Abstract
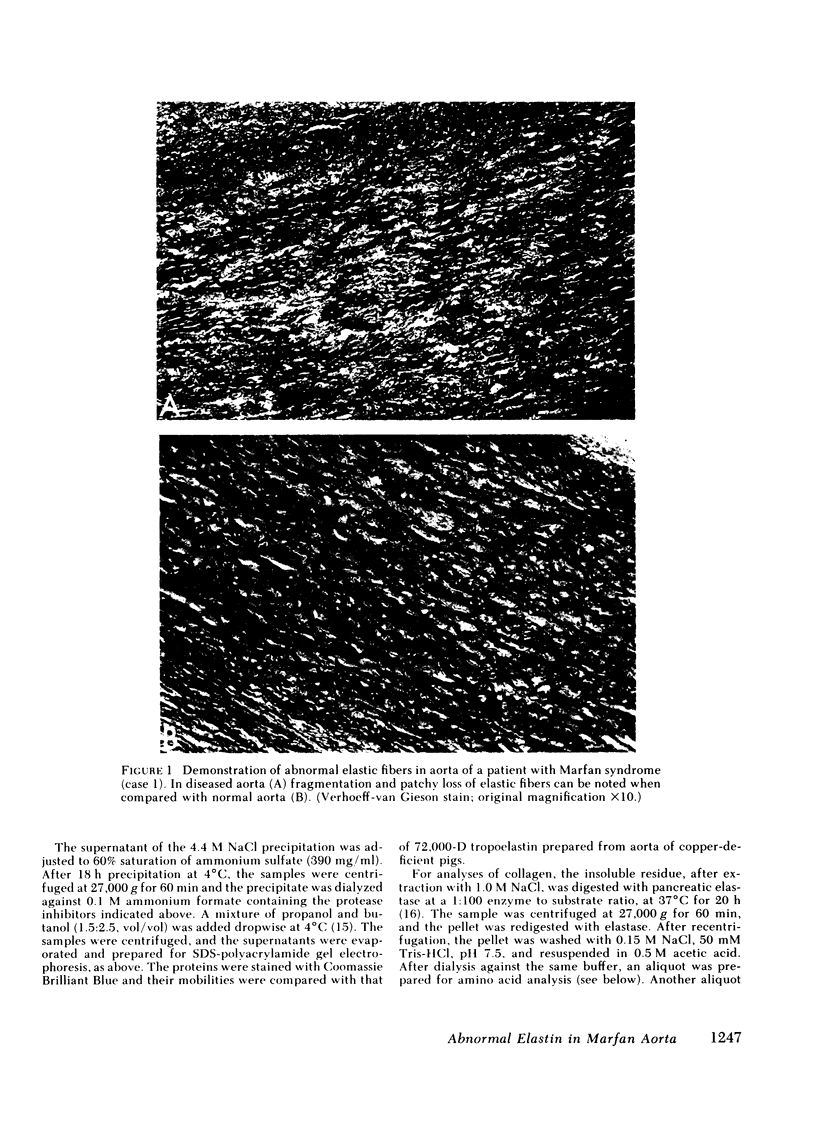
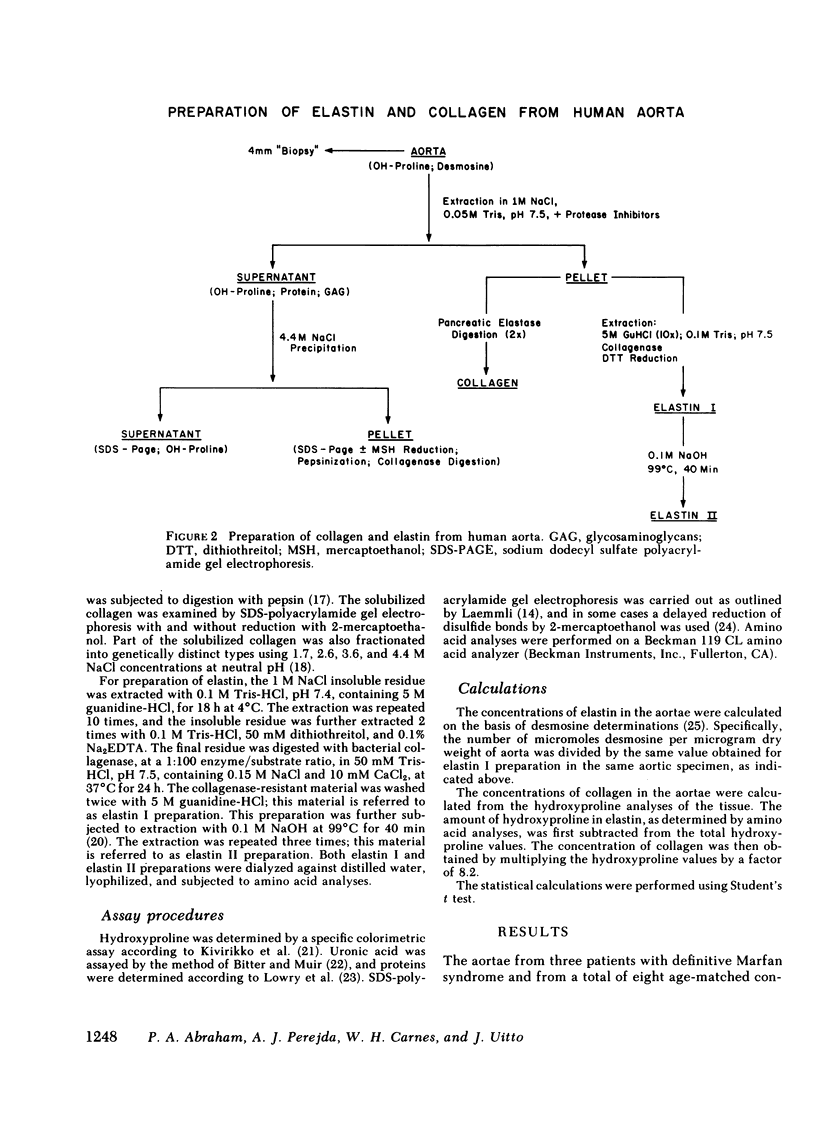
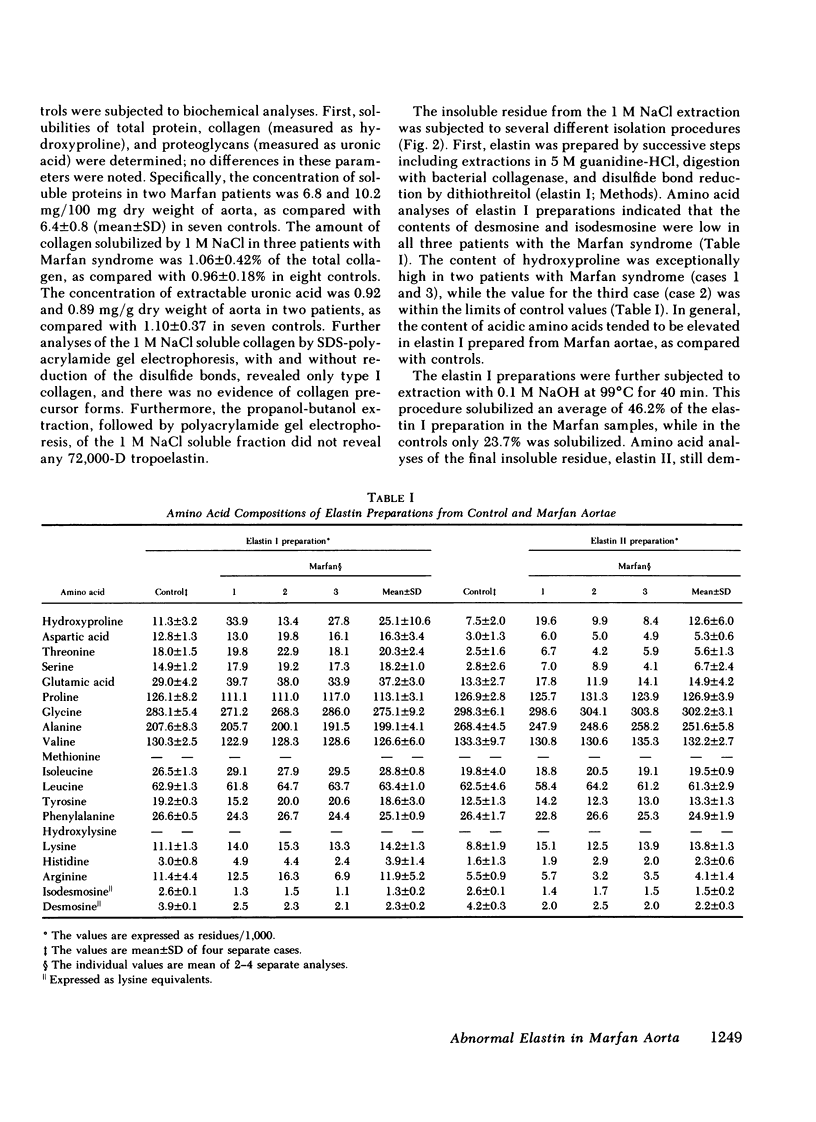
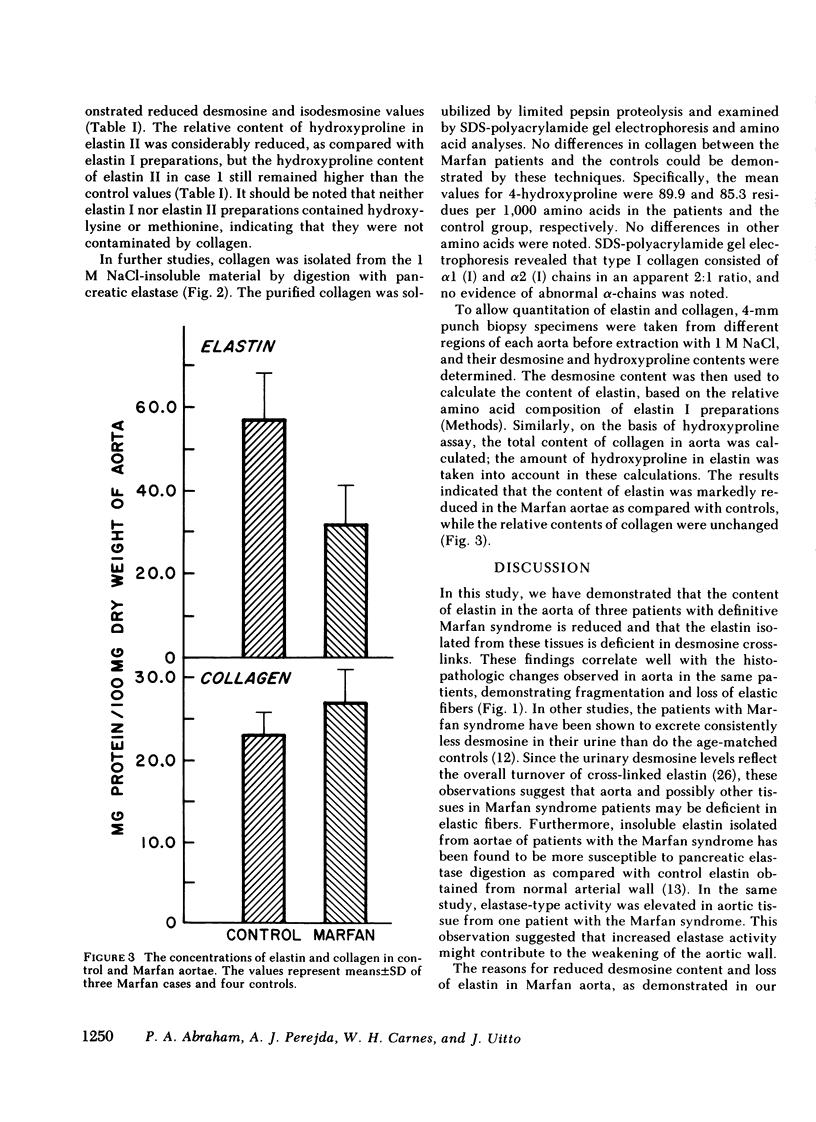
Aortae from three patients with classic presentation of Marfan syndrome, who died of vascular complications, were subjected to biochemical analyses of the connective tissue; for comparison, aortae from eight age-matched controls, without evidence of connective tissue abnormalities, were examined. Elastin was prepared from the aortae by two techniques. First, the tissues were extracted with 5 M guanidine-HCl, bacterial collagenase digestion and reduction with dithiothreitol (elastin I preparation). Secondly, this material was further purified by extraction with 0.1 M NaOH at 99 degrees C (elastin II preparation). Amino acid analyses of both elastin preparations indicated that the values for desmosine and isodesmosine were reduced in Marfan cases to approximately one-half of the control values. A corresponding increase in lysyl residues was noted in elastin II preparations. Also, the concentration of elastin per milligram dry weight of tissue was reduced in Marfan cases. The hydroxyproline content of elastin was increased in two cases with the Marfan syndrome. Recoveries indicated that the alkali treatment solubilized 46.2% of the elastin I preparations in Marfan aortae compared with 23.7% in controls. In contrast to elastin, the concentration and solubility of collagen were unchanged; the amino acid composition and the genetic types of insoluble collagen isolated by limited pepsin proteolysis were the same in both Marfan and control aortae. The results of our study demonstrate that the cross-linking of aortic elastin is reduced in the three patients with Marfan syndrome. Thus, a defect in elastin could explain the vascular fragility observed clinically in these patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Boucek R. J., Noble N. L., Gunja-Smith Z., Butler W. T. The Marfan syndrome: a deficiency in chemically stable collagen cross-links. N Engl J Med. 1981 Oct 22;305(17):988–991. doi: 10.1056/NEJM198110223051705. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Siegel R. C., Peterson K. E., Rowe D. W., Holbrook K. A., Smith L. T., Chang Y. H., Fu J. C. Marfan syndrome: abnormal alpha 2 chain in type I collagen. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7745–7749. doi: 10.1073/pnas.78.12.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouette S., Hornebeck W., Loisance D., Godeau G., Cachera J. P., Robert L. Studies on elastic tissue of aorta in aortic dissections and Marfan syndrome. Pathol Biol (Paris) 1981 Nov;29(9):539–547. [PubMed] [Google Scholar]
- Faris B., Salcedo L. L., Cook V., Johnson L., Foster J. A., Franzblau C. The synthesis of connective tissue protein in smooth muscle cells. Biochim Biophys Acta. 1976 Jan 5;418(1):93–103. doi: 10.1016/0005-2787(76)90330-0. [DOI] [PubMed] [Google Scholar]
- Goldstein R. A., Starcher B. C. Urinary excretion of elastin peptides containing desmosin after intratracheal injection of elastase in hamsters. J Clin Invest. 1978 May;61(5):1286–1290. doi: 10.1172/JCI109045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja-Smith Z., Boucek R. J. Desmosines in human urine. Amounts in early development and in Marfan's syndrome. Biochem J. 1981 Mar 1;193(3):915–918. doi: 10.1042/bj1930915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S., Mandl I., Turino G. M. Determination of the relative amounts of elastin in lung tissues. Biochem Med. 1981 Feb;25(1):74–80. doi: 10.1016/0006-2944(81)90062-4. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I. Urinary excretion of hydroxyproline in health and disease. Int Rev Connect Tissue Res. 1970;5:93–163. doi: 10.1016/b978-0-12-363705-5.50008-7. [DOI] [PubMed] [Google Scholar]
- Krieg T., Müller P. K. The marfan's syndrome. In vitro study of collagen metabolism in tissue specimens of the aorta. Exp Cell Biol. 1977;45(3-4):207–221. [PubMed] [Google Scholar]
- LANSING A. I., ROSENTHAL T. B., ALEX M., DEMPSEY E. W. The structure and chemical characterization of elastic fibers as revealed by elastase and by electron microscopy. Anat Rec. 1952 Dec;114(4):555–575. doi: 10.1002/ar.1091140404. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laitinen O., Uitto J., Iivanainen M., Hannuksela M., Kivirikko K. I. Collagen metabolism of the skin in Marfan's syndrome. Clin Chim Acta. 1968 Sep;21(3):321–326. doi: 10.1016/0009-8981(68)90062-4. [DOI] [PubMed] [Google Scholar]
- Layman D. L., Narayanan A. S., Martin G. R. The production of lysyl oxidase by human fibroblasts in culture. Arch Biochem Biophys. 1972 Mar;149(1):97–101. doi: 10.1016/0003-9861(72)90302-5. [DOI] [PubMed] [Google Scholar]
- Macek M., Hurych J., Chvapil M., Kadlecová V. Study of fibroblasts in Marfan's syndrome. Humangenetik. 1966;3(2):87–97. doi: 10.1007/BF00291289. [DOI] [PubMed] [Google Scholar]
- McKUSICK V. A. The cardiovascular aspects of Marfan's syndrome: a heritable disorder of connective tissue. Circulation. 1955 Mar;11(3):321–342. doi: 10.1161/01.cir.11.3.321. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Martin G. R. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci U S A. 1968 Oct;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest R. E., Moinuddin J. F., Priest J. H. Letter: Collagen of Marfan syndrome is abnormally soluble. Nature. 1973 Oct 5;245(5423):264–266. doi: 10.1038/245264a0. [DOI] [PubMed] [Google Scholar]
- Pyeritz R. E., McKusick V. A. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979 Apr 5;300(14):772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J., Cywinski A. Inhibition of proline hydroxylation does not inhibit secretion of tropoelastin by chick aorta cells. FEBS Lett. 1976 Jun 1;65(2):246–250. doi: 10.1016/0014-5793(76)80490-5. [DOI] [PubMed] [Google Scholar]
- Rucker R. B., Murray J. Cross-linking amino acids in collagen and elastin. Am J Clin Nutr. 1978 Jul;31(7):1221–1236. doi: 10.1093/ajcn/31.7.1221. [DOI] [PubMed] [Google Scholar]
- Sandberg L. B., Bruenger E., Cleary E. G. Tropoelastin purification: improvements using enzyme inhibitors. Anal Biochem. 1975 Mar;64(1):249–254. doi: 10.1016/0003-2697(75)90425-X. [DOI] [PubMed] [Google Scholar]
- Scheck M., Siegel R. C., Parker J., Chang Y. H., Fu J. C. Aortic aneurysm in Marfan's syndrome: changes in the ultrastructure and composition of collagen. J Anat. 1979 Oct;129(Pt 3):645–657. [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini A., Field J. M., Rodger G. W., Spina M. Application of affinity chromatography to the purification of collagenase for the isolation of insoluble elastin. Biochim Biophys Acta. 1975 Mar 28;386(1):80–86. doi: 10.1016/0005-2795(75)90248-2. [DOI] [PubMed] [Google Scholar]
- Siegel R. C. Lysyl oxidase. Int Rev Connect Tissue Res. 1979;8:73–118. doi: 10.1016/b978-0-12-363708-6.50009-6. [DOI] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Uitto J. Collagen polymorphism: isolation and partial characterization of alpha 1(I)-trimer molecules in normal human skin. Arch Biochem Biophys. 1979 Feb;192(2):371–379. doi: 10.1016/0003-9861(79)90105-x. [DOI] [PubMed] [Google Scholar]
- Uitto J., Hoffmann H-P, Prockop D. J. Synthesis of elastin and procallagen by cells from embryonic aorta. Differences in the role of hydroxyproline and the effects of proline analogs on the secretion of the two proteins. Arch Biochem Biophys. 1976 Mar;173(1):187–200. doi: 10.1016/0003-9861(76)90249-6. [DOI] [PubMed] [Google Scholar]




