Abstract
The role of the enzyme hepatic triglyceride lipase was investigated in a primate model, the cynomolgus monkey. Antisera produced against human postheparin hepatic lipase fully inhibited cynomolgus monkey posttheparin plasma hepatic triglyceride lipase activity. Lipoprotein lipase activity was not inhibited by this antisera. Hepatic triglyceride lipase activity in liver biopsies was decreased by 65-90% after intravenous infusion of this antisera into the cynomolgus monkey. After a 3-h infusion of the antisera, analytic ultracentrifugation revealed an increase in mass of very low density lipoproteins (Sf 20-400). Very low density lipoprotein triglyceride isolated by isopycnic ultracentrifugation increased by 60-300%. Analytic ultracentrifugation revealed an increase in mass of lipoproteins with flotation greater than Sf 9 (n = 4). The total mass of intermediate density lipoproteins (Sf 12-20) approximately doubled during the 3 h of in vivo enzyme inhibition. While more rapidly floating low density lipoproteins (Sf 9-12) increased, the total mass of low density lipoproteins decreased after infusion of the antibodies. The changes in high density lipoproteins did not differ from those in control experiments.
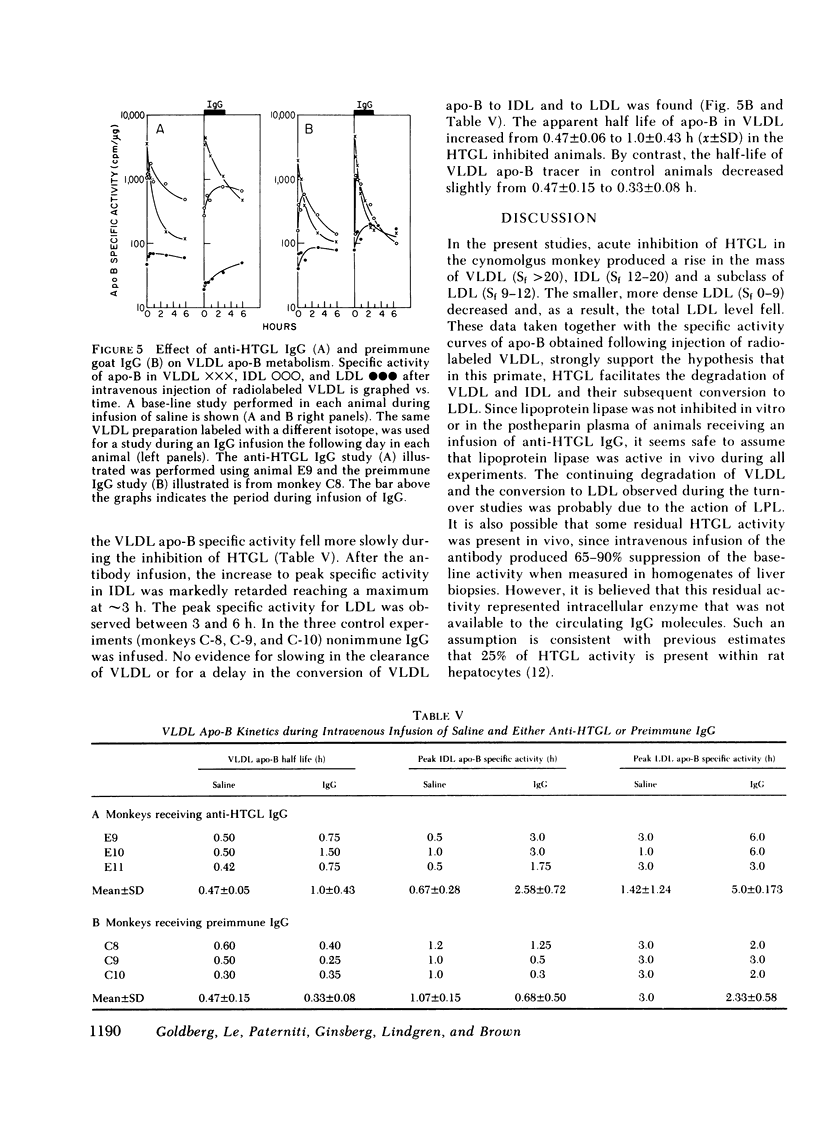
In order to determine whether the increases of plasma concentrations of very low density lipoproteins were due to an increase in the rate of synthesis or a decrease in the rate of clearance of these particles, the metabolism of radiolabeled homologous very low density lipoproteins was studied during intravenous infusion of immunoglobulin G prepared from the antisera against hepatic triglyceride lipase (n = 3) or preimmune goat sera (n = 3). Studies performed in the same animals during saline infusion were used as controls for each immunoglobulin infusion. There was a twofold increase in the apparent half-life of the very low density lipoprotein apolipoprotein-B tracer in animals receiving the antibody, consistent with a decreased catabolism of very low density lipoproteins. Concomitantly, the rise in low density lipoprotein apoprotein-B specific activity was markedly delayed. None of these changes were observed during infusion of preimmune immunoglobulin G.
Hepatic triglyceride lipase participates with lipoprotein lipase in the hydrolysis of the lipid in very low density lipoproteins, intermediate density lipoproteins, and the larger low density lipoproteins (Sf 9-12). Thus, hepatic triglyceride lipase appears to function in a parallel role with lipoprotein lipase in the conversion of very low density and intermediate density lipoproteins to low density lipoproteins (Sf 0-9).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
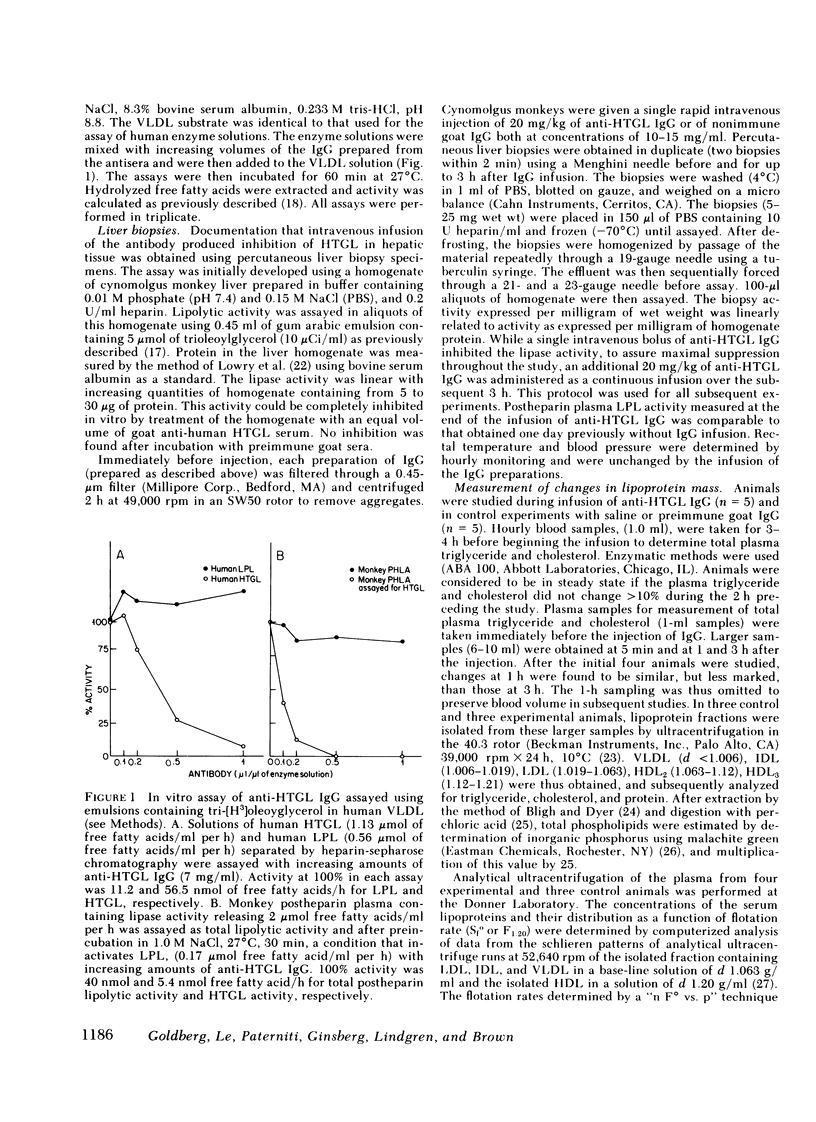
- Augustin J., Freeze H., Tejada P., Brown W. V. A comparison of molecular properties of hepatic triglyceride lipase and lipoprotein lipase from human post-heparin plasma. J Biol Chem. 1978 May 10;253(9):2912–2920. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baginsky M. L., Brown W. V. A new method for the measurement of lipoprotein lipase in postheparin plasma using sodium dodecyl sulfate for the inactivation of hepatic triglyceride lipase. J Lipid Res. 1979 May;20(4):548–556. [PubMed] [Google Scholar]
- Berman M., Hall M., 3rd, Levy R. I., Eisenberg S., Bilheimer D. W., Phair R. D., Goebel R. H. Metabolsim of apoB and apoC lipoproteins in man: kinetic studies in normal and hyperlipoproteininemic subjects. J Lipid Res. 1978 Jan;19(1):38–56. [PubMed] [Google Scholar]
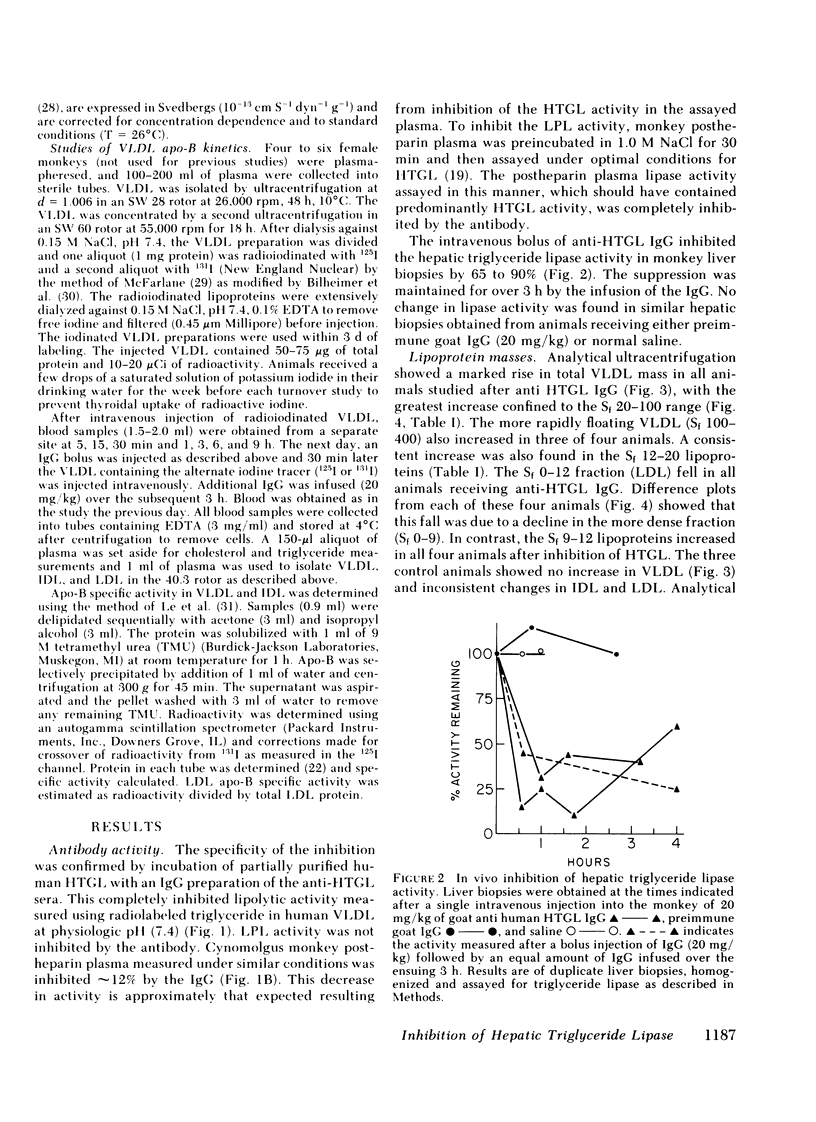
- Berry E. M., Aldini R., Bar-On H., Eisenberg S. Role of the liver in the degradation of very low density lipoproteins: a study of lipolysis by heparin releasable liver lipase and uptake during isolated rat liver perfusion. Eur J Clin Invest. 1981 Jun;11(3):151–159. doi: 10.1111/j.1365-2362.1981.tb01834.x. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
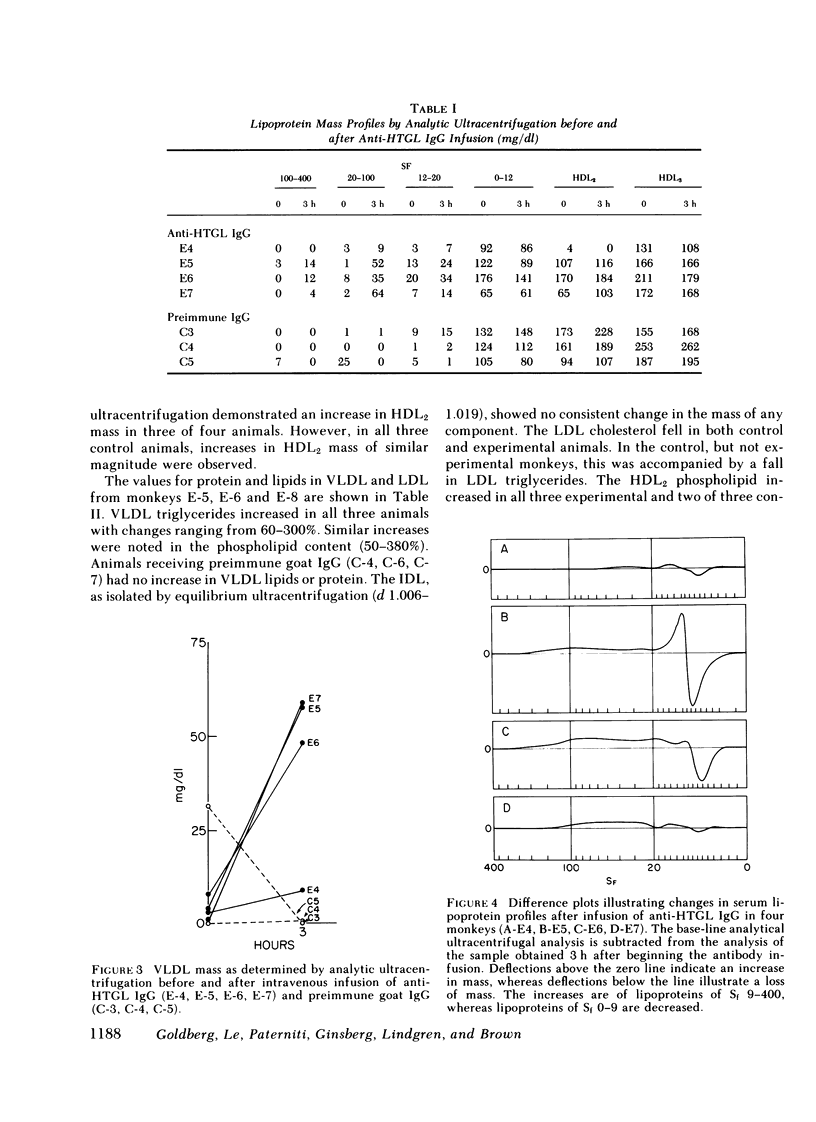
- Boberg J., Augustin J., Baginsky M. L., Tejada P., Brown W. V. Quantitative determination of hepatic and lipoprotein lipase activities from human postheparin plasma. J Lipid Res. 1977 Jul;18(4):544–547. [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Cooper A. D. The metabolism of chylomicron remnants by isolated perfused rat liver. Biochim Biophys Acta. 1977 Sep 28;488(3):464–474. doi: 10.1016/0005-2760(77)90204-1. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. J., Eisenberg S., Fainaru M., Barenholz Y., Olivecrona T. In vitro production of human plasma low density lipoprotein-like particles. A model for very low density lipoprotein catabolism. J Biol Chem. 1979 Jul 10;254(13):6079–6087. [PubMed] [Google Scholar]
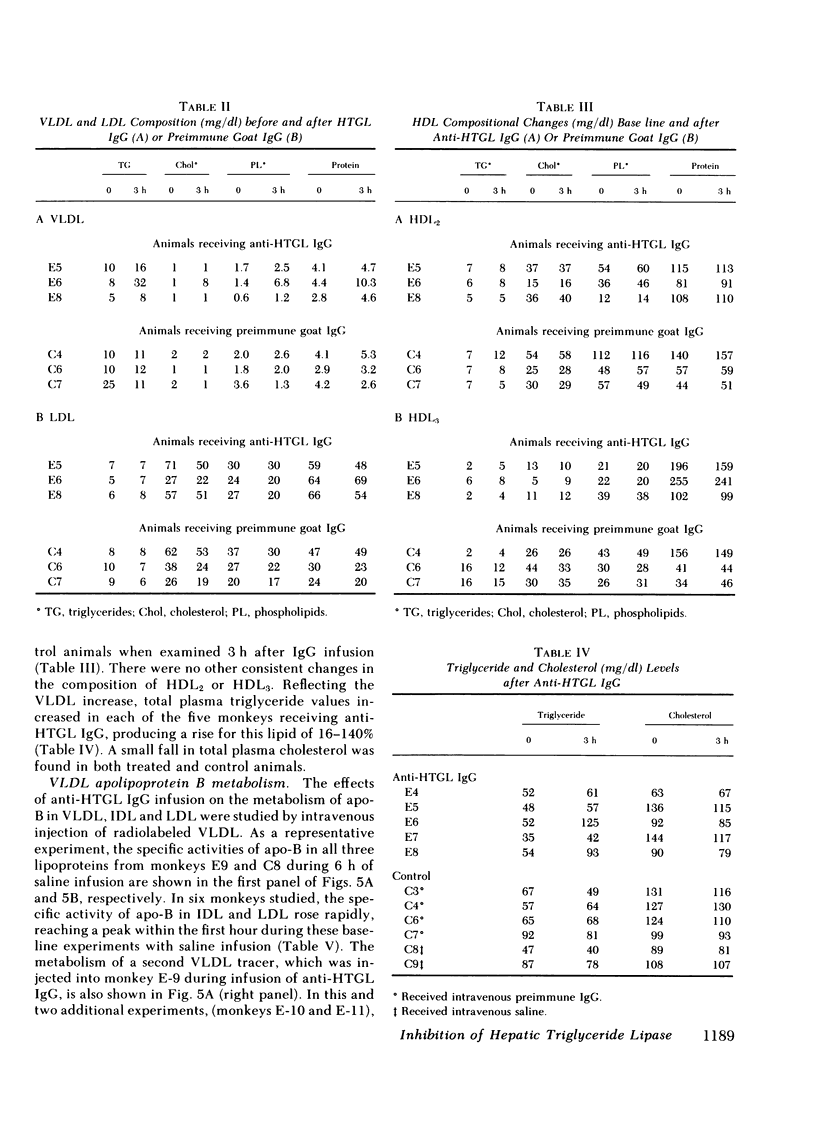
- Eisenberg S., Olivecrona T. Very low density lipoprotein. Fate of phospholipids, cholesterol, and apolipoprotein C during lipolysis in vitro. J Lipid Res. 1979 Jul;20(5):614–623. [PubMed] [Google Scholar]
- Ewing A. M., Freeman N. K., Lindgren F. T. The analysis of human serum lipoprotein distributions. Adv Lipid Res. 1965;3:25–61. [PubMed] [Google Scholar]
- Faergeman O., Sata T., Kane J. P., Havel R. J. Metabolism of apoprotein B of plasma very low density lipoproteins in the rat. J Clin Invest. 1975 Dec;56(6):1396–1403. doi: 10.1172/JCI108220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J. Validation of a procedure for exogenous isotopic labeling of lipoprotein triglyceride with radioactive triolein. Biochim Biophys Acta. 1979 May 25;573(2):255–265. doi: 10.1016/0005-2760(79)90059-6. [DOI] [PubMed] [Google Scholar]
- Ford S., Jr, Schubert W. K., Glueck C. J., Bozian R. C. Familial hyperchylomicronemia. Enzymatic and physiologic studies. Am J Med. 1971 Apr;50(4):536–541. doi: 10.1016/0002-9343(71)90342-1. [DOI] [PubMed] [Google Scholar]
- Grosser J., Schrecker O., Greten H. Function of hepatic triglyceride lipase in lipoprotein metabolism. J Lipid Res. 1981 Mar;22(3):437–442. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J. K., Ehnholm C., Kekki M., Nikkilä E. A. Postheparin plasma lipoprotein lipase and hepatic lipase in normal human subjects: relationship to age, sex and triglyceride metabolism. Adv Exp Med Biol. 1977;82:146–148. doi: 10.1007/978-1-4613-4220-5_22. [DOI] [PubMed] [Google Scholar]
- Jansen H., van Tol A., Hülsmann W. C. On the metabolic function of heparin-releasable liver lipase. Biochem Biophys Res Commun. 1980 Jan 15;92(1):53–59. doi: 10.1016/0006-291x(80)91518-1. [DOI] [PubMed] [Google Scholar]
- Jensen G. L., Baly D. L., Brannon P. M., Bensadoun A. Synthesis and secretion of lipolytic enzymes by cultured chicken hepatocytes. J Biol Chem. 1980 Dec 10;255(23):11141–11148. [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Goldstein J. L. Increased binding of low density lipoprotein to liver membranes from rats treated with 17 alpha-ethinyl estradiol. J Biol Chem. 1979 Nov 25;254(22):11367–11373. [PubMed] [Google Scholar]
- Krauss R. M., Levy R. I., Fredrickson D. S. Selective measurement of two lipase activities in postheparin plasma from normal subjects and patients with hyperlipoproteinemia. J Clin Invest. 1974 Nov;54(5):1107–1124. doi: 10.1172/JCI107855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss R. M., Lindgren F. T., Ray R. M. Interrelationships among subgroups of serum lipoproteins in normal human subjects. Clin Chim Acta. 1980 Jul 1;104(3):275–290. doi: 10.1016/0009-8981(80)90385-x. [DOI] [PubMed] [Google Scholar]
- Kuusi T., Kinnunen P. K., Nikkilä E. A. Hepatic endothelial lipase antiserum influences rat plasma low and high density lipoproteins in vivo. FEBS Lett. 1979 Aug 15;104(2):384–388. doi: 10.1016/0014-5793(79)80858-3. [DOI] [PubMed] [Google Scholar]
- Kuusi T., Nikklä E. A., Virtanen I., Kinnunen P. K. Localization of the heparin-releasable lipase in situ in the rat liver. Biochem J. 1979 Jul 1;181(1):245–246. doi: 10.1042/bj1810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Windmueller H. G., Fredrickson D. S. Comparison of the triglyceride lipase of liver, adipose tissue, and postheparin plasma. J Lipid Res. 1972 May;13(3):356–363. [PubMed] [Google Scholar]
- Le N. A., Melish J. S., Roach B. C., Ginsberg H. N., Brown W. V. Direct measurement of apoprotein B specific activity in 125I-labeled lipoproteins. J Lipid Res. 1978 Jul;19(5):578–584. [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordasini R., Frey F., Flury W., Klose G., Greten H. Selective deficiency of hepatic triglyceride lipase in uremic patients. N Engl J Med. 1977 Dec 22;297(25):1362–1366. doi: 10.1056/NEJM197712222972502. [DOI] [PubMed] [Google Scholar]
- Musliner T. A., Herbert P. N., Kingston M. J. Lipoprotein substrates of lipoprotein lipase and hepatic triacylglycerol lipase from human post-heparin plasma. Biochim Biophys Acta. 1979 Nov 21;575(2):277–288. doi: 10.1016/0005-2760(79)90029-8. [DOI] [PubMed] [Google Scholar]
- Müller P., Felin R., Lambrecht J., Agostini B., Wieland H., Rost W., Seidel D. Hypertriglyceridaemia secondary to liver disease. Eur J Clin Invest. 1974 Dec 5;4(6):419–428. doi: 10.1111/j.1365-2362.1974.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Nicoll A., Lewis B. Evaluation of the roles of lipoprotein lipase and hepatic lipase in lipoprotein metabolism: in vivo and in vitro studies in man. Eur J Clin Invest. 1980 Dec;10(6):487–495. doi: 10.1111/j.1365-2362.1980.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Quarfordt S. H., Frank A., Shames D. M., Berman M., Steinberg D. Very low density lipoprotein triglyceride transport in type IV hyperlipoproteinemia and the effects of carbohydrate-rich diets. J Clin Invest. 1970 Dec;49(12):2281–2297. doi: 10.1172/JCI106448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Miller N. E., Cortese C., Hazzard W., Coltart J., Lewis B. Splanchnic metabolism of plasma apolipoprotein B: studies of artery-hepatic vein differences of mass and radiolabel in fasted human subjects. J Clin Invest. 1981 Jun;67(6):1678–1686. doi: 10.1172/JCI110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J Biol Chem. 1980 Sep 10;255(17):8303–8307. [PubMed] [Google Scholar]



