Abstract
We have previously shown a marked difference in the inflammatory response to human C5a or C5a des arginine (Arg) instilled in rabbit lungs. These studies raised the question of where C5a and C5a des Arg are processes in vivo and what role neutrophils may play in the tissue distribution of these two mediators. After intravenous injection of purified, biologically active 125I-C5a or 125I-C5a des Arg, adult rabbits were serially bled and then killed at various time intervals. Although greater than 50% of the injected radioactivity was cleared from the circulation within 2 min for both mediators, C5a des Arg persisted in the circulation longer than C5a. C5a instillation caused an acute neutropenia, whereas C5a des Arg caused a less severe and more prolonged neutropenia, preceding a neutrophilic response observed with both mediators. Clearance of the mediators was primarily seen in the highly vascularized organs: the lung, spleen, liver, and kidney. A time-dependent accumulation was seen initially in the lung, followed by the spleen, liver, and kidney. Histologic examination showed a marked increase in the number of neutrophils within the lung and spleen. Depletion of circulating neutrophils by nitrogen mustard pretreatment of rabbits showed no change in the amount of labeled mediator bound in the lung, whereas splenic accumulation was dependent on the presence of neutrophils. These results indicate that C5a and C5a des Arg are rapidly removed from the circulation by specific accumulation in vascularized tissues. Clearance by the lung was not affected by neutrophil depletion, whereas clearance by the spleen was dependent on neutrophils. These experiments further suggest there are neutrophil-dependent and neutrophil-independent mechanisms involved in the removal of C5a and C5a des Arg from the circulation and that binding of C5 fragments in the pulmonary vasculature may precede and then induce neutrophil sequestration.
Full text
PDF


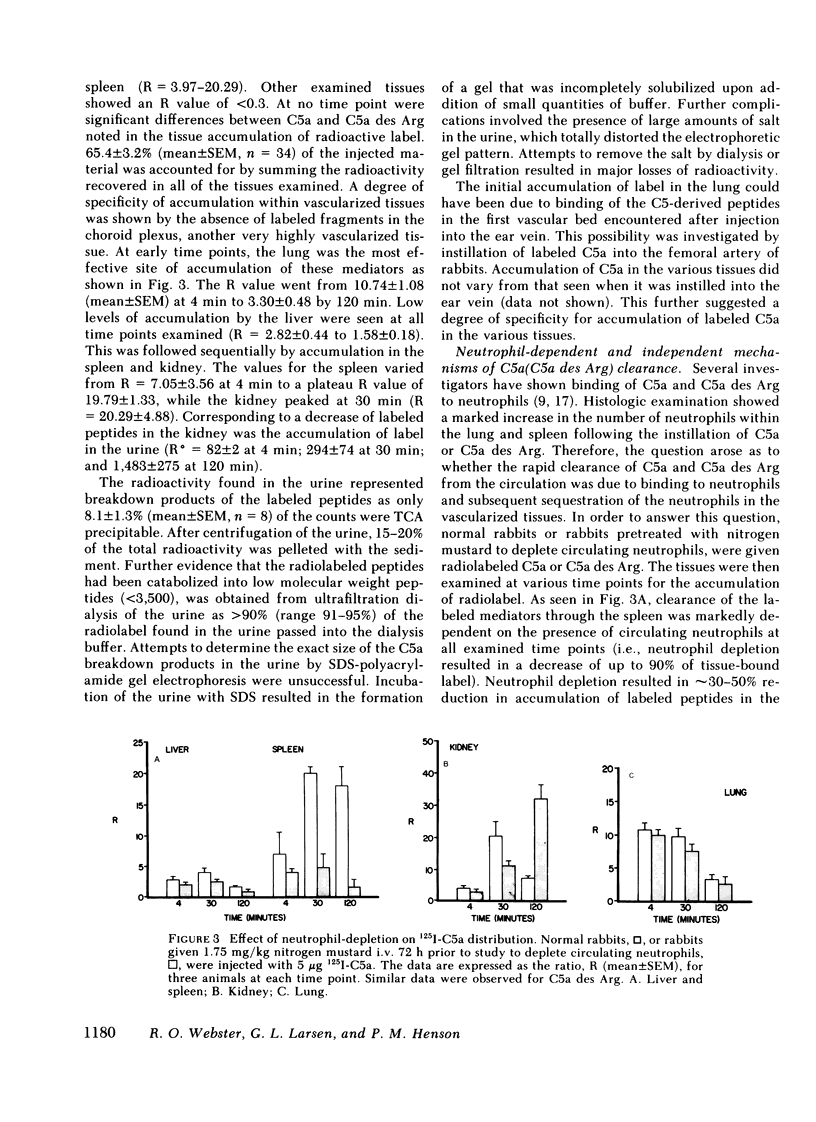
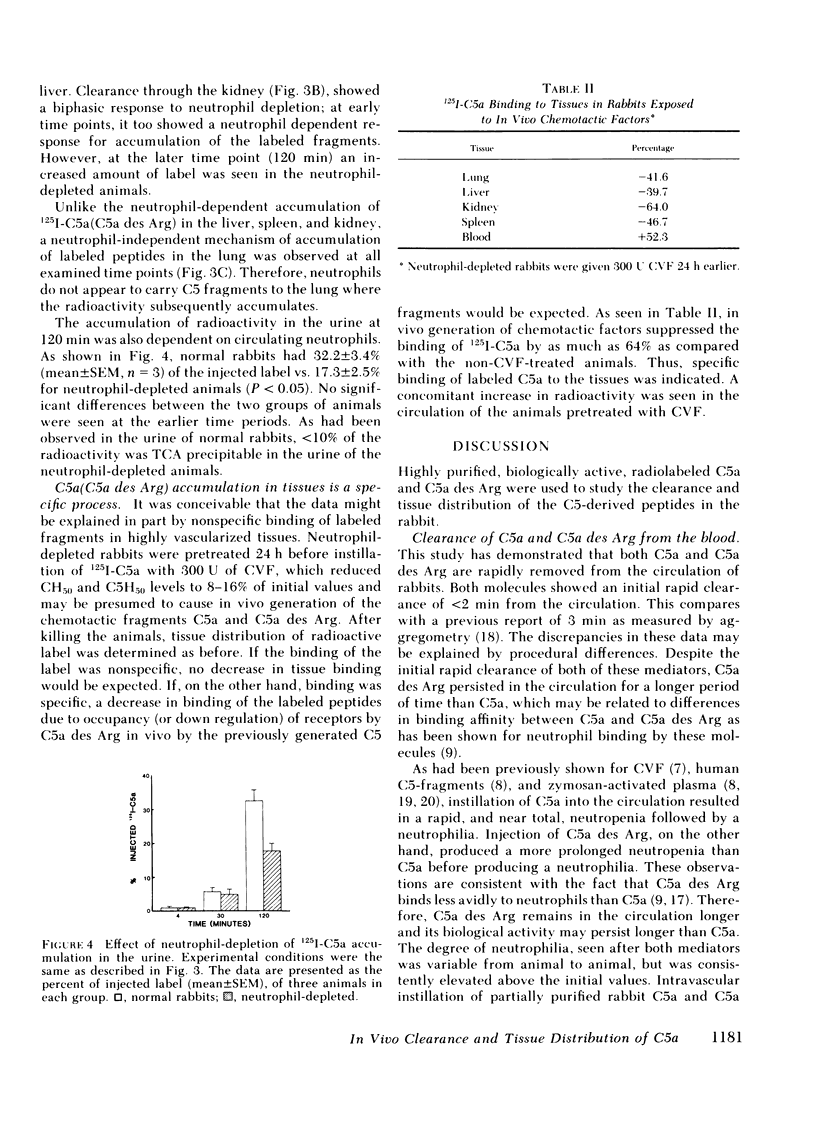


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUDE A. I., CAREY F. J., ZALESKY M. Studies with radioactive endotoxin. II. Correlation of physiologic effects with distribution of radioactivity in rabbits injected with radioactive sodium chromate. J Clin Invest. 1955 Jun;34(6):858–866. doi: 10.1172/JCI103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Human C5a and C5a analogs as probes of the neutrophil C5a receptor. Mol Immunol. 1980 Feb;17(2):151–161. doi: 10.1016/0161-5890(80)90067-x. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Gerard C., Chenoweth D. E., Hugli T. E. Response of human neutrophils to C5a: a role for the oligosaccharide moiety of human C5ades Arg-74 but not of C5a in biologic activity. J Immunol. 1981 Nov;127(5):1978–1982. [PubMed] [Google Scholar]
- Gerard C., Hugli T. E. Anaphylatoxin from the fifth component of porcine complement. Purification and partial chemical characterization. J Biol Chem. 1979 Jul 25;254(14):6346–6351. [PubMed] [Google Scholar]
- Ghebrehiwet B., Müller-Eberhard H. J. C3e: an acidic fragment of human C3 with leukocytosis-inducing activity. J Immunol. 1979 Aug;123(2):616–621. [PubMed] [Google Scholar]
- Giclas P. C., Keeling P. J., Henson P. M. Isolation and characterization of the third and fifth components of rabbit complement. Mol Immunol. 1981 Feb;18(2):113–123. doi: 10.1016/0161-5890(81)90077-8. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Harris P. D., Wayland J. H., Craddock P. R., Jacob H. S. Complement-induced granulocyte aggregation in vivo. Am J Pathol. 1981 Feb;102(2):146–150. [PMC free article] [PubMed] [Google Scholar]
- Hohn D. C., Meyers A. J., Gherini S. T., Beckmann A., Markison R. E., Churg A. M. Production of acute pulmonary injury by leukocytes and activated complement. Surgery. 1980 Jul;88(1):48–58. [PubMed] [Google Scholar]
- Hoover R. L., Folger R., Haering W. A., Ware B. R., Karnovsky M. J. Adhesion of leukocytes to endothelium: roles of divalent cations, surface charge, chemotactic agents and substrate. J Cell Sci. 1980 Oct;45:73–86. doi: 10.1242/jcs.45.1.73. [DOI] [PubMed] [Google Scholar]
- Larsen G. L., McCarthy K., Webster R. O., Henson J., Henson P. M. A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am J Pathol. 1980 Jul;100(1):179–192. [PMC free article] [PubMed] [Google Scholar]
- Lollar P., Owen W. G. Clearance of thrombin from circulation in rabbits by high-affinity binding sites on endothelium. Possible role in the inactivation of thrombin by antithrombin III. J Clin Invest. 1980 Dec;66(6):1222–1230. doi: 10.1172/JCI109973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musson R. A., Morrison D. C., Ulevitch R. J. Distribution of endotoxin (lipopolysaccharide) in the tissues of lipopolysaccharide-responsive and -unresponsive mice. Infect Immun. 1978 Aug;21(2):448–457. doi: 10.1128/iai.21.2.448-457.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Ward P. A. Neutropenia induced by systemic infusion of chemotactic factors. J Immunol. 1977 May;118(5):1586–1589. [PubMed] [Google Scholar]
- Rother K. Leucocyte mobilizing factor: a new biological activity derived from the third component of complement. Eur J Immunol. 1972 Dec;2(6):550–558. doi: 10.1002/eji.1830020615. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Roberts M. F., Ulevitch R. J., Henson P., Dennis E. A. Phospholipase A2 contamination of cobra venom factor preparations. Biologic role in complement-dependent in vivo reactions and inactivation with p-bromophenacyl bromide. Am J Pathol. 1978 Jun;91(3):517–530. [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Cochrane C. G., Henson P. M., Morrison D. C., Doe W. F. Mediation systems in bacterial lipopolysaccharide-induced hypotension and disseminated intravascular coagulation. I. The role of complement. J Exp Med. 1975 Dec 1;142(6):1570–1590. doi: 10.1084/jem.142.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Webster R. O., Larsen G. L., Mitchell B. C., Goins A. J., Henson P. M. Absence of inflammatory lung injury in rabbits challenged intravascularly with complement-derived chemotactic factors. Am Rev Respir Dis. 1982 Mar;125(3):335–340. doi: 10.1164/arrd.1982.125.3.335. [DOI] [PubMed] [Google Scholar]
- Weisdorf D. J., Hammerschmidt D. E., Jacob H. S., Craddock P. R. Rapid in vivo clearance of C5ades arg: a possible protective mechanism against complement-mediated tissue injury. J Lab Clin Med. 1981 Dec;98(6):823–830. [PubMed] [Google Scholar]


