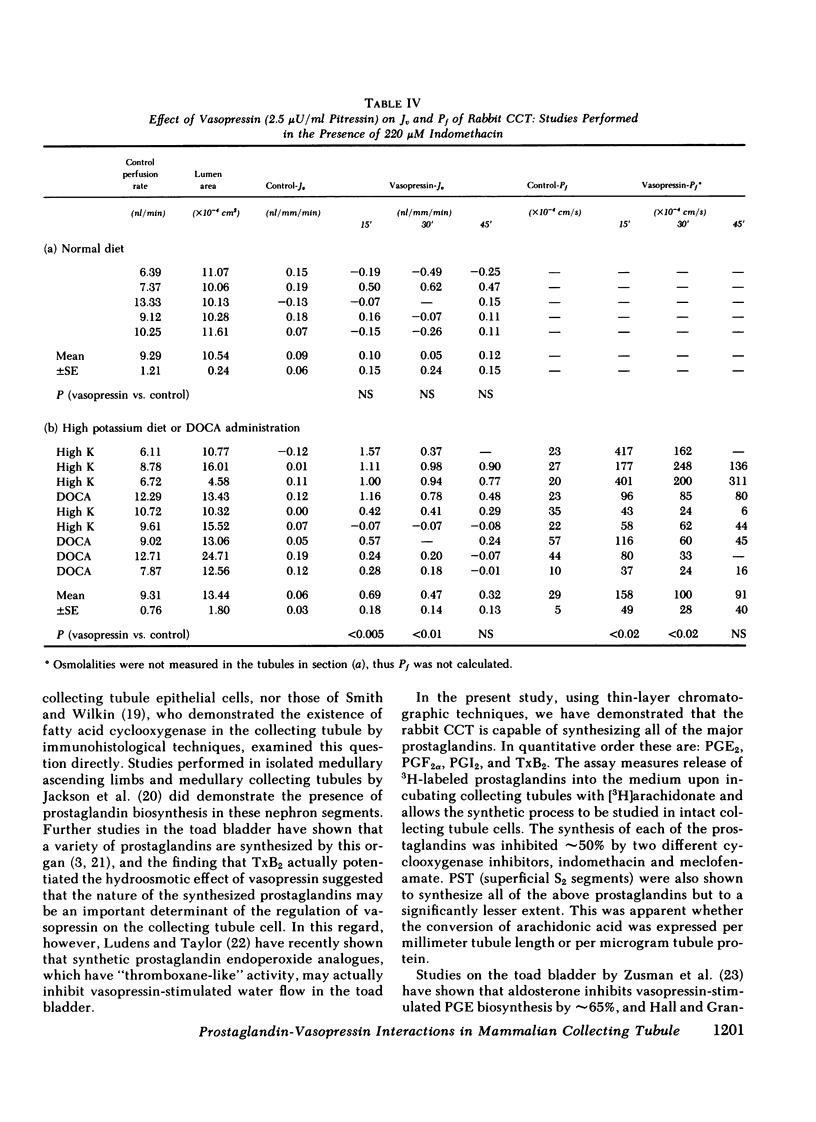
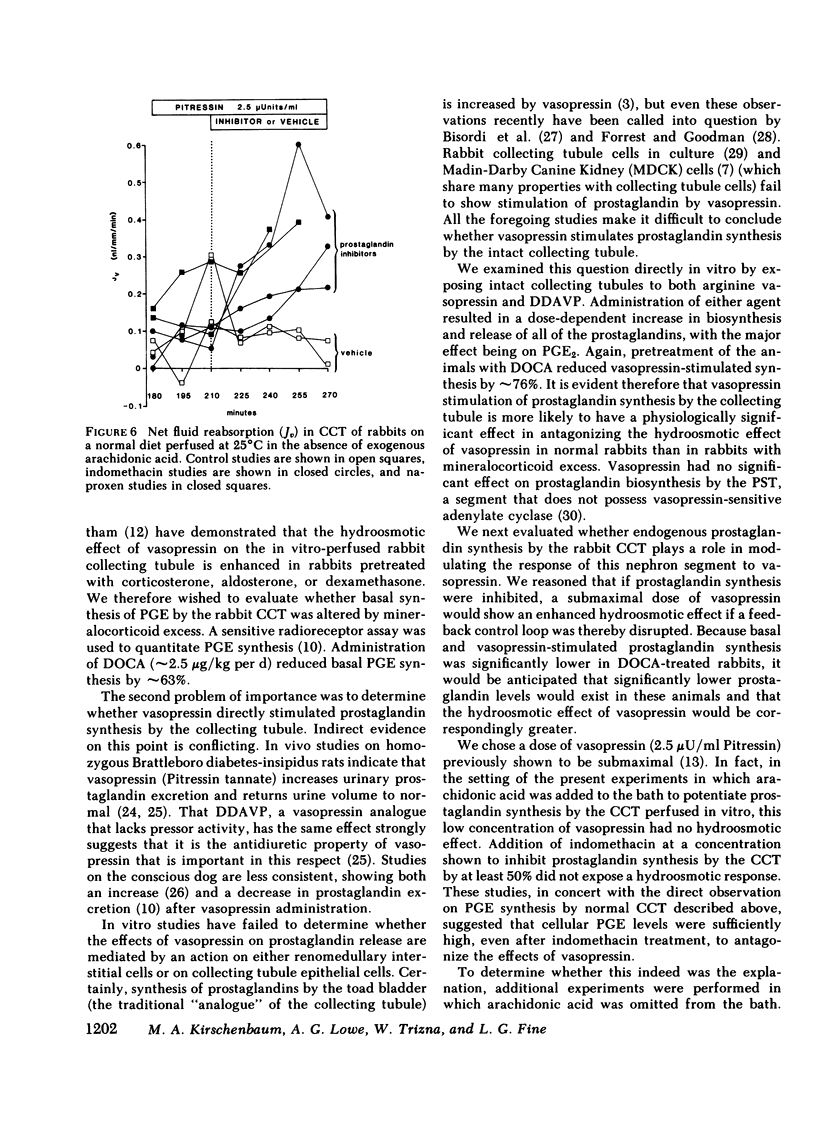
Abstract
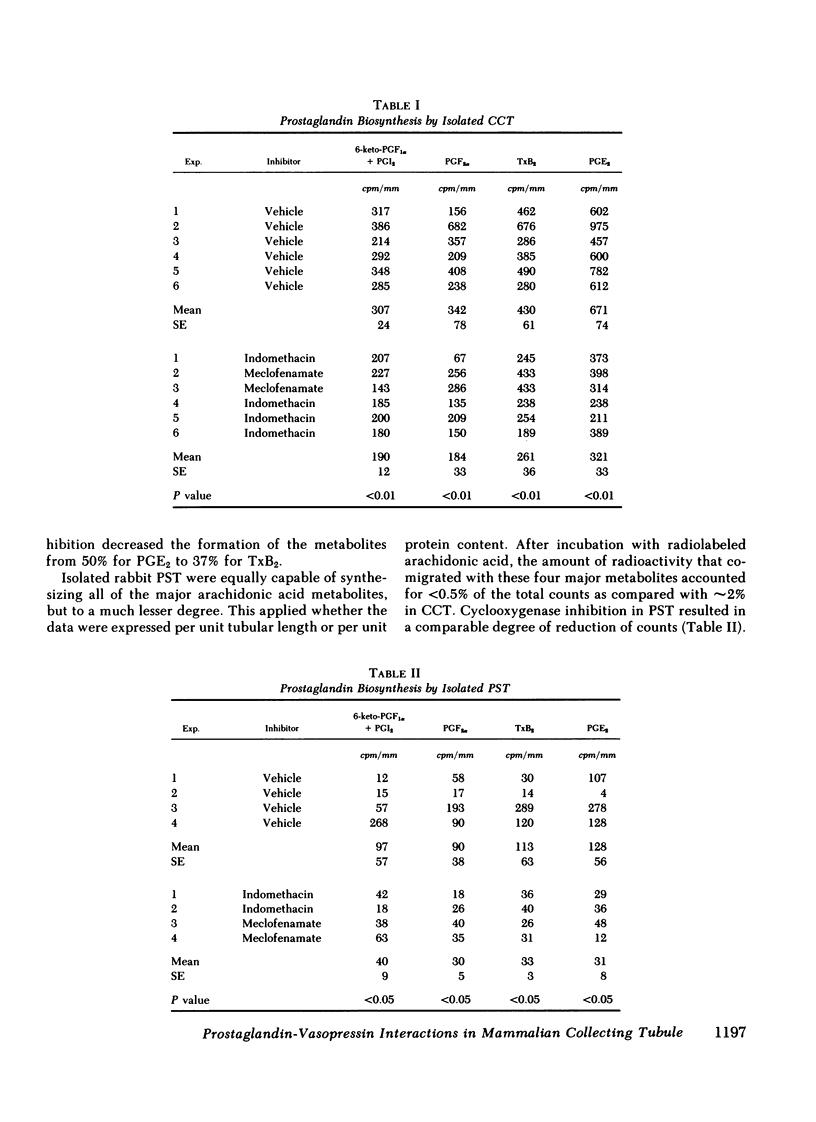
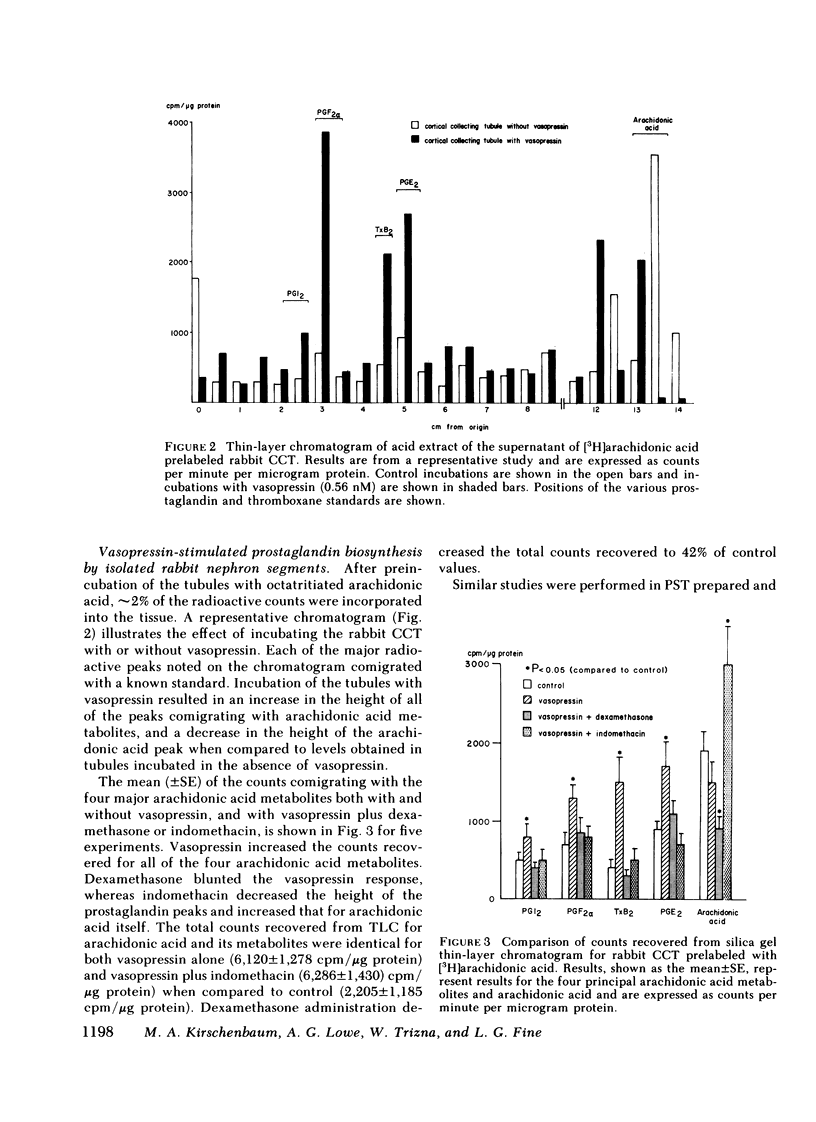
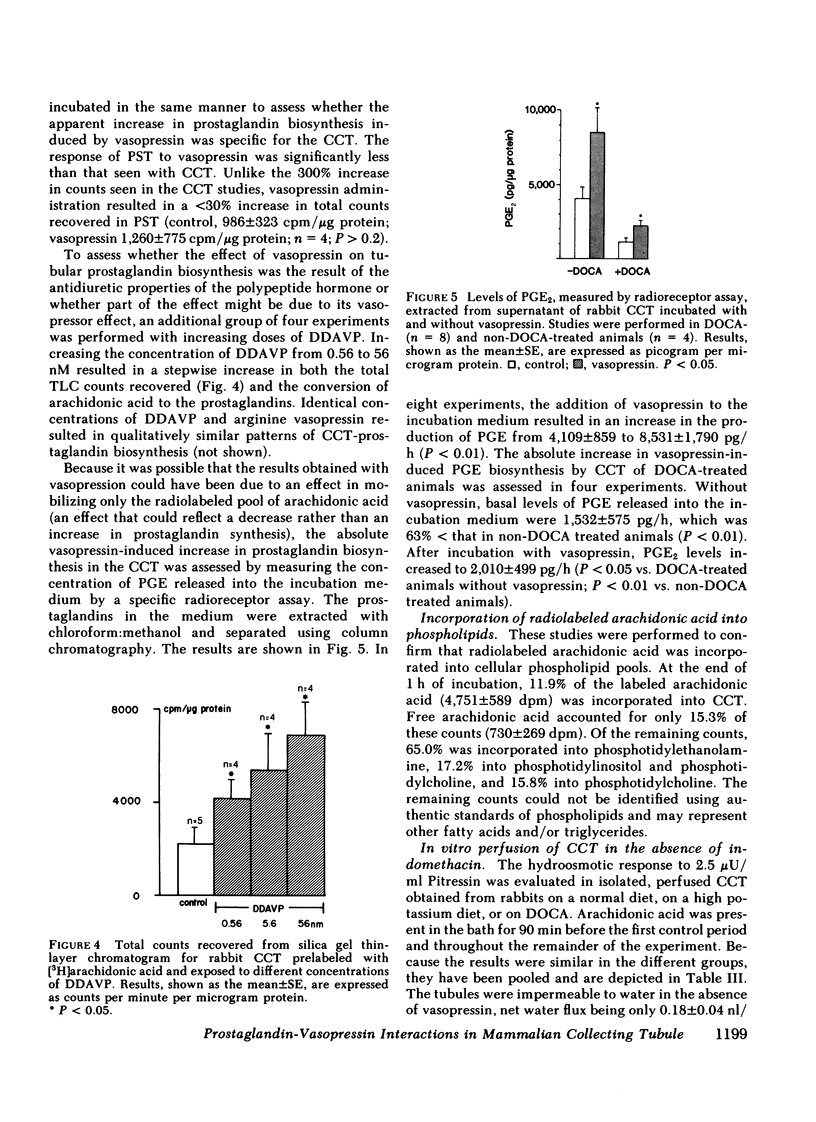
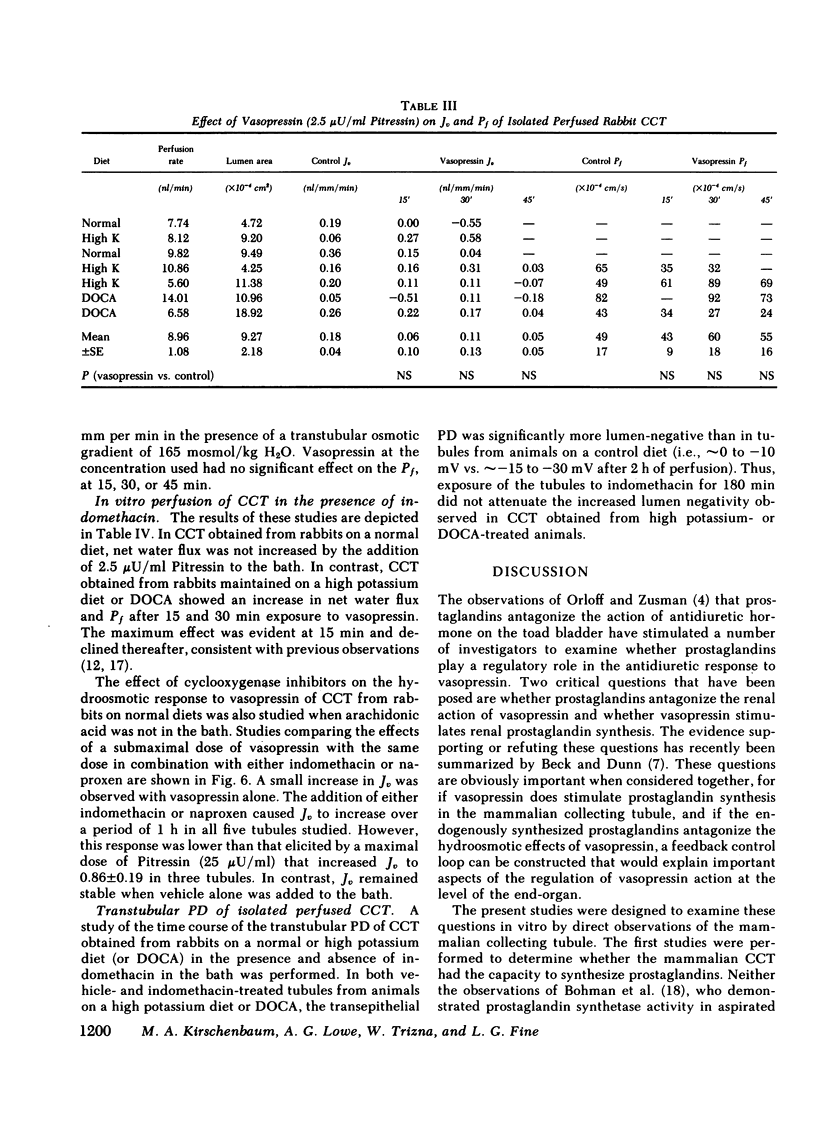
The present studies examined whether vasopressin increases prostaglandin biosynthesis in isolated rabbit cortical collecting tubules (CCT) and whether endogenous prostaglandin biosynthesis plays a role in modulating the response of this nephron segment to vasopressin. Three groups of studies were performed. In the first group, CCT and proximal straight tubules (PST) were incubated with [3H]arachidonic acid, and metabolites were separated and identified using silica gel thin-layer chromatography. CCT were capable of producing all of the major prostaglandins (PG) (PGE2 > thromboxane B2[TxB2] > PGF2α > PGI2). PST produced significantly lesser quantities of these lipids. In the second group, radiolabeled arachidonic acid was incorporated into the phospholipid pool of both CCT and PST, vasopressin was added to the incubation medium, and metabolities were separated and identified as above. Vasopressin stimulated the release of all of the major prostaglandins in CCT but had no effect on PST. PGE release into the incubation medium, as assessed by a radioreceptor assay, increased 108%, and a vasopressin analogue, 1-desamino-8-d-arginine vasopressin, had a quantitatively similar effect. In the third group, a submaximal dose of vasopressin was administered to isolated, perfused CCT studied in the presence and absence of indomethacin to assess whether endogenous prostaglandins play a role in modulating the antidiuretic response to vasopressin. Studies were performed in rabbits on a normal diet and in desoxycorticosterone acetate (DOCA)- or KCl-loaded animals. In the state of mineralocorticoid excess, basal prostaglandin synthesis was 63% lower, and vasopressin-stimulated prostaglandin synthesis 76% lower, than the synthesis observed in rabbits on a normal diet. Cyclooxygenase inhibition exposed a significant hydroosmotic response to a submaximal dose of vasopressin in CCT from DOCA- or KCl-loaded animals. With arachidonic acid in the bath, the same dose of vasopressin failed to elicit a hydroosmotic response in CCT from rabbits on a normal diet even in the presence of a cyclooxygenase inhibitor. However, removal of exogenous arachidonic acid, with a consequently lower rate of prostaglandin synthesis, allowed the cyclooxygenase inhibitor to enhance the hydroosmotic response to vasopressin in these tubules.
We conclude from these studies that the rabbit CCT has the capacity to synthesize all of the major prostaglandins and that the rate of synthesis of these lipids is enhanced by vasopessin. Prostaglandin synthesis by the CCT is postulated to modulate the antidiuretic action of vasopressin via a closed feedback loop. The effectiveness of this feedback regulation is dependent upon the mineralocorticoid status of the animal, which determines the level of basal and vasopressin-stimulated prostaglandin synthesis by the CCT.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Zahid G., Schafer J. A., Troutman S. L., Andreoli T. E. Effect of antidiuretic hormone on water and solute permeation, and the activation energies for these processes, in mammalian cortical collecting tubules: evidence for parallel ADH-sensitive pathways for water and solute diffusion in luminal plasma membranes. J Membr Biol. 1977 Feb 24;31(1-2):103–129. doi: 10.1007/BF01869401. [DOI] [PubMed] [Google Scholar]
- Bisordi J. E., Schlondorff D., Hays R. M. Interaction of vasopressin and prostaglandins in the toad urinary bladder. J Clin Invest. 1980 Dec;66(6):1200–1210. doi: 10.1172/JCI109971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohman S. O. Demonstration of prostaglandin synthesis in collecting duct cells and other cell types of the rabbit renal medulla. Prostaglandins. 1977 Oct;14(4):729–744. doi: 10.1016/0090-6980(77)90201-5. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Knapp D. R., Halushka P. V. Vasopressin stimulates thromboxane synthesis in the toad urinary bladder: effects of imidazole. J Pharmacol Exp Ther. 1979 Sep;210(3):344–348. [PubMed] [Google Scholar]
- Dousa T. P., Valtin H. Cellular actions of vasopressin in the mammalian kidney. Kidney Int. 1976 Jul;10(1):46–63. doi: 10.1038/ki.1976.78. [DOI] [PubMed] [Google Scholar]
- Dunn M. J., Greely H. P., Valtin H., Kintner L. B., Beeuwkes R., 3rd Renal excretion of prostaglandins E2 and F2alpha in diabetes insipidus rats. Am J Physiol. 1978 Dec;235(6):E624–E627. doi: 10.1152/ajpendo.1978.235.6.E624. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Kirschenbaum M. A. Absence of direct effects of prostaglandins on sodium chloride transport in the mammalian nephron. Kidney Int. 1981 Jun;19(6):797–801. doi: 10.1038/ki.1981.83. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Schlondorff D., Trizna W., Gilbert R. M., Bricker N. S. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J Clin Invest. 1978 Jun;61(6):1519–1527. doi: 10.1172/JCI109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkert V. W., Schlondorff D. Prostaglandin synthesis in isolated glomeruli. Prostaglandins. 1979 Jan;17(1):79–86. doi: 10.1016/0090-6980(79)90077-7. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3',5'-monophosphate, and theophylline. J Clin Invest. 1968 May;47(5):1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier F. C., Rollins T. E., Smith W. L. Kinin-induced prostaglandin synthesis by renal papillary collecting tubule cells in culture. Am J Physiol. 1981 Jul;241(1):F94–104. doi: 10.1152/ajprenal.1981.241.1.F94. [DOI] [PubMed] [Google Scholar]
- Hall D. A., Grantham J. J. Temperature effect on ADH response of isolated perfused rabbit collecting tubules. Am J Physiol. 1980 Dec;239(6):F595–F601. doi: 10.1152/ajprenal.1980.239.6.F595. [DOI] [PubMed] [Google Scholar]
- Hassid A., Konieczkowski M., Dunn M. J. Prostaglandin synthesis in isolated rat kidney glomeruli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1155–1159. doi: 10.1073/pnas.76.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B. A., Edwards R. M., Dousa T. P. Vasopressin-prostaglandin interactions in isolated tubules from rat outer medulla. J Lab Clin Med. 1980 Jul;96(1):119–128. [PubMed] [Google Scholar]
- Kirschenbaum M. A., Serros E. R. Effects of alterations in urine flow rate on prostaglandin E excretion in conscious dogs. Am J Physiol. 1980 Feb;238(2):F107–F111. doi: 10.1152/ajprenal.1980.238.2.F107. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ludens J. H., Taylor C. J. Inhibition of ADH-stimulated water flow by stable prostaglandin endoperoxide analogues. Am J Physiol. 1982 Feb;242(2):F119–F125. doi: 10.1152/ajprenal.1982.242.2.F119. [DOI] [PubMed] [Google Scholar]
- McGiff J. C., Crowshaw K., Itskovitz H. D. Prostaglandins and renal function. Fed Proc. 1974 Jan;33(1):39–47. [PubMed] [Google Scholar]
- Morel F. Sites of hormone action in the mammalian nephron. Am J Physiol. 1981 Mar;240(3):F159–F164. doi: 10.1152/ajprenal.1981.240.3.F159. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S., BERGSTROM S. EFFECT OF PROSTAGLANDIN (PGE-1) ON THE PERMEABILITY RESPONSE OF TOAD BLADDER TO VASOPRESSIN, THEOPHYLLINE AND ADENOSINE 3',5'-MONOPHOSPHATE. Nature. 1965 Jan 23;205:397–398. doi: 10.1038/205397a0. [DOI] [PubMed] [Google Scholar]
- Orloff J., Zusman R. Role of prostaglandin E (PGE) in the modulation of the action of vasopressin on water flow in the urinary bladder of the toad and mammalian kidney. J Membr Biol. 1978;40(Spec No):297–304. doi: 10.1007/BF02026012. [DOI] [PubMed] [Google Scholar]
- Schlondorff D., Roczniak S., Satriano J. A., Folkert V. W. Prostaglandin synthesis by isolated rat glomeruli: effect of angiotensin II. Am J Physiol. 1980 Nov;239(5):F486–F495. doi: 10.1152/ajprenal.1980.239.5.F486. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Wilkin G. P. Immunochemistry of prostaglandin endoperoxide-forming cyclooxygenases: the detection of the cyclooxygenases in rat, rabbit, and guinea pig kidneys by immunofluorescence. Prostaglandins. 1977 May;13(5):873–892. doi: 10.1016/0090-6980(77)90217-9. [DOI] [PubMed] [Google Scholar]
- Walker L. A., Gerber J. G., Frolich J. C., Nies A. S. Redistribution of intrarenal blood flow following ADH administration: lack of inhibition by blockade of prostaglandin in cyclooxygenase. Prostaglandins Med. 1978 Oct;1(4):295–303. doi: 10.1016/0161-4630(78)90049-6. [DOI] [PubMed] [Google Scholar]
- Walker L. A., Whorton A. R., Smigel M., France R., Frölich J. C. Antidiuretic hormone increases renal prostaglandin synthesis in vivo. Am J Physiol. 1978 Sep;235(3):F180–F185. doi: 10.1152/ajprenal.1978.235.3.F180. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R., Handler J. S. Effect of adrenal steroids on vasopressin-stimulated PGE synthesis and water flow. Am J Physiol. 1978 Jun;234(6):F532–F540. doi: 10.1152/ajprenal.1978.234.6.F532. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R., Handler J. S. Vasopressin-stimulated prostaglandin E biosynthesis in the toad urinary bladder. Effect of water flow. J Clin Invest. 1977 Dec;60(6):1339–1347. doi: 10.1172/JCI108893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R. Prostaglandin E2 biosynthesis by rabbit renomedullary interstitial cells in tissue culture. Mechanism of stimulation by angiotensin II, bradykinin, and arginine vasopressin. J Biol Chem. 1977 Mar 25;252(6):2069–2071. [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R. Prostaglandin biosynthesis by rabbit renomedullary interstitial cells in tissue culture. Stimulation by angiotensin II, bradykinin, and arginine vasopressin. J Clin Invest. 1977 Jul;60(1):215–223. doi: 10.1172/JCI108758. [DOI] [PMC free article] [PubMed] [Google Scholar]



