies at a physiologically relevant exposure dose.
A. Brain Development
During fetal development, there is a “sensitive period” during which environmental exposures may have persistent effects on the developing brain. Maternal exposures to low doses of BPA (20μg/kg/day, s.c.) have been shown to affect neocortical development in mouse offspring by accelerating neuronal differentiation and migration during the mid-gestational period (Nakamura et al., 2006). In addition, BPA-associated changes in neurogenesis resulted in abnormal neuronal positioning and aberrant connectivity between thalamus and cortex in the adult brain of in utero exposed animals (Nakamura et al., 2007). These effects may have implications for the behavior and physiology of BPA-exposed offspring.
B. Sexual Dimorphism
One of the most striking findings concerning BPA effects on brain development is the loss of sexual dimorphism that has been shown in terms of brain structure and behavior. Kubo et al. found that exposure to BPA (30μg/kg/day in drinking water) during the prenatal and postnatal period of ICR/Jcl mice increased the size of locus coeruleus (LC) in males and decreased LC volume in females, though no effects were found in the sexually dimorphic nucleus of the preoptic area in the hypothalamus (SDN–POA) (Kubo et al., 2003). These sexually dimorphic effects were also observed in measures of avoidance memory and exploratory behavior, with BPA exposure abolishing sex differences in these behaviors at doses of 1.5μg/kg/day (Kubo et al., 2001) and 30μg/kg/day (Kubo et al., 2003), respectively. Likewise, Rubin et al. examined the effect of the extremely low doses of BPA (25 and 250ng/kg/day), administered via an implanted Alzet osmotic pump to pregnant and lactating CD-1 mice, and found that these exposures decreased sex differences in tyrosine hydroxylase (TH) neurons in the anteroventral periventricular preoptic area (APVP) and abolished sex differences in open-field behavior (Rubin et al., 2006). In a related study, exposure to BPA (2.5μg/kg/day in drinking water) during gestation and lactation in Wistar rats resulted in a loss of sex difference in the number of corticotropin-releasing hormone (CRH) neurons in the bed nucleus of the stria terminalis (BNST) and no effect on sex differences in CRH neurons in the preoptic area (POA) (Funabashi et al., 2004). These studies suggest that BPA may diminish sex differences in the brain in a region-specific manner. However, it should be noted that in some studies, BPA is shown to have sex-specific effects without affecting sexual dimorphism. For instance, Tando et al. (2007) found that maternal BPA treatment (3μg/kg/day) during pregnancy and lactation in ddY mice led to a significant reduction in the total volume and density of dopaminergic neurons in the substantia nigra (SN) in adult female offspring, but not in male mice (Tando et al., 2007). Overall, these studies suggest that BPA exposure can alter sex differences in brain and behavior and exert sex-specific effects.
C. Sociosexual and Maternal Behavior
Sexual behavior, social interactions, and maternal behavior have also been found to be effected by developmental BPA exposure. In Sprague-Dawley rats, maternal BPA exposure (40μg/kg/day) during pregnancy or lactation was found associated with impairments in male sexual performance and increased sexual motivation and receptivity in female offspring (Farabollini et al., 2002). In addition, juvenile female rats exposed to the same regime of gestational and lactational BPA treatment, exhibited defeminization in social interactions, including reduced play with males and decreased social grooming (Porrini et al., 2005) and a masculinization of female play behavior, including increased play with females and increased sociosexual exploration (Dessi-Fulgheri et al., 2002). Amongst CD-1 male mice exposed to BPA during fetal development (2 or 20μg/kg/day; gestation days 11–17), increases in aggressive behavior at 8 weeks of age were observed (Kawai et al., 2003). This effect was not associated with increased testosterone levels, but coincided with the age when mice typically reach sexually maturity. Amongst female CD-1 mice, developmental exposure to BPA (10μg/kg/day, oral exposure during gestation days 14–18) negatively affected subsequent maternal behavior, leading to reduced contact with pups (Palanza et al., 2002). Similar reductions in maternal behavior were observed when dams were exposed to the same BPA regimen as adults, and these behavioral changes may be the result of a direct effect of BPA on the neuroendocrine circuits underlying the initiation of postnatal mother-infant interactions (Palanza et al., 2002).
D. Anxiety and Response to Novelty
BPA exposure has been shown to increase anxiety-related behavior in both rats and mice. Consistent with the previous reports of BPA effects on sexual dimorphism, maternal exposure to BPA (10μg/kg/day, oral, gestational day 11 through to postnatal day 8) in CD-1 mice was found to reduce or eliminate sex differences in response to novelty and exploration in open-field and elevated-plus maze tests (Gioiosa et al., 2007). Amongst C57BL/6J mice, prenatal BPA exposure (8mg/kg/day) reduced the amount of time that male offspring spent in the open arms of the elevated-plus maze, while females showed no BPA-induced behavioral changes (Cox et al., 2010). In rats, female offspring of dams exposed to BPA (40μg/kg/day, oral) during pregnancy and lactation spent significantly less time exploring a novel environment compared to control female offspring, though no effect on this behavior was observed in male offspring. However, BPA-treated male rats in this study did exhibit significantly reduced amphetamine-induced hyperactivity (Adriani et al., 2003).
E. Learning and Memory
Impairments in learning have been observed amongst BPA exposed offspring. Male offspring of F344 rats exposed to BPA (100μg/kg/day, orally, from gestational day 3 to postnatal day 20) showed impaired learning of passive and active avoidance tasks at 13 and 15 weeks of age, respectively (Negishi et al., 2004). Similarly, the male offspring of ICR mice exposed to BPA (0.5, 5 and 50mg/kg/day, orally, from gestational day 7 to postnatal day 21) exhibited significant impairments in spatial memory and passive avoidance memory as juveniles (PND 21) and in adulthood (PND 56). Importantly, these changes were associated with decreased protein expression of N-Methyl-D-aspartic acid (NMDA) receptor subunits NR1, NR2A and NR2B, as well as reduced levels of estrogen receptor beta (ERβ) in the hippocampus (Xu et al., 2010), suggesting a neurobiological substrate of BPA-induced cognitive dysfunction.
F. Effects in Humans
In general, there is a lack of epidemiological data on neurotoxic effects of BPA in humans. However, a recent study (Braun et al., 2009) demonstrated that BPA concentration in maternal urine during early pregnancy was associated with higher externalizing behavior scores in 2-year old children, particularly amongst girls. Since externalizing behaviors include hyperactivity and aggression, and are typically more frequent in boys than girls, the results of this study appear consistent with the rodent data suggesting an effect of BPA on sexual dimorphism, though further data will be needed to clarify the nature and magnitude of this effect in humans.
BPA Exposure and Immune Function
The effects of endocrine disruptors such as BPA on immune tissues and the consequences for immune function have been explored in animal studies and suggest that both fetal/postnatal and adult exposure can induce long-term changes. Adult female Balb/c mice administered BPA (0.1mg/g) daily for 2 weeks were found to have reduced IgE and elevated levels of IgG2a, though BPA effects on these antigens were not evident when mice were immunized with ovalbumin. BPA also induced significant changes in cytokine levels, leading to elevated IFN-γ and IL-2, and was associated with increased spleen weight and reduced proliferation of lymphocytes (Alizadeh et al., 2006). Analysis of the specific cytokines targeted by the treatment suggest that BPA may alter the T helper (Th) 1 immune responses (Goto et al., 2007; Yoshino et al., 2003). Both the dose and the timing of exposure to BPA are critical variables in predicting the consequence for immune function. Adult male mice exhibit dose-dependent (5.7, 11.4, 22.8, and 45.6mg/kg) increases in swelling and IL-4, IL-10, and IL-13 production following infection with Leishmania major (Yan et al., 2008). Exposure to BPA was also associated with reduced percentages of T regulatory CD4+CD25+ cells. These immune consequences of BPA were more pronounced when exposure occurred during fetal development. Prenatal exposure to BPA was associated with dose-dependent increases in IFN-γ and IL-4 and reduced CD4+CD25+ cells (Yan et al., 2008). In vitro studies indicate that BPA exposure reduces NF-k B activation in response to immune challenge (Igarashi et al., 2006), an effect that was similarly identified following phenol exposure. Though our knowledge of BPA effects on human immune function is very limited, in a recent analysis, BPA levels were found associated with indices of cell-mediated immune function. The direction of this effect varied dependent on subject age, with increasing BPA being associated with higher antibody levels in subjects over 18 years of age and lower antibody levels in subjects under 18 years of age (Clayton et al., 2010). Overall, the emerging data suggest that BPA exposure alters activity within the immune system and these effects may be most evident and persistent when BPA exposure occurs during early development. The known interactions between immune function and brain development may be an important consideration in studies of BPA-induced effects and maternal immune activation in response to gestational BPA exposure may have implications for placental development and induce changes in the fetal brain. However, it should be noted that very high doses of BPA have been used in the in vivo animal studies of immune function, thus limiting the relevance of these findings for humans.
Cellular and Molecular Effects of BPA
The broad effects of in vivo BPA exposure on neurobiological, behavioral, and immune function has lead to increasing exploration of the cellular and molecular pathways of BPA action. BPA binds both classic estrogen receptors, ERα and ERβ, although with an affinity that is 10,000–100,000 lower than that of endogenous 17β-estradiol (Andersen et al., 1999; Kuiper et al., 1998). Binding to ER can result in either activation or repression of estrogen target genes, depending on the co-regulatory factors that are recruited to the estrogen response elements (EREs) in gene regulatory regions (Stanisic et al., 2010). In terms of estrogenic activity, BPA is best defined as a selective estrogen receptor modulator, because it produces the effects of an estrogen agonist in some tissues, while acting as an estrogen antagonist in other tissues (Gould et al., 1998; Wetherill et al., 2007). Some actions of BPA are very rapid and may be explained by non-genomic actions mediated through a membrane-bound form of ERα and a candidate G-protein coupled membrane estrogen receptor 30 (GPR30). There is also evidence that BPA may have anti-androgenic activity, interfere with thyroid hormone signaling, and bind an orphan nuclear receptor called estrogen-related receptor γ (Sohoni and Sumpter, 1998; Takayanagi et al., 2006; Xu et al., 2005).
BPA has been shown to change the expression of genes in various tissues, including brain and spleen. While these effects are typically attributed to ER-mediated action of BPA, it is still not clear how the low potency at ERs could account for the strong effects observed in many tissues after low-dose BPA exposures. In addition, there is evidence suggesting that in utero BPA exposure results in changes in gene expression that persist into adulthood (Ho et al., 2006; Smith and Taylor, 2007). One possible explanation for these long-term effects could involve molecular mechanisms which lead to persistent changes in gene expression, potentially involving epigenetic pathways. For more detailed discussion on estrogenic action of BPA and its consequences for different tissues and organ systems, we refer the reader to other reviews that more extensively cover this topic (Richter et al., 2007; Wetherill et al., 2007). In the subsequent sections of this review, we will focus on what is known about possible epigenetic actions of BPA, and how these may relate to BPA estrogenic action at molecular level.
The Epigenome as a Plausible Target for Environmental Exposures Occurring During Development
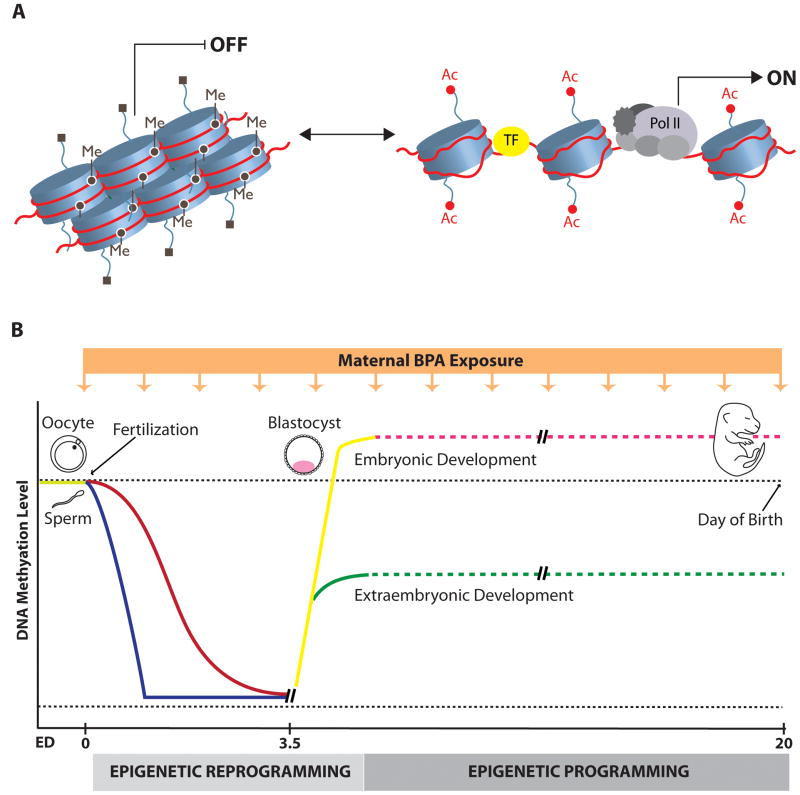
Evidence for the long-term consequences of prenatal BPA exposure, highlighted in the previous sections, suggests that BPA may induce changes in the developing fetus which are maintained into adulthood. In considering the possible mechanisms through which developmental experiences lead to such enduring effects, a novel approach has been to explore the molecular pathways which regulate gene activity and the response of these molecular mechanisms to environmental exposures. Epigenetic mechanisms represent one of the most plausible targets through which environmental toxins, such as BPA, could exert their long-lasting effects. Mammalian development begins with a single totipotent cell (zygote) that subsequently divides and differentiates into many different cell types, which ultimately give rise to an organism. Cell differentiation occurs without change in the DNA sequence, and is based on the establishment of different gene expression programmes in different cell types. One of the main processes that allows for cell-type specific gene expression, without changes in underlying DNA sequence, is called epigenetic gene regulation (Reik, 2007). Epigenetic regulation is possible because DNA in every cell is packaged within a specialized dynamic structure called chromatin, which consists of DNA wrapped around histone proteins (see Figure 1A). When chromatin structure around a genomic region is tightly packed, regardless of DNA sequence, gene expression is repressed. In contrast, a more open chromatin configuration, in which DNA and histones loosely interact, allows access of transcription factors and general transcriptional machinery to the gene regulatory region, leading to the initiation of gene expression (Li et al., 2007). Chromatin structure is largely determined by DNA methylation or various covalent modifications to histone proteins, and these are the major epigenetic mechanisms that control gene expression.
Figure 1. A) Chromatin structure and dynamics.
Chromatin can exist in a closed (left) or more open state (right). The closed chromatin structure does not allow transcription factor access and gene transcription, and is associated with repressive chromatin marks, such as DNA methylation (Me), and deacetylated and methylated histone lysine (H3K9) residues (■). On the contrary, an open and relaxed chromatin state allows access of transcription factors (TF) and general transcriptional machinery (gray ovals) to the gene regulatory region, resulting in the initiation of gene expression. Open chromatin is typically associated with demethylated DNA and highly acetylated histone tails (Ac). B) Proposed epigenetic disruption by BPA during embryonic development. During pre-implementation “epigenetic reprogramming” (zygote to blastocyst stage) methyl marks are actively erased in paternal genome (blue line) immediately after fertilization, followed by passive demethylation of maternal genome (red line) in subsequent cell divisions. Both genomes are de novo methylated resulting in the establishment of DNA methylation patterns and gene expression profiles in the first two cell lineages in the preimplatation embryo: embryonic (yellow line) and extraembryonic (green line) lineages. It is very likely that in more differentiated cells, which are present during later stages of development, genome-wide methylation levels do not change dramatically (presented as dashed lines) though epigenetic “programming”, involving gene-specific changes in both histone modifications and DNA methylation may still be necessary for establishment of gene expression patterns during later lineage commitment. The orange box with the arrows represents maternal exposure to BPA, which may interfere with normal embryonic development and exert deleterious effects during the period of epigenetic reprogramming, while exposures during later stages of development could possibly produce more subtle tissue-specific adverse effects.
In mammals, DNA methylation occurs at position 5 of cytosine residues and predominantly in the context of CpG dinucleotides, many of which are clustered in the GC-rich genomic regions called CpG islands. Methylation of the CpG sites suppresses gene expression, either by directly interfering with the binding of transcription factors, or via inducing repressive chromatin structure in the vicinity of the gene regulatory regions (Klose and Bird, 2006). Histone proteins are also subject to many post-translational modifications that include: acetylation, methylation, phosporylation, ubiquitination, sumoylation and ADP ribosylation. Specific combinations of these modifications can significantly affect chromatin state and mark genes for either enhanced activity or transcriptional silencing (Berger, 2007). It is also known that DNA methylation and histone marks often work in concert to regulate gene expression. For instance, histone methylation (H3K9) can combine with DNA methylation to reinforce the repressive effect on gene activity, and these modifications would be typically accompanied by deacetylation of histones in the same genomic region (Fuks, 2005) (see Figure 1A).
The epigenome refers to DNA methylation and histone modification patterns across the whole genome, and unlike the genome DNA sequence, this pattern varies between different cell types. Also, unlike the very stable genetic code, the epigenome can change in response to environmental cues throughout the life span of an individual. However, it is clear that epigenetic marks are more susceptible to environmental exposures occurring during early prenatal development, when an extensive programming and reprogramming of DNA methylation and histone modification patterns take place in order to establish required cell- and tissue-specific gene expression. In Figure 1B we illustrate the changes in DNA methylation levels that take place during developmental gene programming and highlight how maternal exposure to BPA could disrupt these processes. Epigenetic reprogramming refers to genome-wide erasure and remodeling of DNA methylation and histone modifications, and occurs at two different stages in mammalian development: 1) in the early embryo (from the zygote through the blastocyst stage of pre-implantation development), and 2) during gametogenesis (starting from mid-gestation) (Feng et al., 2010). Post-fertilization epigenetic reprogramming (see Figure 1B) is likely required for the re-establishment of totipotency of the zygote, correct initiation of embryonic gene expression, and early lineage development in the embryo. This period is associated with almost complete erasure of methylation marks within the zygote, followed by de novo methylation of the genome, which coincides with the differentiation of the first two cell lineages of the blastocyst stage. These two lineages are the first to show different epigenetic marks: the embryonic lineage (the inner cell mass) is hypermethylated in comparison to the extraembryonic lineage (trophectoderm), and this seems to be accompanied by different patterns of histone modifications. It seems likely that these early lineages set-up the DNA methylation status of their somatic and placental derivatives. Another wave of epigenetic reprogramming, which also includes a cycle of almost complete demethylation followed by remethylation of the genome, occurs during gametogenesis and is important for the resetting of gene imprinting in germ cells. Imprinted genes show monoallelic parent-of-origin dependent gene expression, and while these parental imprints are maintained in somatic cells of developing embryo, they have to be reestablished in primordial germ cells in a sex specific manner during gametogenesis. Therefore, interference with post-fertilization epigenetic reprogramming has the potential to influence gene programming in developing embryo, while interference with epigenetic reprogramming occurring in germ cells has potential to affect imprinting and the epigenome of future generations of offspring (Reik, 2007; Reik et al., 2001).
Considering the extremely dynamic nature of the epigenome during early embryogenesis and gametogenesis, it is clear that exposure to environmental agents that affect epigenetic mechanisms during these periods have the potential to exert significant effects on embryonic development. Currently, it is unclear how epigenetic regulation is involved in the control of gene expression in later developmental stages, though it is very likely that epigenetic modifications do not change as dynamically in later developmental stages when compared to embryonic development (see Figure 1B) (Reik, 2007). However, evidence has emerged indicating that both histone modifications and DNA methylation still actively participate in controlling gene expression in later lineage commitment, as shown for epigenetic “programming” of stem cells into neuronal precursors followed by differentiation into specific neuron or glia cells (Golebiewska et al., 2009; Liu and Casaccia, 2010; Miller and Gauthier, 2007). Therefore, it seems likely that across the entire developmental period, organisms are vulnerable to epigenetic disruption, and that any agent with the ability to affect the epigenome can induce adverse developmental effects, consequently increasing the risk of adult-onset disease. A critical question is whether the epigenome can be altered in response to prenatal BPA exposure, a topic that will be discussed in the following section.
Epigenetic Mechanisms of BPA-Induced Effects
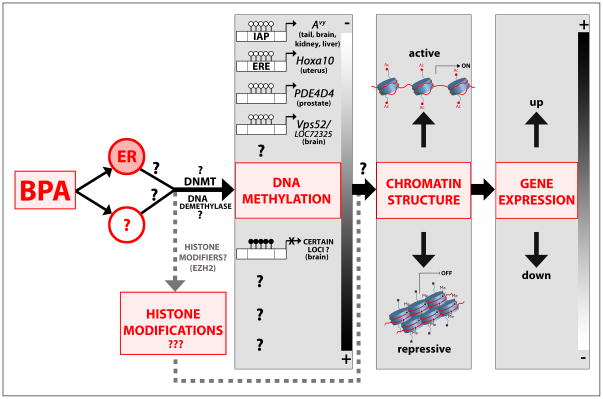
Evidence for BPA-induced epigenetic alterations is summarized in Figure 2. The first convincing study suggesting that BPA can induce epigenetic changes came from Dolinoy et al. (2007), using the Agouti viable yellow (Avy) mouse model. These mice are a well characterized animal model for studying epigenetic effects of environmental agents, due to a meta-stable, DNA methylation sensitive, Avy allele in the Agouti gene locus that determines coat color (Rosenfeld, 2010). The expression of the Agouti gene varies depending on the extent of DNA methylation in the intracisternal A particle (IAP) retrotransposon inserted upstream of the Agouti gene. Accordingly, the coat color of Avy mice ranges from pure yellow (hypomethylation of the Avy IAP) to pseudoagouti brown (hypermethylation of the Avy IAP). Importantly, prior to studies on BPA effects, it was shown that developmental exposure to an environmental factor such as maternal diet, which is rich in methyl group donors (such as folate) or supplemented with phytoestrogen genistein, is able to increase Avy IAP methylation in the offspring, and shift the coat color distribution in these animals toward pseudoagouti brown (Cooney et al., 2002; Dolinoy et al., 2006). Using this mouse model, it was demonstrated that BPA exposure during pregnancy had a DNA hypomethylating effect on the epigenome of the offspring (Dolinoy et al., 2007). Maternal exposure to BPA (phytoestrogen-free AIN-93G diet supplemented with 50mg/kg BPA) shifted the coat color distribution of offspring toward yellow by decreasing methylation at specific CpG sites in Avy allele. Methylation levels observed in tail DNA samples were highly correlated with methylation levels in brain, kidney and liver from the same animals, implying that BPA-induced changes in Avy IAP methylation were established before germ layer differentiation in the embryonic stem cells. BPA also induced CpG hypomethylation in the CabpIAP meta-stable epiallele, which contains an IAP insert within the CDK5 binding-protein gene. Interestingly, BPA-induced changes in DNA methylation and phenotype in Avy offspring were reversed when, in addition to BPA treatment, pregnant mice received a diet enriched with methyl group donors or genistein.
Figure 2. Possible model for BPA-induced epigenetic effects.
In utero BPA exposure may influence the epigenome acting through ER and/or other signaling pathways. It has been demonstrated that BPA induces hypomethylation of the CpG sites in the gene regulatory regions of several genes (Agouti Avy locus, Hoxa10, PDE4D4, Vps52, LOC72325) in different tissues resulting in up-regulation of the corresponding gene expression. Hypermethylating effects of BPA have been shown at several loci in the fetal brain in a genome-wide study, but the association with changes in gene expression was not explored. It is yet unknown whether the activities and/or expression of DNMTs and putative DNA demethylase(s) may be affected by BPA. In addition, there is very limited information on how BPA influences histone modifications and chromatin structure. We propose that BPA may affect both DNA methylation and histone modifications in specific gene regulatory regions, which work in concert to change local chromatin structure, leading to corresponding up- or down-regulation of gene expression.
Though this study provides evidence suggesting that maternal exposure to BPA has a potential to change the epigenome in developing offspring, it is important to emphasize that no comparable Agouti or Cabp gene containing a retroviral insert, is present in the human genome (Rosenfeld, 2010). However, studies supporting the hypothesis of epigenetic action of BPA are not limited to Avy mice (see Figure 2). Neonatal BPA exposure (10μg/kg/day, s.c.) induces hypomethylation of specific CpG sites in the regulatory CpG island of phosphodiesterase type 4 variant 4 (PDE4D4) gene, associated with elevated expression of this gene in the adult prostate and increased susceptibility to prostate cancer with aging (Ho et al., 2006). In addition, high-dose in utero BPA treatment (5mg/kg, i.p., gestational days 9–16) increased the expression of the developmental homeobox gene Hoxa10 in the uterus of female offspring at 2 weeks of age (Bromer et al., 2010). These changes in gene expression were associated with significant demethylation of specific CpG sites in both the promoter and intron of the Hoxa10 gene. Importantly, Hoxa10 DNA methylation was not altered in the uteri of adult mice treated with the comparable dose of BPA, implying that BPA might have greater impact on the epigenome during a critical developmental window (i.e. during fetal development). Genome-wide effects of BPA on DNA methylation in brain tissue have also been explored. Maternal exposure to a low dose of BPA (20μg/kg, from gestational day 0 to days 12–14) was associated with either hypo- or hyper-methylation of the promoter-associated CpG islands in multiple loci in the fetal mouse forebrain (E12.5 or E14.5) (Yaoi et al., 2008). Gene-specific changes were confirmed at 13 loci, although the methylation status of the specific CpG sites could not be determined because the methodology used in this study is not able to detect methylation changes at a single-base resolution. However, the authors showed that in two genes, encoding protein-transport-related proteins, changes in DNA methylation state were associated with altered gene expression profiles. Currently, BPA effects on histone modifications have been examined in only one study. BPA was found to increase expression of the histone methyltransferase EZH2 (Enhancer of Zeste Homolog 2) mRNA and protein levels in a human breast cancer cell line, and EZH2 protein level in mammary glands of 6-week old mice exposed to BPA in utero (5mg/kg, i.p., during gestational days 9–16) (Doherty et al., 2010). Both in vitro and in vivo, these changes were accompanied by an increase in histone H3 trimethylation at lysine 27, which is the main histone modification catalyzed by EZH2 and is typically associated with gene repression (Vire et al., 2006; Zhu et al., 2009).
Future Directions in Studies of the Epigenetic Effects of BPA
Though there have been limited investigations of the epigenetic consequences of developmental exposure to BPA, the emerging data suggest that this chemical may induce changes in the DNA methylation status of genes, associated with altered gene expression. While the results of these studies are important as an initial indicator that BPA has a potential to alter the epigenome, further studies will be needed to confirm these initial findings, and expand the number of target genes explored. In particular, based on the neurodevelopmental and immune consequences of BPA exposure, analysis of epigenetic alterations and changes in gene expression in genes involved in the control of limbic, cognitive, neuroendocrine, and immune functions will be particularly relevant. In addition, it will be of great importance to explore the potential mechanisms by which BPA may affect the epigenome (see Figure 2). Currently, it is unclear whether epigenetic actions of BPA are related to its action at steroid hormone receptors. It is well-established that many downstream effector proteins of steroid receptor signaling pathways have the ability to modify histones and remodel chromatin, leading to changes in chromatin structure and consequent up- or down-regulation of steroid hormone-responsive genes (Biddie, 2010). There is some evidence suggesting that estrogen is able to dynamically change the methylation status of its target genes (Metivier et al., 2008). Therefore, it would not be surprising that the epigenetic effects of BPA could be explained, in part, through effects on steroid receptors, especially ERs. This hypothesis is also supported by evidence that other endocrine disuptors, with either estrogenic or androgenic activity, can alter the epigenome (Alworth et al., 2002; Anway et al., 2005; Li et al., 1997). An additional possibility to consider is that the epigenetic actions of BPA, which might result from steroid signaling-independent pathways, could facilitate the estrogenic actions of BPA. For example, Bromer et al. (2010) demonstrated that BPA decreases DNA methylation of the estrogen-responsive element in the Hoxa10 gene, resulting in an increase of binding of ERα to this target region and elevated responsiveness to estrogens. BPA may also enhance estrogenic activity through up-regulation of ER receptors in target tissues. The promoter region of the gene that encodes ERα is regulated by DNA methylation-mediated mechanisms both in neurons and during cancer progression (Champagne and Curley, 2008). Previous studies have shown that BPA may increase ERα expression in the brain and spleen (Kawai et al., 2007; Miao et al., 2008), and our preliminary data show that this occurs in a dose-dependent and a brain region-specific manner (Kundakovic and Champagne, unpublished data). Considering that ERα gene expression can be changed through altered DNA methylation both during prenatal (Westberry et al., 2010) and in the early postnatal period (Champagne et al., 2006), it would be interesting to explore if this mechanism also can affect ERα expression in response to early life BPA exposure. If this mechanism is relevant to BPA-induced effects, an intriguing possibility would be to assess whether these effects could be augmented using a maternal diet rich in methyl donors (as already shown for the Agouti gene and coat color phenotype in Avy mice), or though variation in postnatal mother-infant interactions, which have likewise been found to induce epigenetic changes in the ERα gene (Champagne et al., 2006).
Regardless of whether the epigenetic actions of BPA are carried out through steroid receptor-dependent or –independent pathways, one significant gap in our current knowledge is regarding the direct effector proteins that execute changes in DNA methylation. Though the evidence we have described suggests a hypomethylating effect of BPA, it is not known whether this effect is associated with changes in the expression or the activity of the enzymes that methylate DNA (DNA methyltransferases - DNMTs) or induced through promoting the activity of a yet to be identified protein with DNA demethylase activity. Interestingly, a recent study demonstrated that estradiol infusion can affect DNMT expression in the dorsal hippocampus (Zhao et al., 2010). However, Bromer et al. (2010) did not detect any change in DNMT expression that accompanied demethylation of the Hox10 gene in the uterus of 2-week old animals that were exposed to BPA in utero. Thus there are critical questions that need to be addressed to expand our knowledge of BPA’s potential epigenetic mechanisms of action: Do changes in DNMT levels or activity occur during specific developmental periods? If so, could these changes persist until adulthood in some tissues? Can active demethylation be somehow involved in BPA activity? What is the effect of BPA on histone modifications at the level of single genes, and how could this relate to corresponding changes in DNA methylation? Future research in this area will be needed to determine how BPA affects the epigenome and the consequence of these effects for disease susceptibility in adulthood.
Transgenerational Implications of Environmentally-Induced Epigenetic Marks
In the previous sections, we have focused our discussion on the effects of maternal exposure to BPA on neurodevelopmental, behavioral, and immune consequences in the first generation of offspring (F1). However, there is increasing concern that the adverse effects of endocrine disruptors can extend to several generations following only one gestational exposure. One of the best examples of this phenomenon emerged from studies of DES, a synthetic estrogen that was prescribed from the late 1940s to early 1970s for prevention of miscarriages in pregnant women. In humans, prenatal DES treatment is now known to be associated with an increased risk of reproductive anomalies and reproductive tract tumors, not only in individuals exposed to DES in utero, but also in the subsequent generation of offspring (Veurink et al., 2005). In mice, perinatal exposure to DES resulted in genital tract abnormalities and cancers in the first (F1) and second (F2) generation offspring. It has been suggested that these abnormalities may be associated with aberrant DNA methylation in developmental and cancer-related genes, implying that epigenetic alterations might underlie transgenerational adverse effects of DES (Newbold et al., 1998, 2000; Veurink et al., 2005). It is important to keep in mind that when mothers are exposed to a toxicant during pregnancy, the mother (F0 generation), the developing embryo (F1 generation), and the developing germ line of the F2 generation experience direct exposure to the compound (Jirtle and Skinner, 2007). Thus EDCs could possibly directly affect the epigenome of the F2 generation and the F2 epigenome may be particularly sensitive to epigenetic dysregulation during the period of dynamic re-programming of parental gene imprints occuring at the time of gametogenesis. However, the adverse effects of EDCs may even extend beyond the F2 generation, inducing changes in offspring that have not had direct exposure to the chemical. Traditional views on the transgenerational inheritance of phenotypes have focused on genetic transmission through the germ line. It was believed that epigenetic reprogramming events ensured that epigenetic marks acquired by the previous generation would be erased in primordial germ cells and early embryo so that a new organism would develop solely based on inherited genetic make-up. However, there is now evidence that DNA methylation at certain loci can escape reprogramming during development, providing the basis for the hypothesis that transgenerational inheritance may occur through epigenetic mechanisms both in rodents and humans (Lange and Schneider, 2010; Reik, 2007). In studies of the consequences of transient gestational exposure to the anti-androgenic fungicide, vinclozolin, during the period of gonadal sex determination, a decreased spermatogenic capacity and male subfertility were observed in F1 offspring and in three subsequent generations (F2–F4) (Anway et al., 2005). These abnormalities were associated with aberrant DNA methylation patterns in two genes in the sperm of F1–F3 generations (Anway et al., 2005) and genome-wide DNA methylation changes in the promoter regions of F3 generation sperm (Guerrero-Bosagna et al., 2010), and it has been proposed that transmission of the transgenerational phenotype occurred epigenetically through the male germ line. In addition, adult animals from all examined generations (F1–F4) developed disease states and tissue abnormalities, such as prostate and kidney disease, tumors and immune cell defects (Anway et al, 2006). These studies strongly imply that exposures to EDCs may have cumulative adverse effects on future generations, and that these effects could be mediated through epigenetic mechanisms. Therefore, in studies on the adverse effects of BPA, it would be appropriate and informative to incorporate a transgenerational study design which would allow for exploration of the possible mechanisms through which exposure to this compound could affect multiple generations.
Conclusions & Future Directions
Developmental BPA exposure can induce adverse effects on brain development, sexual differentiation and behavior, as well as immune function, and these effects may even extend to future generations. Though there is emerging evidence supporting the role of epigenetic mechanisms in these BPA-induced effects, further studies are needed to examine the correlation between BPA-induced epigenetic alterations, changes in gene expression, and phenotypic outcomes. Of particular importance will be the exploration of molecular and behavioral changes that occur in response to low-dose developmental BPA exposures, which may be relevant to environmental BPA exposures in humans (vom Saal and Welshons, 2006). Clearly, translating the findings from animal studies to health risks of environmental exposures to humans is not straightforward. One of the main obstacles in studying environmentally-induced or disease-associated epigenetic marks in humans, particularly when studying neurodevelopmental phenotypes, is the cell- and tissue-specificity of epigenetic changes that would require analysis of target tissues. Therefore, it will be important to determine whether epigenetic markers in more accessible tissues, such as blood lymphocytes, correlate with epigenetic markers in target tissues, such as the brain. Though speculative, in the case of BPA, the immune consequences of exposure may possibly allow for the use of immune activation and lymphocyte gene transcription patterns to predict the degree of early life exposure and impact of BPA in non-immune tissues. Carefully designed and well-controlled animal studies involving detailed tissue collection from BPA exposed animals, coupled with comprehensive analyses of gene expression and DNA methylation profiles in various tissues, will serve as a valuable tool for identifying clinical epigenetic markers that may be predicative of BPA effects in humans.
Acknowledgments
Funding: This research was supported by Grant Number DP2OD001674 from the Office of the Director, National Institutes of Health
References
- Adriani W, Seta DD, Dessi-Fulgheri F, Farabollini F, Laviola G. Altered profiles of spontaneous novelty seeking, impulsive behavior, and response to D-amphetamine in rats perinatally exposed to bisphenol A. Environ Health Perspect. 2003;111:395–401. doi: 10.1289/ehp.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh M, Ota F, Hosoi K, Kato M, Sakai T, Satter MA. Altered allergic cytokine and antibody response in mice treated with Bisphenol A. J Med Invest. 2006;53:70–80. doi: 10.2152/jmi.53.70. [DOI] [PubMed] [Google Scholar]
- Alworth LC, Howdeshell KL, Ruhlen RL, Day JK, Lubahn DB, Huang TH, Besch-Williford CL, vom Saal FS. Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol Appl Pharmacol. 2002;183:10–22. doi: 10.1006/taap.2002.9459. [DOI] [PubMed] [Google Scholar]
- Andersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, Bjerregaard P, Christiansen LB, Gissel B, Hummel R, Jorgensen EB, Korsgaard B, Le Guevel R, Leffers H, McLachlan J, Moller A, Nielsen JB, Olea N, Oles-Karasko A, Pakdel F, Pedersen KL, Perez P, Skakkeboek NE, Sonnenschein C, Soto AM, et al. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999;107(Suppl 1):89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147:S43–49. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Bernal AJ, Jirtle RL. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol. 2010;88:938–944. doi: 10.1002/bdra.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddie SC. Chromatin architecture and the regulation of nuclear receptor inducible transcription. J Neuroendocrinol. 2010 doi: 10.1111/j.1365-2826.2010.02079.x. [DOI] [PubMed] [Google Scholar]
- Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem. 2010;398:571–576. doi: 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24:2273–2280. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Maternal regulation of estrogen receptor alpha methylation. Curr Opin Pharmacol. 2008;8:735–739. doi: 10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Clayton EM, Todd M, Dowd JB, Aiello AE. The Impact of Bisphenol A and Triclosan on Immune Parameters in the US Population, NHANES 2003–2006. Environ Health Perspect. 2010 doi: 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115:1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010;58:754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(Suppl 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: An epigenetic mechanism linking endocrine disruptors to breast cancer. Hormones and Cancer. 2010;1:146–155. doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect. 2002;110(Suppl 3):409–414. doi: 10.1289/ehp.02110s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science. 2010;330:622–627. doi: 10.1126/science.1190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15:490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology. 2004;29:475–485. doi: 10.1016/s0306-4530(03)00055-6. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52:307–316. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Golebiewska A, Atkinson SP, Lako M, Armstrong L. Epigenetic landscaping during hESC differentiation to neural cells. Stem Cells. 2009;27:1298–1308. doi: 10.1002/stem.59. [DOI] [PubMed] [Google Scholar]
- Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front Neuroendocrinol. 2008;29:358–374. doi: 10.1016/j.yfrne.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Takano-Ishikawa Y, Ono H, Yoshida M, Yamaki K, Shinmoto H. Orally administered bisphenol A disturbed antigen specific immunoresponses in the naive condition. Biosci Biotechnol Biochem. 2007;71:2136–2143. doi: 10.1271/bbb.70004. [DOI] [PubMed] [Google Scholar]
- Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142:203–214. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi A, Ohtsu S, Muroi M, Tanamoto K. Effects of possible endocrine disrupting chemicals on bacterial component-induced activation of NF-kappaB. Biol Pharm Bull. 2006;29:2120–2122. doi: 10.1248/bpb.29.2120. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Cummings A. Mode of action: inhibition of androgen receptor function--vinclozolin-induced malformations in reproductive development. Crit Rev Toxicol. 2005;35:721–726. doi: 10.1080/10408440591007377. [DOI] [PubMed] [Google Scholar]
- Kawai K, Murakami S, Senba E, Yamanaka T, Fujiwara Y, Arimura C, Nozaki T, Takii M, Kubo C. Changes in estrogen receptors alpha and beta expression in the brain of mice exposed prenatally to bisphenol A. Regul Toxicol Pharmacol. 2007;47:166–170. doi: 10.1016/j.yrtph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Kawai K, Nozaki T, Nishikata H, Aou S, Takii M, Kubo C. Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol A. Environ Health Perspect. 2003;111:175–178. doi: 10.1289/ehp.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kubo K, Arai O, Ogata R, Omura M, Hori T, Aou S. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behavior in the rat. Neurosci Lett. 2001;304:73–76. doi: 10.1016/s0304-3940(01)01760-8. [DOI] [PubMed] [Google Scholar]
- Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kwack SJ, Kim KB, Kim HS, Yoon KS, Lee BM. Risk assessment of soybean-based phytoestrogens. J Toxicol Environ Health A. 2009;72:1254–1261. doi: 10.1080/15287390903212212. [DOI] [PubMed] [Google Scholar]
- Lange UC, Schneider R. What an epigenome remembers. Bioessays. 2010;32:659–668. doi: 10.1002/bies.201000030. [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, McLachlan JA, Negishi M. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57:4356–4359. [PubMed] [Google Scholar]
- Liu J, Casaccia P. Epigenetic regulation of oligodendrocyte identity. Trends Neurosci. 2010;33:193–201. doi: 10.1016/j.tins.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan JA. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev. 2001;22:319–341. doi: 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, Benes V, Jeltsch A, Gannon F, Salbert G. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- Miao S, Gao Z, Kou Z, Xu G, Su C, Liu N. Influence of bisphenol a on developing rat estrogen receptors and some cytokines in rats: a two-generational study. J Toxicol Environ Health A. 2008;71:1000–1008. doi: 10.1080/15287390801907467. [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Mills PK, Yang R. Regression analysis of pesticide use and breast cancer incidence in California Latinas. J Environ Health. 2006;68:15–22. quiz 43–14. [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Sugimoto T, Fushiki S. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci Lett. 2007;420:100–105. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Yaoi T, Fujiwara Y, Sugimoto T, Fushiki S. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of Bisphenol A. J Neurosci Res. 2006;84:1197–1205. doi: 10.1002/jnr.21020. [DOI] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Suzaki S, Maeda H, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ Health Perspect. 2004;112:1159–1164. doi: 10.1289/ehp.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19:1655–1663. doi: 10.1093/carcin/19.9.1655. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 2000;21:1355–1363. [PubMed] [Google Scholar]
- Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110(Suppl 3):415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrini S, Belloni V, Della Seta D, Farabollini F, Giannelli G, Dessi-Fulgheri F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull. 2005;65:261–266. doi: 10.1016/j.brainresbull.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS. Animal models to study environmental epigenetics. Biol Reprod. 2010;82:473–488. doi: 10.1095/biolreprod.109.080952. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J. 2007;21:239–246. doi: 10.1096/fj.06-6635com. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Stanisic V, Lonard DM, O’Malley BW. Modulation of steroid hormone receptor activity. Prog Brain Res. 2010;181:153–176. doi: 10.1016/S0079-6123(08)81009-6. [DOI] [PubMed] [Google Scholar]
- Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol Lett. 2006;167:95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Tando S, Itoh K, Yaoi T, Ikeda J, Fujiwara Y, Fushiki S. Effects of pre- and neonatal exposure to bisphenol A on murine brain development. Brain Dev. 2007;29:352–356. doi: 10.1016/j.braindev.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Welshons WV, Vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod Toxicol. 2008;25:169–176. doi: 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veurink M, Koster M, Berg LT. The history of DES, lessons to be learned. Pharm World Sci. 2005;27:139–143. doi: 10.1007/s11096-005-3663-z. [DOI] [PubMed] [Google Scholar]
- Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ Res. 2006;100:50–76. doi: 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zhang J, Wang YM, Ye YP, Luo QQ. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm Behav. 2010;58:326–333. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Yan H, Takamoto M, Sugane K. Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect. 2008;116:514–519. doi: 10.1289/ehp.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376:563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Yoshino S, Yamaki K, Yanagisawa R, Takano H, Hayashi H, Mori Y. Effects of bisphenol A on antigen-specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice. Br J Pharmacol. 2003;138:1271–1276. doi: 10.1038/sj.bjp.0705166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci U S A. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Edwards RJ, Boobis AR. Increased expression of histone proteins during estrogen-mediated cell proliferation. Environ Health Perspect. 2009;117:928–934. doi: 10.1289/ehp.0800109. [DOI] [PMC free article] [PubMed] [Google Scholar]