Abstract
Endometrial cancer is the most common invasive gynecologic malignancy in developed countries. The most prevalent endometrioid tumors are linked to excessive estrogen exposure and hyperplasia. However, molecular mechanisms and signaling pathways underlying their etiology and pathophysiology remain poorly understood. We have shown that protein kinase Cα (PKCα) is aberrantly expressed in endometrioid tumors and is an important mediator of endometrial cancer cell survival, proliferation, and invasion. In this study, we demonstrate that expression of active, myristoylated PKCα conferred ligand-independent activation of estrogen-receptor- (ER-) dependent promoters and enhanced responses to estrogen. Conversely, knockdown of PKCα reduced ER-dependent gene expression and inhibited estrogen-induced proliferation of endometrial cancer cells. The ability of PKCα to potentiate estrogen activation of ER-dependent transcription was attenuated by inhibitors of phosphoinositide 3-kinase (PI3K) and Akt. Evidence suggests that PKCα and estrogen signal transduction pathways functionally interact, to modulate ER-dependent growth and transcription. Thus, PKCα signaling, via PI3K/Akt, may be a critical element of the hyperestrogenic environment and activation of ER that is thought to underlie the development of estrogen-dependent endometrial hyperplasia and malignancy. PKCα-dependent pathways may provide much needed prognostic markers of aggressive disease and novel therapeutic targets in ER positive tumors.
1. Introduction
Endometrial cancer is the most common invasive gynecological malignancy in the United States, accounting for 45,000 new cancer cases and over 7,500 deaths annually [1]. However, molecular mechanisms underlying its etiology and pathophysiology are poorly understood. Endometrial carcinomas are derived from glandular epithelium and typically divided into two subtypes based on clinical, histological, and molecular characteristics [2, 3]. Type I tumors, comprising 80% of cases, are generally well or moderately differentiated with endometrioid morphology and are associated with chronic unopposed estrogen exposure and hyperplasia. By contrast, type II tumors are more heterogeneous, poorly differentiated and may be estrogen independent, arising in a background of atrophic endometrium [2, 4]. The prevalence of advanced stage, high-grade tumors, of both types, with recurrent metastatic disease is increasing [5, 6]. Such cancers typically have a poorer prognosis and are refractory to current therapeutic regimens [7].
Endometrioid tumors retain expression of estrogen (ER) and progesterone (PR) receptors [8], and estrogen is a critical regulator of endometrial proliferation [9, 10]. Indeed, the majority of endometrial cancers are thought to arise due to unopposed estrogen action leading to hyperplasia and malignant transformation [2, 11]. However, our understanding of the molecular mechanisms underlying the pathophysiology of endometrial cancer lags far behind that of other hormone-dependent malignancies such as breast, prostate and ovarian cancer [2, 8, 12, 13].
The protein kinase C (PKC) family has been implicated in the regulation of numerous signal transduction pathways, modulating cell growth, differentiation, and survival [14–16]. In endometrial cancer cells and primary endometrial epithelium, expression of PKCα is increased in response to treatment with estrogen and tamoxifen and may underlie the proliferative actions of these agents in the endometrium [17, 18]. We have previously shown that PKCα is aberrantly expressed in human endometrial tumors [19, 20] and is a critical regulator of endometrial cancer cell survival, proliferation, transformation, invasion, and response to chemotherapy [21, 22]. In addition, we demonstrated that knockdown of PKCα inhibits growth of estrogen-dependent endometrial cancers in an in vivo model [20].
In this study, we present evidence that, in type I endometrial cancer cells, PKCα induces hormone-independent activation of ER, potentiates estrogen transcriptional responses, and regulates estrogen-dependent proliferation and gene expression. Thus, PKCα signaling may be a critical element of the supraphysiologic activation of ER thought to underlie the development of endometrial hyperplasia and malignancy.
2. Materials and Methods
2.1. Cell Lines
Ishikawa and HEC-50 endometrial carcinoma cells were a generous gift from Dr. Leslie (University of Iowa). Ishikawa cells expressing luciferase (luc) or PKCα shRNAs have been described [21]. Unless stated otherwise, all cell lines were maintained in 5% CO2, phenol red free DMEM, supplemented with charcoal stripped 10% fetal bovine serum, 10 units/mL penicillin, 10 μg/mL streptomycin, and 200 μM L-glutamine. Prior to estrogen treatment (100 nM Estradiol, Sigma Aldrich, St. Louis, MO, USA), cells were transferred to phenol red free DMEM containing 1x SR-1 serum replacement (Sigma Aldrich, St. Louis, MO, USA). Cell lines used were authenticated by analysis of DNA microsatellite short tandem repeats (STRS), as described previously [23].
2.2. Cell Proliferation
Cell number and viability were determined from subconfluent cultures using a Vi-Cell Coulter Counter (Beckman-Coulter, Inc., Fullerton, CA, USA) as described in [20].
2.3. Luciferase Reporter Assays
The ERE-luc and pS2-luc promoter reporter constructs have been described in [24–26]. Myristoylated PKCα vector [27] was obtained from Addgene (Cambridge, MA). Cells (2.0 × 105) were transiently transfected with 0.5 μg ERE-Luc or pS2-luc reporter plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) as per the manufacturers protocol. 0.5 μg pCMVβ, encoding β-galactosidase under control of the CMV constitutive promoter, was included as a control for transfection efficiency and cell number. Total DNA was kept constant by addition of empty vectors. Promoter activity was determined by Luciferase and β-galactosidase assays, as described in [28].
2.4. RNA Isolation and Quantitative RTPCR
RNA was isolated from 106 cells using a Qiagen RNeasy kit (Qiagen, Germantown, MD, USA) according to the manufacturer's directions and quantitated using a NanoDrop ND1000 spectrophotometer. Aliquots were evaluated by chromatography using an Agilent RNA 6000 Nano LabChip kit (Agilent Technologies, Santa Clara, CA, USA) on an Agilent Bioanalyzer 2100 system. cDNAs were prepared using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) as per the manufacturers instructions. The samples were amplified by real-time PCR using iQ SYBR green supermix (Bio-Rad, Hercules, CA, USA) on a Bio-Rad CFX96 C1000 Thermal Cycler using the following conditions: 10 minutes at 95°C and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Negative control RNA samples were not reverse transcribed or did not lack PCR template. Results were analyzed with qbasePLUS software (Bio-Rad Hercules, CA, USA), and changes in expression, relative to β-actin and rpl13a controls, were estimated using the ΔCT method [29]. Primer pair sequences (forward and reverse, 5′ to 3′) were as follows: β-Actin: AGCCTCGCCTTTGCCGA and GCGCGGCGATATCATCATC; RPL13A: TACCAGAAAGTTTGCTTACGTGGG and TGCCTGTTTCCGTAACCTCAAG; PRKCA: GCTTCCAGTGCCAAGTTTGC and GCACCCGGACAAGAAAAAGTAA; LTF: ATGGTGGTTTCATATACGAGGCA and GCCACGGCATAATAGTGAGTT; c-FOS: AAAAGGAGAATCCGAAGGGAAA and GTCTGTCTCCGCTTGGAGTGTAT; pS2 (TFF1): AGGCCCAGACAGAGACGTGTAC and CGTCGAAACAGCAGCCCTTA. Primers were designed using Primer3 software (http://primer3.wi.mit.edu) and obtained from Eurofins MWG Operon (Huntsville, AL, USA) or Integrated DNA Technologies (Coralville, IA, USA).
2.5. Statistical Analysis
Data were expressed as mean ± standard deviation or standard error of the mean and analyzed using Student's t-test. P values <0.05 were considered significantly different.
3. Results
To investigate the functional role of PKCα signal transduction in the regulation of ER-dependent transcription, Ishikawa endometrial cancer cells were transiently transfected with a myristoylated PKCα construct (myrPKCα) that is targeted to membranes and thereby rendered constitutively active [21, 27]. As shown in Figure 1, expression of myrPKCα, in the absence of estrogen, resulted in a dose-dependent activation of transcription from a promoter containing 3 copies of a canonical estrogen response element (ERE) fused to luciferase [30]. Treatment of Ishikawa cells with estradiol (E2) increased the activity of the ERE promoter approximately 30-fold (Figure 2(a)). In the presence of activated myrPKCα, E2-stimulated ERE promoter activity was further increased over 170-fold. Thus, PKCα induced hormone-independent activity of an ERE and potentiated the effect of estrogen. Similar results were obtained using the pS2 (TFF1) promoter, an endogenous E2 regulated gene [31] (Figure 2(b)). myrPKCα expression induced a marked increase in basal pS2 promoter activity and enhanced the stimulatory effect of E2. Treatment with E2 had no effect on the level of myrPKCα expression in Ishikawa cells (not shown).
Figure 1.
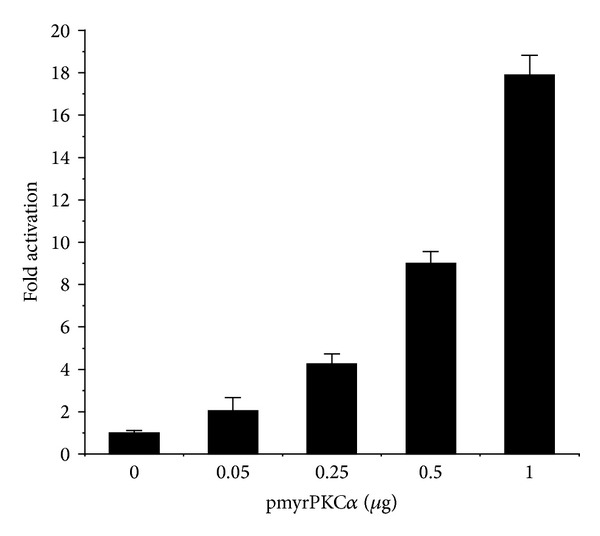
PKCα activates an estrogen responsive promoter. Ishikawa cells were transiently transfected with 0.5 μg pEREluc, 0.3 μg pCMVβ, and the indicated amounts of pmyrPKCα or vector control (pCDNA3). Luciferase activity was normalized to β-galactosidase and promoter activity expressed as fold increase over control. Data are mean ± s.d. (n = 3).
Figure 2.
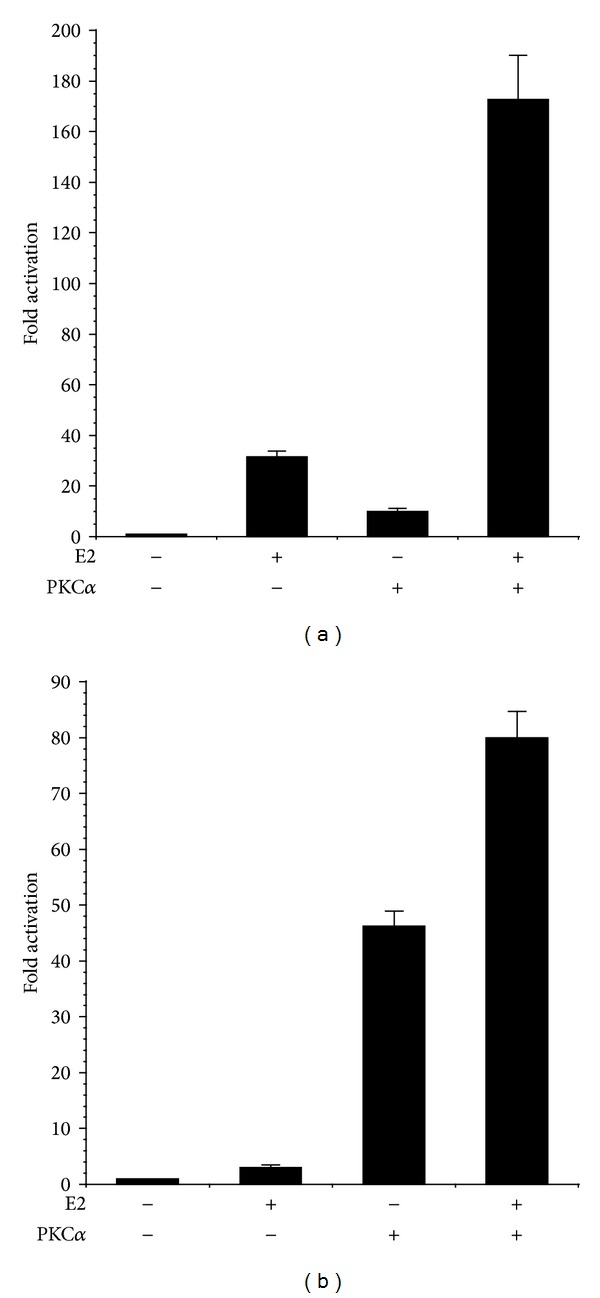
PKCα enhances ER-dependent promoter activity. Ishikawa cells were transiently transfected with (a) 0.5 μg pEREluc or (b) 0.5 μg pPS2luc and 0.3 μg pCMVβ in the presence or absence of 0.5 μg pmyrPKCα or vector control (pCDNA3). Cells were treated with ±100 nM estradiol (E2), as indicated. Luciferase activity was normalized to β-galactosidase and promoter activity expressed as fold increase over control. Data are mean ± s.e.m of 6 experiments conducted in triplicate.
In HEC-50 endometrial cancer cells, which lack estrogen receptor (ER) [32], activity of the ERE and pS2 promoters was minimal (Figure 3). Expression of active PKCα or treatment with E2 (in the presence or absence of myrPKCα) had no effect on pS2 or ERE promoter activity, indicating that the effects of PKCα and E2 are dependent on ER expression (Figure 3). Accordingly, transfection of HEC-50 cells with pHEGO encoding ERα reconstituted ERE and pS2 transcriptional responses to both E2 and myrPKCα (Figure 4). Expression of ERα in HEC-50 cells also restored the enhancement of E2-stimulated promoter activity by PKCα. (Figure 4). Together, these results (Figures 1–4) indicate that PKCα signaling induces ligand-independent activation of ER-dependent transcription and thereby potentiates responses to E2.
Figure 3.
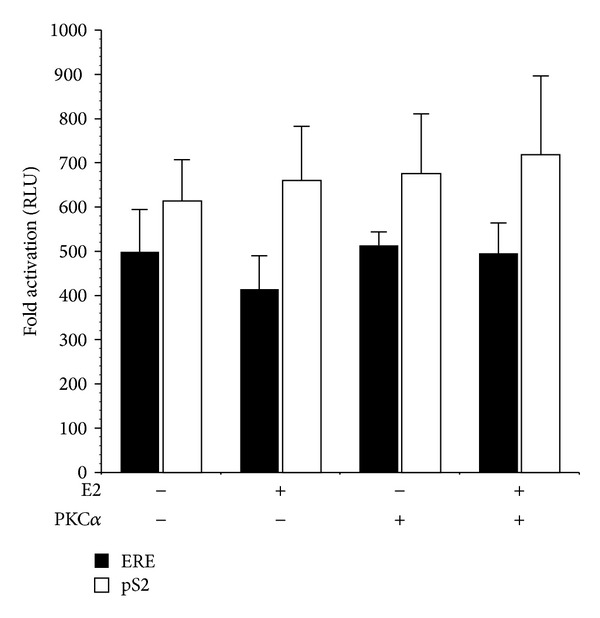
Estrogen and PKCα responses are ER dependent. HEC-50 cells, lacking ER, were transiently transfected with 0.5 μg pEREluc or 0.5 μg pPS2luc and 0.3 μg pCMVβ in the presence or absence of 0.5 μg pmyrPKCα or vector control (pCDNA3). Cells were treated with ±100 nM estradiol (E2), as indicated. Luciferase activity was normalized to β-galactosidase and promoter activity expressed as Relative Light Units (RLU). Data are mean ± s.e.m of 4 experiments conducted in triplicate.
Figure 4.
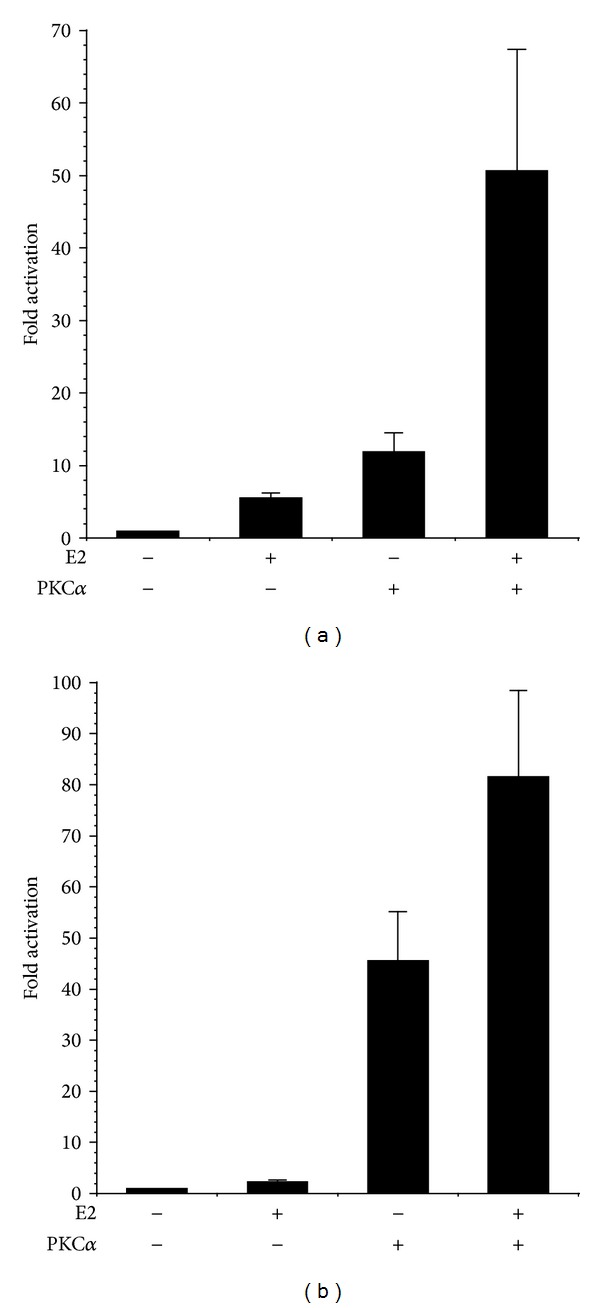
Reconstitution of PKCα regulated, ER-dependent transcription in HEC-50 cells. Cells were transiently transfected with 0.5 μg pHEGO (ERα) and (a) 0.5 μg pEREluc or (b) 0.5 μg pPS2luc and 0.3 μg pCMVβ in the presence or absence of 0.5 μg pmyrPKCα or vector control (pCDNA3). Cells were treated with ±100 nM estradiol (E2), as indicated. Promoter activity was determined as in Figure 2. Data are mean ± s.e.m of 6 experiments conducted in triplicate.
Activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway is one of the most critical steps in endometrial carcinogenesis [11] and has been shown to mediate ligand-independent activation of ER [33, 34]. Moreover, we have previously implicated PKCα in the regulation of Akt in endometrial cancer cells [22]. To investigate the role of PI3K/Akt signaling in PKCα regulation of transcription, we treated Ishikawa cells with pharmacological inhibitors of PI3K (LY 29004) or Akt (Akt-I-1/2) [35, 36] and examined their effects on the ERE promoter (Figure 5). Treatment of Ishikawa cells with LY29004 or Akt-I-1/2 significantly inhibited the ability of myrPKCα to enhance E2 activation of the ERE promoter (Figure 5(a)). Similar results were obtained in HEC-50 cells transfected with ERα (Figure 5(b)). LY29004 and Akt-I-1/2 treatment resulted in the expected decrease in phosphorylation of Akt and GSK3, respectively, and did not impact expression of myrPKCα (not shown). Thus, the effects of PKCα on E2- and ER-dependent transcription are mediated, in part, by the PI3K/Akt pathway.
Figure 5.
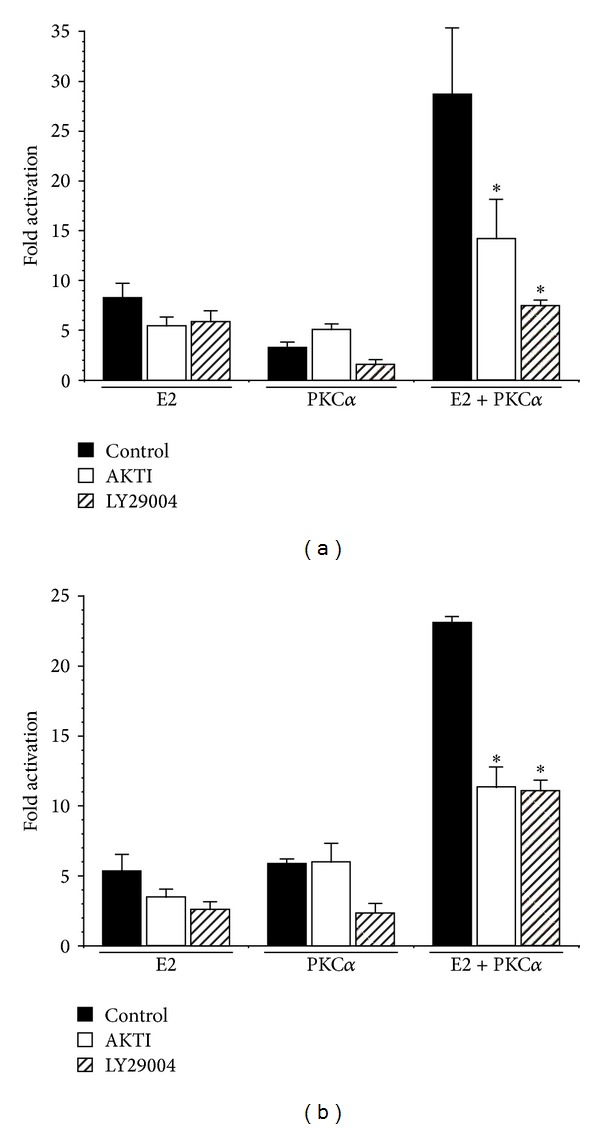
PKCα effects on ER-dependent transcription are mediated by the PI3-kinase/Akt pathway. (a) Ishikawa cells were transiently transfected with 0.5 μg pEREluc and 0.3 μg pCMVβ, in the presence or absence of 0.5 μg pmyrPKCα. (b) HEC-50 cells were transiently transfected with ERα (0.5 μg pHEGO), 0.5 μg pEREluc, and 0.3 μg pCMVβ ± 0.5 μg pmyrPKCα or pCDNA3. Cells were treated with ±100 nM estradiol (E2) in the presence or absence of the Akt and PI3K inhibitors, Akt-I-1/2 (1 μM) and LY29004 (10 μM), respectively. Promoter activity was determined as in Figure 2 and expressed as fold increase over the appropriate inhibitor or diluent control. Results are mean ± s.d. (n = 6). *P < 0.05.
To confirm the results, using the ERE and pS2 promoter constructs, we examined expression of a panel of estrogen-dependent genes implicated in endometrial neoplastic transformation [33, 34]. Levels of pS2 (TFF1), lactotransferrin (Ltf), and c-fos mRNA were determined by real-time reverse transcription PCR, in Ishikawa cells stably expressing shRNA to knockdown PKCα. Control cells were transduced with shRNA targeting luciferase [20]. As shown in Figure 6, knockdown of PKCα in Ishikawa cells significantly reduced expression of the estrogen-dependent genes pS2, ltf, and c-fos. PKCα shRNA expressing cells also exhibited the expected decrease in PKCα mRNA levels (Figure 6).
Figure 6.
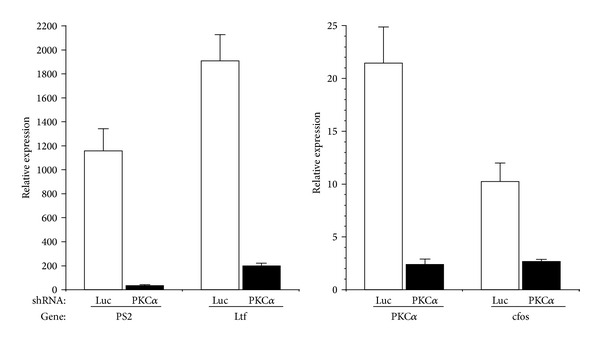
Knockdown of PKCα reduces ER-dependent gene expression. Ishikawa cells were stably transduced with shRNAs targeting PKCα or luciferase (luc). RNA was isolated and analyzed by real time reverse transcription PCR, using primers specific for the indicated gene, as described in Section 2. ΔCt values were calculated relative to a control gene (rp13) and relative levels expressed as 2Δct. Data are mean ± s.e.m (n = 6).
Estrogen is a critical regulator of type I endometrial cancer growth and stimulates proliferation of Ishikawa cells [9, 37–39]. We therefore determined the effect of PKCα knockdown on estrogen-dependent proliferation. E2 treatment stimulated proliferation of Ishikawa cells expressing a control shRNA targeting luciferase, reflected by an increase in the number of viable cells (Figure 7). Knockdown of PKCα significantly reduced the E2-dependent increase in cell number at 72 h and essentially abrogated the E2 proliferative response at 144 h. Cell viability (89%–96%) was not significantly different between cell lines and was not affected by E2 treatment.
Figure 7.
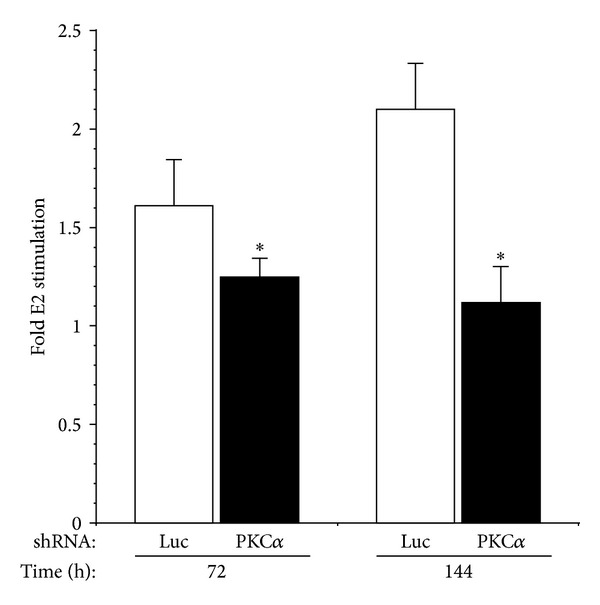
Knockdown of PKCα inhibits estrogen-stimulated growth of Ishikawa cells. Cells were stably transfected with shRNAs targeting PKCα or luciferase. Control (luc) or PKCα knockdown cell lines were treated with ±100 nM estrogen (E2) and harvested at the indicated time points. Cell number and viability were determined using a Beckmann Coulter Vi-CELL analyzer. Results are expressed as the fold increase in cell number induced by estrogen treatment. Data are mean ± s.e.m (n = 6). *P ≤ 0.05.
Together, these results indicate that PKCα is a critical regulator of ER-dependent gene expression and modulates both E2-stimulated transcription and cell proliferation in ER positive endometrial cancer cells.
4. Discussion
Estrogen, acting through ER, is a major contributor to endometrial proliferation. Indeed, hormone-dependent, type I endometrial cancers are thought to arise due to excess estrogen stimulation, unopposed by progesterone, promoting mitogenesis, atypical hyperplasia, and the transition to malignant adenocarcinoma [4, 8, 11]. In this study, we have shown that activation of PKCα is a critical element of such an estrogenic environment, resulting in estrogen-independent activation of ER-dependent transcription and potentiating the effects of estrogen on both gene expression and endometrial cancer cell proliferation. The primary effect of PKCα is to stimulate basal, unliganded ER transactivation, thereby amplifying estrogen-stimulated promoter activity and enhancing levels of genes linked to endometrial hyperplasia and malignancy.
To confirm the observed interaction of PKCα and ER signaling on estrogen responsive promoters, we examined levels of a subset of estrogen responsive genes (lactotransferrin, pS2/TFF1, and c-fos) implicated in proliferation of normal and transformed endometrial cells and linked to the development of endometrial carcinoma [11, 33, 34, 40, 41]. Knockdown of PKCα in endometrial cancer cells reduced expression of these genes (Figure 6) consistent with their regulation by both ER and PKCα. Accordingly, treatment of breast and endometrial cancer cells with phorbol esters, to activate PKC, has been shown to induce expression of pS2 and c-fos and augment their increased levels observed in response to estrogen treatment [41–43].
Cyclin D1 is also an important mediator of estrogen-dependent endometrial cell proliferation and is over expressed in endometrioid tumors [9, 37]. Consistent with interaction of E2 and PKCα mitogenic signaling pathways, we previously demonstrated that PKCα activates the cyclin D1 promoter in endometrial cancer cells [20]. In addition, expression of the cyclin-dependent kinase (CDK) inhibitor p21 is decreased in endometrial cancers, correlating with poorer prognosis [44, 45]. Estrogen-induced Ishikawa cell proliferation paralleled a decline in p21 protein expression [9], whilst progesterone mediated growth inhibition was linked to elevated p21 levels [46]. Expression of p21 was also upregulated in response to knockdown of PKCα [20], suggesting that the CDK inhibitor is a target of both PKCα and estrogen signaling pathways, regulating endometrial cancer cell proliferation.
The PI3K/Akt pathway is commonly dysregulated in type I endometrial cancers. More than 80% of endometrioid carcinomas exhibit loss of the tumor suppressor PTEN and/or activating mutations in PI3K [47–49]. PTEN heterozygous mice develop endometrial hyperplasia and adenocarcinoma, characteristic of human endometrioid tumors [11, 33, 34]. Endometrial tumorigenesis in this model is associated with upregulation of estrogen-stimulated gene expression and ligand-independent activation of ER [34], mediated by Akt [33]. Consistent with these results, we have shown that PKCα is required to maintain Akt activity in endometrial cancer cells [20] and that amplification of estrogen/ER mediated transcription by PKCα is dependent upon the PI3K/Akt pathway (Figure 5).
Phosphorylation of ER has been implicated in regulation of its transcriptional activity and DNA binding [50, 51]. Phosphorylation of serine 167, by Akt, induces activation of ER [33], and phosphorylation of serines 104, 106, and 118 modulates ER interaction with co activators [52]. PKCα-dependent ER phosphorylation and its functional role in endometrial cancer cells remain to be established; however, these latter sites match the consensus substrate sequence for PKC and, since PKCα regulates Akt activity [20], suggest that the effects of PKCα may be mediated by direct or indirect phosphorylation of ER.
5. Conclusions
In summary, we have shown that activation of PKCα induces estrogen-independent activation of ER-dependent gene expression and potentiates the effects of estrogen on transcription. Evidence also implicates PKCα in the regulation of estrogen-dependent endometrial cancer cell proliferation. Thus, PKCα-dependent signal transduction is a critical component of the environment of excessive estrogen and supraphysiologic activation of ER, which is thought to underlie the development of endometrial hyperplasia and endometrioid adenocarcinoma. Furthermore, estrogen exposure may increase PKCα expression and/or activity in endometrial cancer cells [17, 18, 53], providing a potential positive feedback loop to amplify estrogen and ER-dependent responses.
The incidence of endometrial cancer continues to rise, and, despite advances in hormonal and chemotherapy, overall survival has not significantly improved [54–56]. Thus, there is an evident need to develop novel, molecular targeted therapies. PKCα is a critical element in the estrogen, PI3K/Akt, and growth factor/ERK-dependent signal transduction pathways regulating the growth of type I tumors [20–22]. Hence, inhibition of PKCα-dependent signaling would enable the simultaneous targeting of multiple estrogen-dependent and -independent pathways implicated in the development and progression of endometrial carcinogenesis. PKCα specific inhibitors [57–59] may provide novel avenues, for primary or adjunct therapeutic intervention, to target tumors resistant to current regimens.
Conflict of Interests
The authors have no conflict of interests.
Acknowledgments
This paper is funded by the Cancer League of Colorado, University of Colorado Cancer Center, and NCI CA125427.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. Journal of Clinical Oncology. 2006;24(29):4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 3.Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Archiv. 2004;444(3):213–223. doi: 10.1007/s00428-003-0947-3. [DOI] [PubMed] [Google Scholar]
- 4.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 5.Masciullo V, Amadio G, Lo Russo D, et al. Controversies in the management of endometrial cancer. Obstetrics and Gynecology International. 2010;2010:7 pages. doi: 10.1155/2010/638165.638165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. American Journal of Obstetrics and Gynecology. 2008;198(2):218.e1–218.e6. doi: 10.1016/j.ajog.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 7.Ellenson LH, Wu T-C. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5(6):533–538. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Ryan AJ, Susil B, Jobling TW, Oehler MK. Endometrial cancer. Cell and Tissue Research. 2005;322(1):53–61. doi: 10.1007/s00441-005-1109-5. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe J, Kamata Y, Seo N, Okayasu I, Kuramoto H. Stimulatory effect of estrogen on the growth of endometrial cancer cells is regulated by cell-cycle regulators. Journal of Steroid Biochemistry and Molecular Biology. 2007;107(3–5):163–171. doi: 10.1016/j.jsbmb.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Dardes RC, Schafer JM, Pearce ST, Osipo C, Chen B, Jordan VC. Regulation of estrogen target genes and growth by selective estrogen-receptor modulators in endometrial cancer cells. Gynecologic Oncology. 2002;85(3):498–506. doi: 10.1006/gyno.2002.6659. [DOI] [PubMed] [Google Scholar]
- 11.Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annual Review of Pathology. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
- 12.Berchuck A, Boyd J. Molecular basis of endometrial cancer. Cancer. 1995;76(supplement 10):2034–2040. doi: 10.1002/1097-0142(19951115)76:10+<2034::aid-cncr2820761321>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Llauradó M, Ruiz A, Majem B, et al. Molecular bases of endometrial cancer: new roles for new actors in the diagnosis and the therapy of the disease. Molecular and Cellular Endocrinology. 2012;358(2):244–255. doi: 10.1016/j.mce.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chemical Reviews. 2001;101(8):2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 15.Nishizuka Y. The protein kinase C family and lipid mediators for transmembrane signaling and cell regulation. Alcoholism Clinical and Experimental Research. 2001;25(5):3s–7s. doi: 10.1097/00000374-200105051-00003. [DOI] [PubMed] [Google Scholar]
- 16.Toker A. Signaling through protein kinase C. Frontiers in Bioscience. 1998;3:D1134–D1147. doi: 10.2741/a350. [DOI] [PubMed] [Google Scholar]
- 17.Tonetti DA, O’Regan R, Tanjore S, England G, Jordan VC. Antiestrogen stimulated human endometrial cancer growth: laboratory and clinical considerations. Journal of Steroid Biochemistry and Molecular Biology. 1998;65(1–6):181–189. doi: 10.1016/s0960-0760(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Chen Y, Liang J, et al. Hypomethylation-linked activation of PAX2 mediates tamoxifen-stimulated endometrial carcinogenesis. Nature. 2005;438(7070):981–987. doi: 10.1038/nature04225. [DOI] [PubMed] [Google Scholar]
- 19.Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP. Analysis of protein kinase C delta (PKCδ) expression in endometrial tumors. Human Pathology. 2008;39(1):21–29. doi: 10.1016/j.humpath.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haughian JM, Reno EM, Thorne AM, Bradford AP. Protein kinase C alpha-dependent signaling mediates endometrial cancer cell growth and tumorigenesis. International Journal of Cancer. 2009;125(11):2556–2564. doi: 10.1002/ijc.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haughian JM, Bradford AP. Protein kinase C alpha (PKCα) regulates growth and invasion of endometrial cancer cells. Journal of Cellular Physiology. 2009;220(1):112–118. doi: 10.1002/jcp.21741. [DOI] [PubMed] [Google Scholar]
- 22.Haughian JM, Jackson TA, Koterwas DM, Bradford AP. Endometrial cancer cell survival and apoptosis is regulated by protein kinase C α and δ . Endocrine-Related Cancer. 2006;13(4):1251–1267. doi: 10.1677/erc.1.01278. [DOI] [PubMed] [Google Scholar]
- 23.Korch C, Spillman MA, Jackson TA, et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecologic Oncology. 2012;127(1):241–248. doi: 10.1016/j.ygyno.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu D, Kiriyama Y, Lee KY, Giguère V. Transcriptional regulation of the estrogen-inducible pS2 breast cancer marker gene by the ERR family of orphan nuclear receptors. Cancer Research. 2001;61(18):6755–6761. [PubMed] [Google Scholar]
- 25.Kudoh M, Susaki Y, Ideyama Y, et al. Inhibitory effect of a novel non-steroidal aromatase inhibitor, YM511 on the proliferation of MCF-7 human breast cancer cell. Journal of Steroid Biochemistry and Molecular Biology. 1996;58(2):189–194. doi: 10.1016/0960-0760(96)00023-4. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto N, Jinno N, Kitamura S. Activation of estrogen response element dependent transcription by thyroid hormone with increase in estrogen receptor levels in a rat pituitary cell line, GH3. Journal of Endocrinology. 2004;181(1):77–83. doi: 10.1677/joe.0.1810077. [DOI] [PubMed] [Google Scholar]
- 27.Hodges RR, Raddassi I, Zoukbri D, Toker A, Kazlauskas A, Dartt DA. Effect of overexpression of constitutively active PKCα on rat lacrimal gland protein secretion. Investigative Ophthalmology and Visual Science. 2004;45(11):3974–3981. doi: 10.1167/iovs.04-0508. [DOI] [PubMed] [Google Scholar]
- 28.Jackson TA, Schweppe RE, Koterwas DM, Bradford AP. Fibroblast growth factor activation of the rat PRL promoter is mediated by PKCδ . Molecular Endocrinology. 2001;15(9):1517–1528. doi: 10.1210/mend.15.9.0683. [DOI] [PubMed] [Google Scholar]
- 29.Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Current Protocols in Molecular Biology. 2006;73(chapter 15):15.8.1–15.8.28. doi: 10.1002/0471142727.mb1508s73. [DOI] [PubMed] [Google Scholar]
- 30.Wang SY, Ahn BS, Harris R, Nordeen SK, Shapiro DJ. Fluorescence anisotropy microplate assay for analysis of steroid receptor-DNA interactions. BioTechniques. 2004;37(5):807–817. doi: 10.2144/04375RR01. [DOI] [PubMed] [Google Scholar]
- 31.Demirpence E, Semlali A, Oliva J, et al. An estrogen-responsive element-targeted histone deacetylase enzyme has an antiestrogen activity that differs from that of hydroxytamoxifen. Cancer Research. 2002;62(22):6519–6528. [PubMed] [Google Scholar]
- 32.Albitar L, Pickett G, Morgan M, Davies S, Leslie KK. Models representing type I and type II human endometrial cancers: Ishikawa H and Hec50co cells. Gynecologic Oncology. 2007;106(1):52–64. doi: 10.1016/j.ygyno.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 33.Vilgelm A, Lian Z, Wang H, et al. Akt-mediated phosphorylation and activation of estrogen receptor α is required for endometrial neoplastic transformation in Pten+/- mice. Cancer Research. 2006;66(7):3375–3380. doi: 10.1158/0008-5472.CAN-05-4019. [DOI] [PubMed] [Google Scholar]
- 34.Lian Z, De Luca P, Di Cristofano A. Gene expression analysis reveals a signature of estrogen receptor activation upon loss of Pten in a mouse model of endometrial cancer. Journal of Cellular Physiology. 2006;208(2):255–266. doi: 10.1002/jcp.20681. [DOI] [PubMed] [Google Scholar]
- 35.Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Patents on Anti-Cancer Drug Discovery. 2010;5(1):29–57. doi: 10.2174/157489210789702208. [DOI] [PubMed] [Google Scholar]
- 36.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochemical Journal. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiozawa T, Miyamoto T, Kashima H, Nakayama K, Nikaido T, Konishi I. Estrogen-induced proliferation of normal endometrial glandular cells is initiated by transcriptional activation of cyclin D1 via binding of c-Jun to an AP-1 sequence. Oncogene. 2004;23(53):8603–8610. doi: 10.1038/sj.onc.1207849. [DOI] [PubMed] [Google Scholar]
- 38.Kashima H, Shiozawa T, Miyamoto T, et al. Autocrine stimulation of IGF1 in estrogen-induced growth of endometrial carcinoma cells: involvement of the mitogen-activated protein kinase pathway followed by up-regulation of cyclin D1 and cyclin E. Endocrine-Related Cancer. 2009;16(1):113–122. doi: 10.1677/ERC-08-0117. [DOI] [PubMed] [Google Scholar]
- 39.Holinka CF, Anzai Y, Hata H, Kimmel N, Kuramoto H, Gurpide E. Proliferation and responsiveness to estrogen of human endometrial cancer cells under serum-free culture conditions. Cancer Research. 1989;49(12):3297–3301. [PubMed] [Google Scholar]
- 40.Albright CD, Kaufman DG. Lactoferrin: a tamoxifen-responsive protein in normal and malignant human endometrial cells in culture. Experimental and Molecular Pathology. 2001;70(2):71–76. doi: 10.1006/exmp.2000.2354. [DOI] [PubMed] [Google Scholar]
- 41.Fujimoto J, Hori M, Ichigo S, Morishita S, Tamaya T. Clinical implication of fos and jun expressions and protein kinase activity in endometrial cancers. European Journal of Gynaecological Oncology. 1995;16(2):138–146. [PubMed] [Google Scholar]
- 42.Fujimoto J, Hori M, Ichigo S, Morishita S, Tamaya T. Estrogen induces expression of c-fos and c-jun via activation of protein kinase C in an endometrial cancer cell line and fibroblasts derived from human uterine endometrium. Gynecological Endocrinology. 1996;10(2):109–118. doi: 10.3109/09513599609097900. [DOI] [PubMed] [Google Scholar]
- 43.Inadera H. Estrogen-induced genes, WISP-2 and pS2, respond divergently to protein kinase pathway. Biochemical and Biophysical Research Communications. 2003;309(2):272–278. doi: 10.1016/j.bbrc.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Palazzo JP, Mercer WE, Kovatich AJ, Mchugh M. Immunohistochemical localization of p21(WAF1/CIP1) in normal, hyperplastic, and neoplastic uterine tissues. Human Pathology. 1997;28(1):60–66. doi: 10.1016/s0046-8177(97)90280-x. [DOI] [PubMed] [Google Scholar]
- 45.Pijnenborg JMA, van de Broek L, Dam de Veen GC, et al. TP53 overexpression in recurrent endometrial carcinoma. Gynecologic Oncology. 2006;100(2):397–404. doi: 10.1016/j.ygyno.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 46.Kawaguchi M, Watanabe J, Hamano M, et al. Medroxyprogesterone acetate stimulates cdk inhibitors, p21 and p27, in endometrial carcinoma cells transfected with progesterone receptor-B cDNA. European Journal of Gynaecological Oncology. 2006;27(1):33–38. [PubMed] [Google Scholar]
- 47.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 48.Hayes MP, Wang H, Espinal-Witter R, et al. PIK3CA and PTEN mutations in uterine endometrioid carcinoma and complex atypical hyperplasia. Clinical Cancer Research. 2006;12(20):5932–5935. doi: 10.1158/1078-0432.CCR-06-1375. [DOI] [PubMed] [Google Scholar]
- 49.Latta E, Chapman WB. PTEN mutations and evolving concepts in endometrial neoplasia. Current Opinion in Obstetrics and Gynecology. 2002;14(1):59–65. doi: 10.1097/00001703-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Molecular Endocrinology. 2007;21(10):2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- 51.Weigel NL, Moore NL. Kinases and protein phosphorylation as regulators of steroid hormone action. Nuclear Receptor Signaling. 2007;5, article e005 doi: 10.1621/nrs.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Molecular Endocrinology. 2006;20(12):3120–3132. doi: 10.1210/me.2006-0068. [DOI] [PubMed] [Google Scholar]
- 53.Yang JZ, O’Flatharta C, Harvey BJ, Thomas W. Membrane ERα-dependent activation of PKCα in endometrial cancer cells by estradiol. Steroids. 2008;73(11):1110–1122. doi: 10.1016/j.steroids.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhry P, Asselin E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocrine-Related Cancer. 2009;16(2):363–380. doi: 10.1677/ERC-08-0266. [DOI] [PubMed] [Google Scholar]
- 55.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nature Reviews Clinical Oncology. 2011;8(5):261–271. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 56.Weigelt B, Banerjee S. Molecular targets and targeted therapeutics in endometrial cancer. Current Opinion in Oncology. 2012;24(5):554–563. doi: 10.1097/CCO.0b013e328354e585. [DOI] [PubMed] [Google Scholar]
- 57.Goekjian PG, Jirousek MR. Protein kinase C inhibitors as novel anticancer drugs. Expert Opinion on Investigational Drugs. 2001;10(12):2117–2140. doi: 10.1517/13543784.10.12.2117. [DOI] [PubMed] [Google Scholar]
- 58.Hofmann J. Protein kinase C isozymes as potential targets for anticancer therapy. Current Cancer Drug Targets. 2004;4(2):125–146. doi: 10.2174/1568009043481579. [DOI] [PubMed] [Google Scholar]
- 59.Swannie HC, Kaye SB. Protein kinase C inhibitors. Current Oncology Reports. 2002;4(1):37–46. doi: 10.1007/s11912-002-0046-7. [DOI] [PubMed] [Google Scholar]


