Abstract
Detailed studies of apolipoprotein E (apoE)-containing lipoproteins in abetalipoproteinemia have been performed in an attempt to resolve the apparent paradox of a suppressed low density lipoprotein (LDL) receptor pathway in the absence of apoB-containing lipoproteins. It was hypothesized that apoE-containing high density lipoproteins (HDL) in abetalipoproteinemia might functionally substitute for LDL in regulation of cholesterol metabolism in these patients.
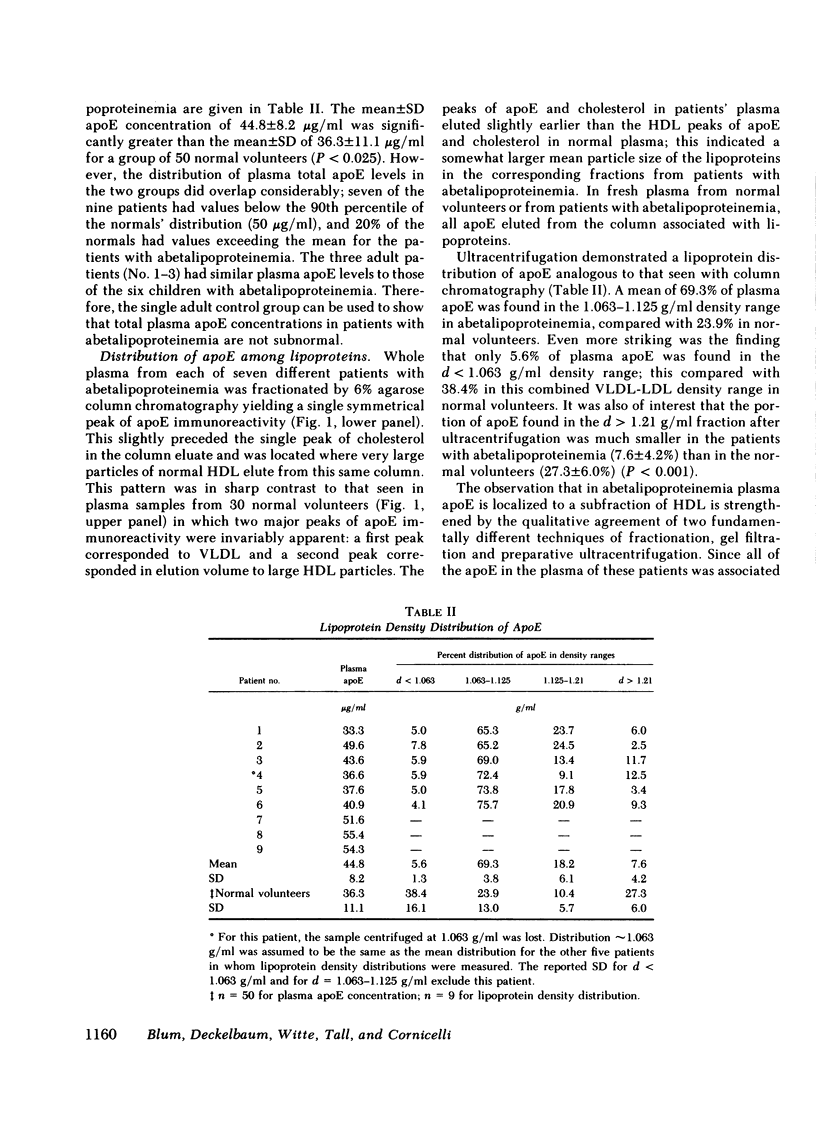
The mean (±standard deviation) plasma concentration of apoE in nine patients with abetalipoproteinemia was 44.8±8.2 μg/ml, slightly higher than the corresponding value for a group of 50 normal volunteers, 36.3±11 μg/ml. Fractionation of plasma lipoproteins by agarose column chromatography or by ultracentrifugation indicated that in abetalipoproteinemia, plasma apoE was restricted to a subfraction of HDL. This was in contrast to the results obtained with plasma from 30 normal volunteers, in whom apoE was distributed between very low density lipoproteins (VLDL) and HDL. Consequently, the mean apoE content of HDL in abetalipoproteinemia (44.8 μg/ml) was more than twice that found in the normal volunteers (20.3 μg/ml).
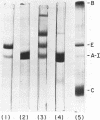
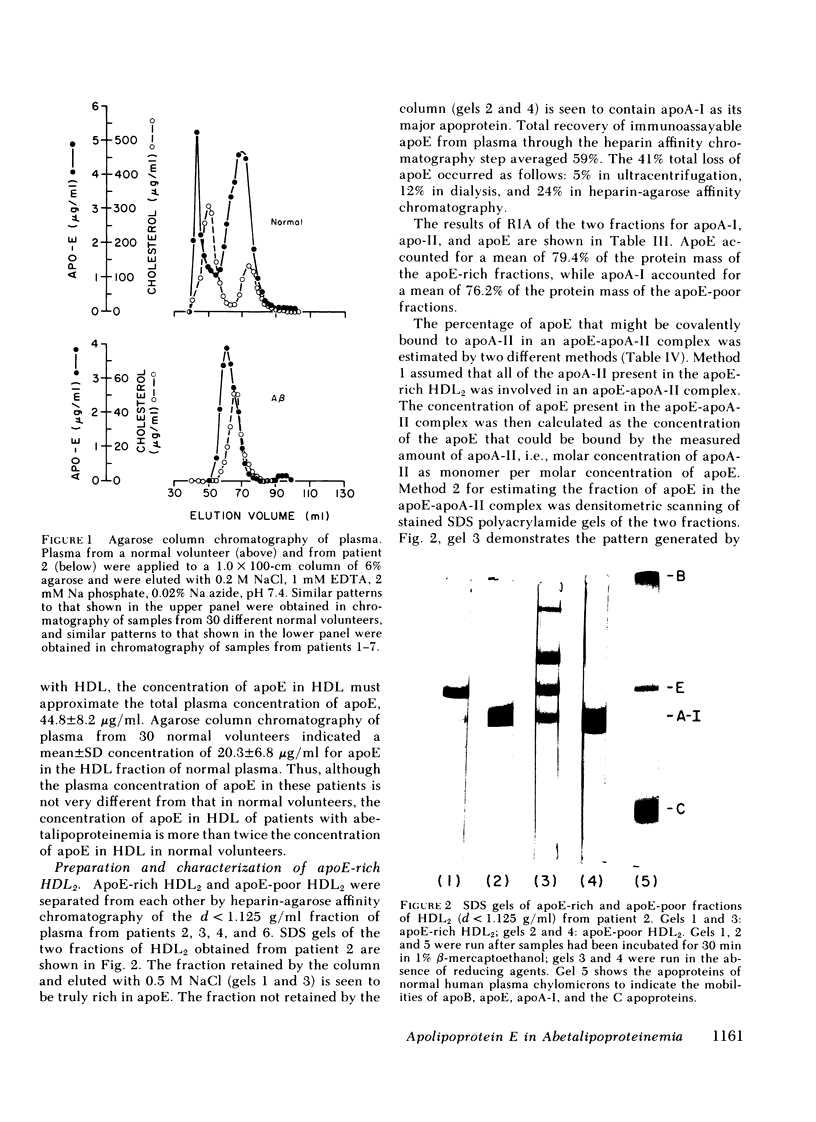
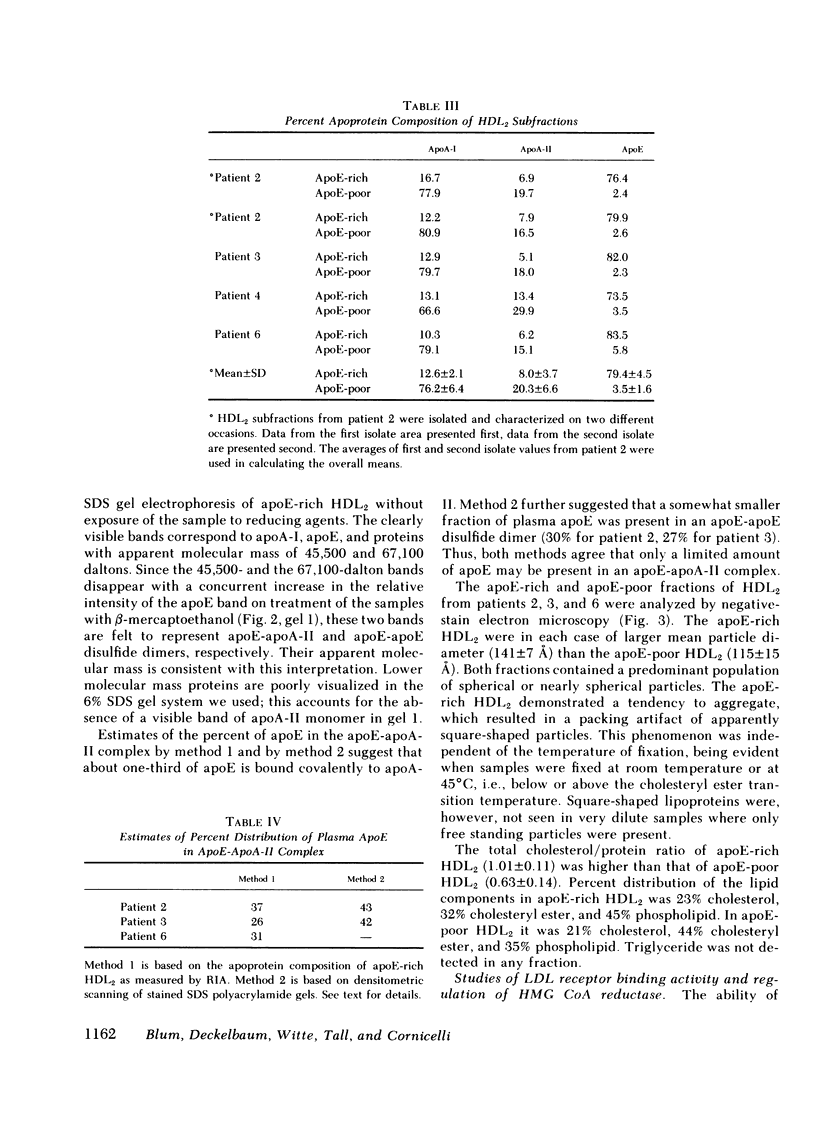
ApoE-rich and apoE-poor subfractions of HDL2 were isolated by heparin-agarose affinity chromatography. ApoE comprised a mean of 81% of the protein mass of the apoE-rich subfraction. Compared with the apoE-poor subfraction, the apoE-rich HDL2 was of larger mean particle diameter (141±7 vs. 115±15 Å) and had a higher ratio of total cholesterol/protein (1.01±0.11 vs. 0.63±0.14).
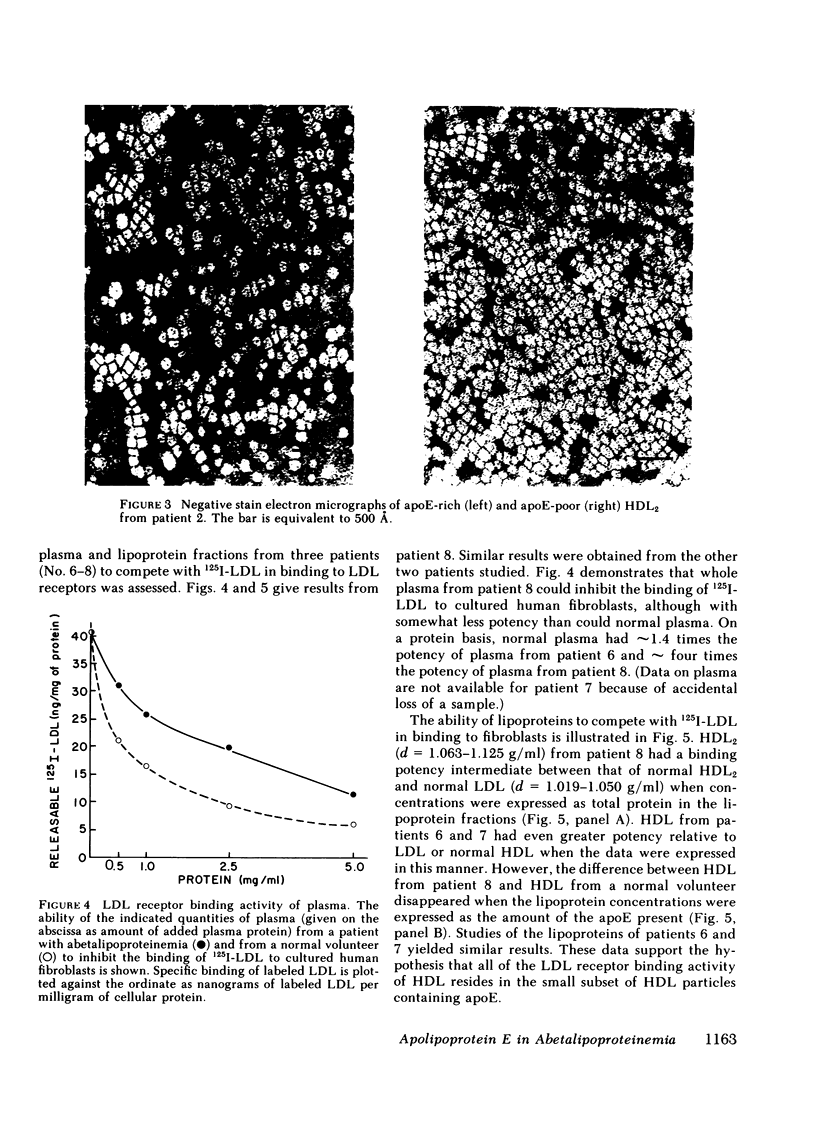
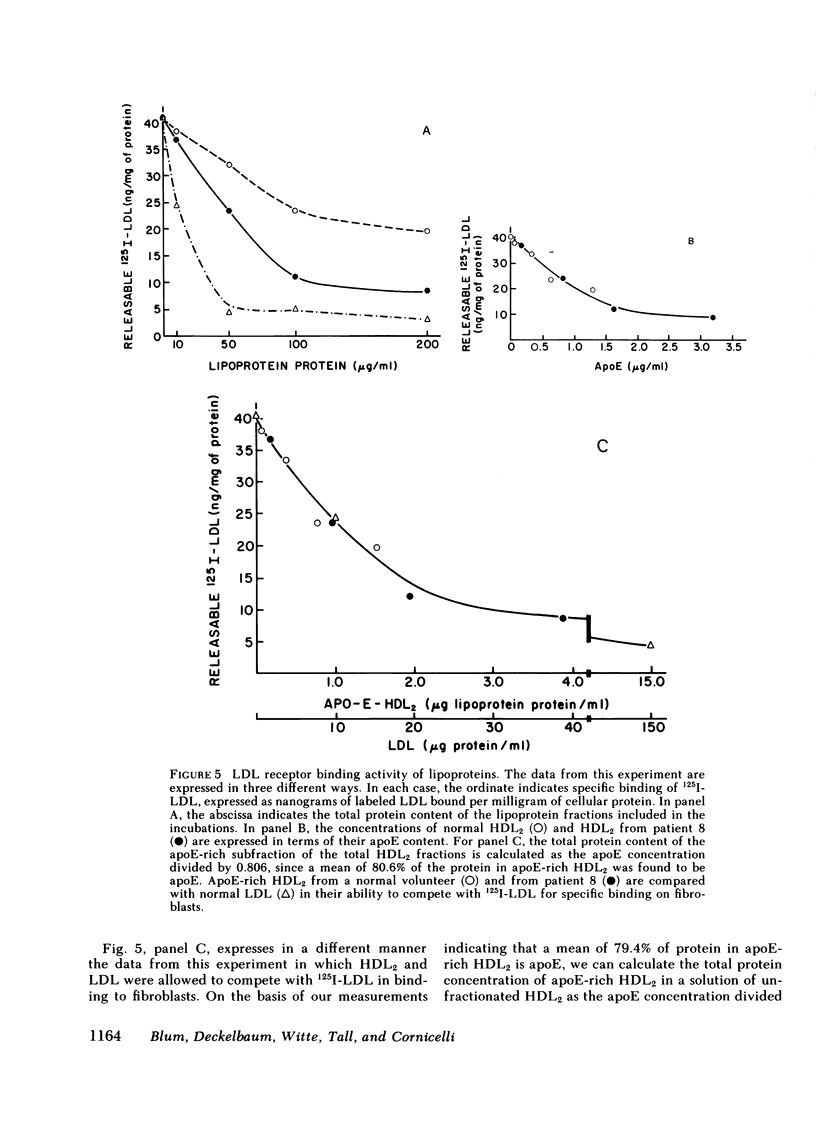
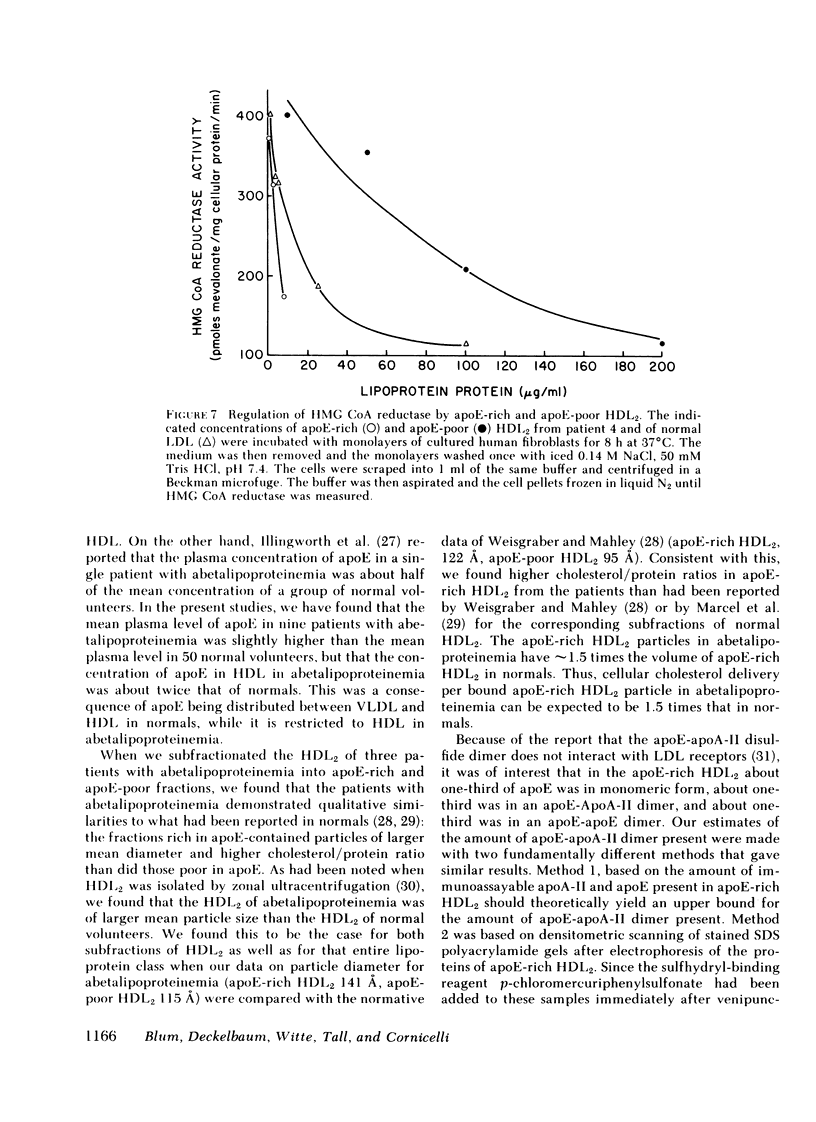
Plasma and HDL fractions from three patients were studied with respect to their ability to compete with 125I-LDL in specific binding to receptors on cultured human fibroblasts. The binding activity of plasma from patients (per milligram of protein) was about half that of plasma from normal volunteers. All binding activity in the patients' plasma was found to reside in the HDL fraction. The binding activity of the patients' HDL (on a total protein basis) was intermediate between that of normal HDL and normal LDL. However, the large differences in binding between patients' HDL and normal HDL entirely disappeared when data were expressed in terms of the apoE content of these lipoproteins. This suggested that the binding activity was restricted to that subfraction of HDL particles that contain apoE. These apoE-rich HDL particles had calculated binding potencies per milligram of protein 10-25 times that of normal LDL. Direct binding studies using 125I-apoE-rich HDL2 and 125I-apoE-poor HDL2, confirmed the suggestion that binding is restricted to the subfraction of HDL particles containing apoE. The apoE-rich HDL2 were found to be very potent inhibitors of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase activity in cultured fibroblasts, providing direct evidence of the ability of these lipoproteins to regulate cholesterol metabolism.
On the basis of binding potencies of apoE-rich HDL, apoE concentrations, and the composition of apoE-rich HDL, it could be calculated that apoE-rich HDL in abetalipoproteinemia have a capacity to deliver cholesterol to tissues via the LDL receptor pathway equivalent to an LDL concentration of 50-150 mg/dl of cholesterol. Thus, these apoE-rich lipoproteins are capable of producing the suppression of cholesterol synthesis and LDL receptor activity previously observed in abetalipoproteinemia.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr 3-Hydroxy-3-methylglutaryl coenzyme A reductase from avian liver. Catalytic properties. Biochim Biophys Acta. 1979 Jan 29;572(1):83–94. doi: 10.1016/0005-2760(79)90202-9. [DOI] [PubMed] [Google Scholar]
- Bersot T. P., Mahley R. W., Brown M. S., Goldstein J. L. Interaction of swine lipoproteins with the low density lipoprotein receptor in human fibroblasts. J Biol Chem. 1976 Apr 25;251(8):2395–2398. [PubMed] [Google Scholar]
- Bierman E. L., Albers J. Regulation of low density lipoprotein receptor activity by cultured human arterial smooth muscle cells. Biochim Biophys Acta. 1977 Jul 20;488(1):152–160. doi: 10.1016/0005-2760(77)90133-3. [DOI] [PubMed] [Google Scholar]
- Blum C. B., Aron L., Sciacca R. Radioimmunoassay studies of human apolipoprotein E. J Clin Invest. 1980 Dec;66(6):1240–1250. doi: 10.1172/JCI109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Analysis of a mutant strain of human fibroblasts with a defect in the internalization of receptor-bound low density lipoprotein. Cell. 1976 Dec;9(4 Pt 2):663–674. doi: 10.1016/0092-8674(76)90130-6. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975 Nov;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- CHIAMORI N., HENRY R. J. Study of the ferric chloride method for determination of total cholesterol and cholesterol esters. Am J Clin Pathol. 1959 Apr;31(4):305–309. doi: 10.1093/ajcp/31.4.305. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Faust J. R., Beaudet A. L., Brown M. S. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975 Nov 10;250(21):8487–8495. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Brown M. S., Innerarity T. L., Mahley R. W. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J Biol Chem. 1980 Mar 10;255(5):1839–1848. [PubMed] [Google Scholar]
- Guertler L. S., St Clair R. W. Low density lipoprotein receptor activity on skin fibroblasts from rhesus monkeys with diet-induced or spontaneous hypercholesterolemia. J Biol Chem. 1980 Jan 10;255(1):92–99. [PubMed] [Google Scholar]
- Ho Y. K., Brown M. S., Kayden H. J., Goldstein J. L. Binding, internalization, and hydrolysis of low density lipoprotein in long-term lymphoid cell lines from a normal subject and a patient with homozygous familial hypercholesterolemia. J Exp Med. 1976 Aug 1;144(2):444–455. doi: 10.1084/jem.144.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Faust J. R., Bilheimer D. W., Brown M. S., Goldstein J. L. Regulation of cholesterol synthesis by low density lipoprotein in isolated human lymphocytes. Comparison of cells from normal subjects and patients with homozygous familial hypercholesterolemia and abetalipoproteinemia. J Exp Med. 1977 Jun 1;145(6):1531–1549. doi: 10.1084/jem.145.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth D. R., Connor W. E., Alaupovic P. High density lipoprotein metabolism in a patient with abetalipoproteninemia. Ann Nutr Metab. 1981;25(1):1–10. doi: 10.1159/000176473. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Connor W. E., Buist N. R., Jhaveri B. M., Lin D. S., McMurry M. P. Sterol balance in abetalipoproteinemia: studies in a patient with homozygous familial hypobetalipoproteinemia. Metabolism. 1979 Nov;28(11):1152–1160. doi: 10.1016/0026-0495(79)90155-0. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Connor W. E., Lin D. S., Diliberti J. Lipid metabolism in abetalipoproteinemia: a study of cholesterol absorption and sterol balance in two patients. Gastroenterology. 1980 Jan;78(1):68–75. [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W., Weisgraber K. H., Bersot T. P. Apoprotein (E--A-II) complex of human plasma lipoproteins. II. Receptor binding activity of a high density lipoprotein subfraction modulated by the apo(E--A-II) complex. J Biol Chem. 1978 Sep 10;253(17):6289–6295. [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J Biol Chem. 1979 May 25;254(10):4186–4190. [PubMed] [Google Scholar]
- Kayden H. J., Hatam L., Beratis N. G. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and the esterification of cholesterol in human long term lymphoid cell lines. Biochemistry. 1976 Feb 10;15(3):521–528. doi: 10.1021/bi00648a011. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Faust J. R., Brown M. S., Goldstein J. L. Low density lipoprotein receptors in bovine adrenal cortex. I. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured adrenocortical cells. Endocrinology. 1979 Mar;104(3):599–609. doi: 10.1210/endo-104-3-599. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Interaction of canine and swine lipoproteins with the low density lipoprotein receptor of fibroblasts as correlated with heparin/manganese precipitability. J Biol Chem. 1977 Jun 10;252(11):3980–3986. [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P., Hardman D. A., Hamilton R. L., Dalal K. B. Normotriglyceridemic abetalipoproteinemia. absence of the B-100 apolipoprotein. J Clin Invest. 1981 May;67(5):1441–1450. doi: 10.1172/JCI110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel Y. L., Vezina C., Emond D., Suzue G. Heterogeneity of human high density lipoprotein: presence of lipoproteins with and without apoE and their roles as substrates for lecithin:cholesterol acyltransferase reaction. Proc Natl Acad Sci U S A. 1980 May;77(5):2969–2973. doi: 10.1073/pnas.77.5.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myant N. B., Reichl D., Lloyd J. K. Sterol balance in a patient with abetalipoproteinaemia. Atherosclerosis. 1978 Apr;29(4):509–512. doi: 10.1016/0021-9150(78)90180-6. [DOI] [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Arnold K. S., Mahley R. W. Rate and equilibrium constants for binding of apo-E HDLc (a cholesterol-induced lipoprotein) and low density lipoproteins to human fibroblasts: evidence for multiple receptor binding of apo-E HDLc. Proc Natl Acad Sci U S A. 1979 May;76(5):2311–2315. doi: 10.1073/pnas.76.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichl D., Myant N. B., Brown M. S., Goldstein J. L. Biologically active low density lipoprotein in human peripheral lymph. J Clin Invest. 1978 Jan;61(1):64–71. doi: 10.1172/JCI108926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichl D., Myant N. B., Lloyd J. K. Surface binding and catabolism of low-density lipoprotein by circulating lymphocytes from patients with abetalipoproteinaemia, with observations on sterol synthesis in lymphocytes from one patient. Biochim Biophys Acta. 1978 Jul 25;530(1):124–131. doi: 10.1016/0005-2760(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Reichl D., Myant N. B., Pflug J. J. Concentration of lipoproteins containing apolipoprotein B in human peripheral lymph. Biochim Biophys Acta. 1977 Oct 24;489(1):98–105. doi: 10.1016/0005-2760(77)90236-3. [DOI] [PubMed] [Google Scholar]
- Reichl D., Simons L. A., Myant N. B., Pflug J. J., Mills G. L. The lipids and lipoproteins of human peripheral lymph, with observations on the transport of cholesterol from plasma and tissues into lymph. Clin Sci Mol Med. 1973 Sep;45(3):313–329. doi: 10.1042/cs0450313. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Aggerbeck L. P., Kruski A. W., Lim C. T., Kayden H. J. A study of the abnormal lipoproteins in abetalipoproteinemia. J Clin Invest. 1974 Feb;53(2):440–453. doi: 10.1172/JCI107578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., Weinstein D. B., Stein Y., Steinberg D. Binding, internalization, and degradation of low density lipoprotein by normal human fibroblasts and by fibroblasts from a case of homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1976 Jan;73(1):14–18. doi: 10.1073/pnas.73.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W. Subfractionation of human high density lipoproteins by heparin-Sepharose affinity chromatography. J Lipid Res. 1980 Mar;21(3):316–325. [PubMed] [Google Scholar]
- Witte L. D., Cornicelli J. A. Platelet-derived growth factor stimulates low density lipoprotein receptor activity in cultured human fibroblasts. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5962–5966. doi: 10.1073/pnas.77.10.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]