Abstract
Saccades are a potentially important biomarker of Huntington disease (HD) progression, as saccadic abnormalities can be detected both cross-sectionally and longitudinally. Although vertical saccadic impairment was reported decades ago, recent studies have focused on horizontal saccades. This study investigated antisaccade (AS) and memory guided saccade (MG) impairment in both the horizontal and vertical directions in individuals with the disease-causing CAG expansion (CAG+; n = 74), using those without the expansion (CAG−; n = 47) as controls. Percentage of errors, latency, and variability of latency were used to measure saccadic performance. We evaluated the benefits of measuring saccades in both directions by comparing effect sizes of horizontal and vertical measures, and by investigating the correlation of saccadic measures with underlying gray matter loss. Consistent with previous studies, AS and MG impairments were detected prior to the onset of manifest disease. Furthermore, the largest effect sizes were found for vertical saccades. A subset of participants (12 CAG−, 12 premanifest CAG+, 7 manifest HD) underwent magnetic resonance imaging, and an automated parcellation and segmentation procedure was used to extract thickness and volume measures in saccade-generating and inhibiting regions. These measures were then tested for associations with saccadic impairment. Latency of vertical AS was significantly associated with atrophy in the left superior frontal gyrus, left inferior parietal lobule, and bilateral caudate nuclei. This study suggests an important role for measuring vertical saccades. Vertical saccades may possess more statistical power than horizontal saccades, and the latency of vertical AS is associated with gray matter loss in both cortical and subcortical regions important in saccade function.
Keywords: Huntington disease, Premanifest, Saccades, MRI, Gray matter atrophy
Introduction
Huntington disease (HD) is an autosomal dominant disorder caused by an expanded number of CAG repeats in the Huntington gene [1]. The Unified Huntington Disease Rating Scale (UHDRS) is often used to classify participants in research studies. While the UHDRS emphasizes motor abnormalities, cognitive [2, 3], saccade [4, 5], and structural brain [6, 7] abnormalities can be detected before a UHDRS-based diagnosis of HD (i.e. during the “premanifest” period of the disease). These abnormalities are potential biomarkers that offer insights into the brain systems that are first to be affected, and the subsequent progression of disease.
A saccade is a rapid eye movement that shifts gaze from one location to another. An extensive, systematic study of ocular motor function in manifest HD [8] found that the most profound changes were in the ability to initiate voluntary saccades and to avoid interruption of fixation by inappropriate saccades. Subsequent studies in premanifest and manifest HD have found abnormalities in antisaccade (AS) and memory guided (MG) measures of latency, variability of latency, and error rates [4, 5, 9–12]. For some MG tasks, the rate of decline may increase as individuals approach their predicted age of onset [13]. Further supporting the use of saccades in premanifest HD, Antoniades et al. [14] recently showed that in premanifest HD the latency of saccades directed toward a visual stimulus are significantly slower after only 1 year, while the latency of hand-tapping (another common measurement of motor function in HD) remained steady up to 3 years later. However, despite early evidence in one isolated report that vertical saccade abnormalities may be more prominent than those of horizontal saccades [8], recent studies have instead focused on horizontal saccades.
The value of quantifying early behavioral changes, such as those seen with saccadic measures, is most enhanced when observed behavioral alterations can point to regions of underlying brain abnormalities. Striatal atrophy has long been recognized as a cardinal feature of HD progression [15, 16], and can be detected using structural MRI fifteen or more years prior to disease onset [6, 7, 17]. However, atrophy is not limited to the striatum, and has also been detected in the thalamus and multiple cortical regions (reviewed in Bohanna et al. [15] and Rosas et al. [18]). Cortical atrophy may begin in posterior regions, and progress anteriorly [17, 19, 20]. Given the wide cortico-subcortical network thought to support saccadic eye movements, there is likely to be a systematic relationship across the spectrum of disease severity between regional gray matter loss and saccadic abnormalities in CAG-expanded (CAG+) individuals. However such a hypothesized association between gray matter changes and saccade performance in HD has not yet been tested.
This study examines the relationship between regional loss of cerebral gray matter and measures of saccadic impairment that significantly differentiate between a group without the CAG expansion (CAG−) and CAG+ groups, including measures of the relatively understudied vertical saccades. By understanding these relationships, clinically assessable markers can potentially be derived that are closely associated with the loss of both striatal and cortical tissue in the premanifest period.
Methods
Participants
Study participants were recruited primarily from individuals who had taken part in previous HD research studies at Indiana University [4, 10, 21]. The study was approved by the institutional review board at Indiana University, and all participants gave informed consent prior to their inclusion in the study. All participants had a parent diagnosed with HD. One hundred twenty-one participants completed the saccade protocol, with a subset of 31 participants having magnetic resonance imaging (MRI) consistent with our initial goal of 30–35 subjects enrolled in the imaging protocol. All participants in the saccade protocol were given the opportunity to participate in the imaging protocol; however, many were unable to stay the additional day required to accommodate MRI scheduling or were anxious about being in the scanner. All those who were imaged characterized themselves as right-handed. No participant reported a concurrent neurologic illness, a major Axis-I psychiatric diagnosis (e.g. schizophrenia, bipolar disorder), or current alcohol or drug abuse/dependence. Participants were asked not to disclose their CAG status, if known, to study staff.
Clinical evaluation and study group assignment
Molecular testing was used to determine the number of CAG repeats in the Huntington gene [22]. Participants with 2 alleles of fewer than 28 repeats were considered to be CAG unexpanded (CAG−; n = 47; 12 in imaging subset), while those having at least 1 allele with more than 38 CAG repeats were considered CAG expanded (CAG+; n = 74; 19 in imaging subset).
Experienced movement disorder neurologists administered the motor portion of the Unified Huntington Disease Rating Scale-99 [23] (UHDRS). The neurologists were aware that the participants were at-risk for HD, but were blinded to the results of all other study assessments. On the basis of the motor examination only, the neurologist assigned an overall confidence rating (UHDRS diagnosis confidence level) that represented the likelihood of motor abnormalities attributable to HD. Those CAG+ subjects with a confidence rating from 0 to 3 (<99% confidence of HD) were classified as premanifest (preHD; n = 49; 12 in imaging subset), while those receiving a 4 (≥99% confidence) were considered to have manifest HD (HD; n = 25; 7 in imaging subset). Estimated onset was defined as the age at which a person had a 50% probability of having manifest disease, and the estimated time to onset (TTO) was calculated for each preHD participant [24, 25]. PreHD subjects were further classified into two similarly sized subgroups: (1) far from estimated onset (Far; TTO > 13 years; n = 25) and (2) near to estimated onset (Near; TTO < 13 years; n = 24). Because of the small sample, dichotomization of the preHD group was not used in the imaging data analysis.
Eye movement recording and analysis
Saccade testing and analysis was performed as previously described [21]. Briefly, the vertical and horizontal positions of the participant’s pupils were recorded at 250 Hz. Four sensors monitored head movements, and eye positions were adjusted for small head movements (EyelinkII, SR Research Ltd, spatial resolution <0.1°). Position of the peripheral stimulus (±7.5 and ±15° horizontally; ±10° vertically) and the timing of stimulus presentation (2–3 s per trial) were randomized. More specifically for the MG task, the peripheral stimulus was presented for 50 ms, followed by a 1–2 s delay before withdrawal of the central fixation stimulus and saccade generation to the remembered position. The two saccadic tasks were administered in both the horizontal and vertical directions.
The AS task required making a saccade to the mirror opposite location of a peripheral stimulus, and the MG task required making a saccade to a remembered location of a peripheral stimulus (Online Resource 1). Each of the tasks consisted of 24 trials.
For each of the tasks, response latency (correct responses only), variability (i.e. standard deviation) of latency (correct responses only), and percentage of errors (the number of trials performed with a mistake/the total number of trials completed) were measured or calculated for each individual [21]. Criteria for a correct trial included (1) saccade initiation between 100 and 700 ms following withdrawal of the fixation stimulus, (2) a saccade made in the appropriate direction, and (3) no saccades made prior to or immediately following an otherwise appropriate saccade. The most common error for the AS task was an initial saccade made toward the peripheral stimulus; a premature saccade (made prior to removal of fixation stimulus) and a saccade in a wrong direction were the most common errors for the MG task. Any trials not meeting the stated requirements were flagged as an error. Latency was not calculated for subjects who made errors in more than 60% of the trials in a particular task (i.e. fewer than 10 correct saccades).
The saccade measures were tested for group differences (CAG−, Far, Near, HD) using analysis of covariance (ANCOVA) in SAS v9.13. A significant ANCOVA test (p ≤ 0.05) was followed by one-tailed t tests between CAG− and Far groups, Far and Near groups, and Near and HD groups. Age, gender, and education were included in the model as covariates when they had a significant effect (p ≤ 0.05).
To further compare the ability of horizontal and vertical saccadic measures to detect impairment in HD, we investigated the effect sizes of each measure. The effect size was measured as Cohen’s d [26], which is computed as the difference in the group means divided by the pooled standard deviation. The difference was calculated such that a difference in the expected direction would yield a positive value, while a difference in the unexpected direction would yield a negative value. We used the same comparisons as those used in the post hoc t tests mentioned above (CAG− and Far groups, Far and Near groups, and Near and HD groups).
Image acquisition and analysis
A subset of participants (12 CAG−, 12 preHD, 7 HD) was imaged in a Siemens (Erlangen, Germany) 3T Magnetom Trio-Tim scanner with a 12-channel head-coil array. These participants were selected prior to the study visit based on their willingness to participate in the imaging substudy. A whole-brain, high resolution (1.0 × 1.0 × 1.2 mm voxels) structural image volume was acquired using a 3D magnetization prepared rapid gradient echo (MPRAGE) sequence with imaging parameters optimized according to the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol [27].
An automated parcellation and segmentation procedure in FreeSurfer V4 [28–31] was implemented to extract cortical thickness and volume measures. Analyses focused on FreeSurfer segmented and parcellated structures that overlap with or are inclusive of regions known to mediate saccade function: dorsolateral prefrontal cortex (DLPFC) in the rostral middle frontal gyrus (rMFG), frontal eye fields (FEF) in caudal MFG (cMFG), supplementary eye fields (SEF) in superior frontal gyrus (SFG), inferior parietal lobule (IPL), and the caudate nucleus (reviewed in McDowell et al. [32]). The rostral and caudal anterior cingulate cortex (rACC, cACC) were also included because of their monitoring role in volitional saccades [32]. ANCOVA was used to test for group differences (CAG−, preHD, HD) in thickness and volume. A significant ANCOVA test (p ≤ 0.05) was followed by two-tailed t tests for all groupwise comparisons. Age, gender, and intracranial volume (ICV) were included in the model as covariates when they had significant effects (p ≤ 0.05).
Structural-saccadic relationships
A Spearman nonparametric correlation was used to test for an association between saccade impairment and brain atrophy in saccade-related regions. Only saccadic measures and structural regions with a significant group difference (p ≤ 0.05 by post hoc analysis) were used. This assured that relationships were identified between brain regions and saccadic measures where abnormalities are detectable. This data reduction approach excludes areas in which there may be a significant relationship between tissue volume and saccadic function, but no obvious neurodegeneration or saccadic impairment. Gender, age, and ICV were included in the model as partial variables. We examined whether multiple significant associations might be due to the association (as measured by Spearman nonparametric correlation) between structural regions known to be involved in saccade performance (see “Results”).
Results
In the large primary sample, the 4 study groups (CAG−, Far, Near, HD) did not significantly differ in education, gender, race, or handedness (p ≥ 0.6; Table 1), although the Far group was significantly younger than the other three groups (p ≤ 0.0005). In the subset of participants that underwent imaging, there were no significant group (CAG−, preHD, HD) differences (p ≥ 0.1).
Table 1.
Participant demographics
| CAG− | PreHD
|
HD | |||
|---|---|---|---|---|---|
| Far | (Imaged subset) | Near | |||
| Number of participants | 47 (12) | 25 | (12) | 24 | 25 (7) |
| Agea (mean years ± SD) | 47.1 ± 11.2 (46.1 ± 11.4) | 36.0 ± 11.1 | (43.9 ± 14.4) | 47.6 ± 11.7 | 48.9 ± 11.7 (45.5 ± 15.0) |
| Education (mean years ± SD) | 15.2 ± 2.3 (14.8 ± 2.1) | 15.3 ± 2.3 | (16.6 ± 4.1) | 16.1 ± 3.2 | 15.3 ± 2.6 (14.0 ± 1.5) |
| Male:female | 12:35 (5:7) | 8:17 | (5:7) | 10:14 | 8:17 (2:5) |
| Race (% Caucasian) | 100 (100) | 100 | (100) | 100 | 100 (100) |
| Handedness (% right) | 89.4 (100) | 88.0 | (100) | 87.5 | 88.0 (100) |
| CAG repeats in larger allele (mean ± SD) | 19.9 ± 3.0 (20.2 ± 3.3) | 42.4 ± 2.5 | (42.5 ± 2.2) | 42.9 ± 2.9 | 43.7 ± 4.0 (45.4 ± 5.5) |
| Estimated time to onset (mean years ± SD) | 18.1 ± 3.4 | (13.1 ± 6.4) | 9.0 ± 2.1 | ||
Data for imaged participants are listed in parentheses
The far group was significantly younger than all other groups for the larger non-imaging sample (p ≤ 0.0005)
Saccade abnormalities and effect sizes
Unadjusted group performance (i.e. not reflecting inclusion of covariates in the analytic model; mean ± SD) is reported in Table 2. There was a significant difference (p ≤ 0.05) in the performance of the Far and Near groups (Table 3) for: AS, percentage of horizontal errors, and latency of correct horizontal and vertical AS; and MG, percentage of horizontal errors. Additionally, there was a significant difference between the Near and HD groups for the measures: AS, percentage of horizontal and vertical errors; and MG, variability of latency of correct vertical saccades. There were no significant differences between the CAG− and Far groups for any saccadic measure.
Table 2.
Group differences in saccade performance
| Unadjusted mean ± SD
|
||||
|---|---|---|---|---|
| CAG− | Far | Near | HD | |
| Antisaccades | ||||
| % Errors | ||||
| Hrz | 20.3 ± 18.9 | 16.3 ± 22.4 | 29.4 ± 22.8 | 43.8 ± 28.5 |
| Vrt | 30.6 ± 19.3 | 25.8 ± 23.0 | 32.2 ± 25.5 | 52.3 ± 29.2 |
| Latency (ms) | ||||
| Hrz | 288.9 ± 47.7 | 283.4 ± 49.9 | 309.5 ± 44.8 | 321.0 ± 54.6 |
| Vrt | 304.6 ± 47.2 | 300.2 ± 51.2 | 344.3 ± 75.5 | 371.8 ± 56.1 |
| Variability of latency (ms) | ||||
| Hrz | 50.5 ± 23.8 | 60.0 ± 32.5 | 62.1 ± 28.5 | 73.6 ± 74.0 |
| Vrt | 55.4 ± 25.7 | 73.1 ± 48.8 | 73.1 ± 58.5 | 99.1 ± 75.6 |
| Memory guided saccades | ||||
| % Errors | ||||
| Hrz | 24.8 ± 15.0 | 25.5 ± 20.6 | 38.0 ± 24.8 | 39.8 ± 23.4 |
| Vrt | 17.6 ± 17.3 | 27.5 ± 16.0 | 29.4 ± 20.7 | 33.7 ± 25.7 |
| Latency (ms) | ||||
| Hrz | 299.9 ± 64.1 | 309.7 ± 60.8 | 326.5 ± 59.4 | 357.9 ± 82.0 |
| Vrt | 319.2 ± 68.0 | 306.3 ± 63.3 | 339.5 ± 67.3 | 374.3 ± 105.3 |
| Variability of latency (ms) | ||||
| Hrz | 82.6 ± 40.6 | 104.1 ± 59.1 | 106.9 ± 47.8 | 111.5 ± 50.3 |
| Vrt | 94.8 ± 46.6 | 91.9 ± 67.3 | 110.1 ± 55.3 | 150.4 ± 70.8 |
Table 3.
p values and effect sizes for group comparisons of saccade performance
| Measure | Direction | ANCOVA | CAG− versus Far
|
Far versus Near
|
Near versus HD
|
|||
|---|---|---|---|---|---|---|---|---|
| p value | p value | Effect size | p value | Effect size | p value | Effect size | ||
| Antisaccades | ||||||||
| % Errors | Hrz | < 0.0001 | 0.3 | −0.20 | 0.02 | 0.59 | 0.02 | 0.57 |
| Vrt | 0.0004 | 0.2 | −0.24 | 0.2 | 0.27 | 0.002 | 0.75 | |
| Latency (ms) | Hrz | 0.03 | 0.2 | −0.12 | 0.02 | 0.56 | 0.2 | 0.24 |
| Vrt | 0.0002 | 0.3 | −0.09 | 0.005 | 0.71 | 0.09 | 0.42 | |
| Variability of latency (ms) | Hrz | 0.2 | NS | 0.36 | NS | 0.07 | NS | 0.22 |
| Vrt | 0.02 | 0.07 | 0.52 | 0.5 | −0.002 | 0.06 | 0.40 | |
| Memory guided saccades | ||||||||
| % Errors | Hrz | 0.005 | 0.5 | 0.04 | 0.01 | 0.56 | 0.5 | 0.08 |
| Vrt | 0.04 | 0.1 | 0.60 | 0.2 | 0.11 | 0.4 | 0.19 | |
| Latency (ms) | Hrz | 0.01 | 0.4 | 0.16 | 0.1 | 0.29 | 0.07 | 0.45 |
| Vrt | 0.05 | 0.2 | −0.20 | 0.06 | 0.52 | 0.1 | 0.43 | |
| Variability of latency (ms) | Hrz | 0.1 | NS | 0.46 | NS | 0.05 | NS | 0.10 |
| Vrt | 0.03 | 0.4 | −0.05 | 0.1 | 0.30 | 0.004 | 0.68 | |
Bolded values indicate effect sizes >0.5
NS indicates that ANCOVA was not significant, precluding further post hoc testing. Hrz horizontal, Vrt vertical
When comparing the most closely related groups (CAG− and Far, Far and Near, and Near and HD), all significant findings were of medium effect sizes (0.5 ≤ d ≤ 0.8). The largest of these were found for vertical saccade measures: percentage of vertical AS errors (d = 0.75, Near vs. HD), vertical AS latency (d = 0.71, Far vs. Near), and variability of vertical MG latency (d = 0.68, Near vs. HD, Table 3). Several other measures also had effect sizes larger than 0.5: percentage of horizontal AS errors (Far vs. Near, Near vs. HD), horizontal AS latency (Far vs. Near), variability of vertical AS latency (CAG− vs. Far), percentage of horizontal MG errors (Far vs. Near), percentage of vertical MG errors (CAG− vs. Far), and vertical MG latency (Far vs. Near).
Gray matter loss
Consistent with previous studies, there was a significant group effect on thickness and volume for several cortical and subcortical regions (Table 4). Compared to that of CAG− subjects, preHD cortical thickness was significantly reduced in several frontal (bilateral SFG, left rMFG and cMFG) and parietal (bilateral IPL) regions. Bilateral volume loss was also apparent in the caudate nucleus. HD had a thinner right IPL and smaller bilateral caudate volume than preHD subjects. While there was a significant difference between CAG− and HD for all above mentioned regions, there was no significant loss of thickness in the rACC or cACC.
Table 4.
Gray matter atrophy in saccade-related brain regions
| Unadjusted mean ± SD
|
Post hoc | |||
|---|---|---|---|---|
| CAG− | Premanifest CAG+ | Manifest HD | ||
| Frontal lobe thickness (mm) | ||||
| Superior frontal gyrus | ||||
| Left1 | 2.62 ± 0.14 | 2.51 ± 0.13 | 2.48 ± 0.06 | a, b |
| Right1 | 2.54 ± 0.12 | 2.44 ± 0.11 | 2.43 ± 0.07 | a, b |
| Rostral middle frontal gyrus | ||||
| Left1, 2 | 2.34 ± 0.12 | 2.23 ± 0.09 | 2.20 ± 0.11 | a, b |
| Right1 | 2.19 ± 0.11 | 2.16 ± 0.11 | 2.13 ± 0.03 | |
| Caudal middle frontal gyrus | ||||
| Left1 | 2.49 ± 0.10 | 2.39 ± 0.11 | 2.32 ± 0.08 | a, b |
| Right1 | 2.44 ± 0.10 | 2.42 ± 0.12 | 2.35 ± 0.04 | |
| Parietal lobe thickness (mm) | ||||
| Inferior parietal lobule | ||||
| Left1 | 2.43 ± 0.11 | 2.29 ± 0.12 | 2.20 ± 0.13 | a, b |
| Right1 | 2.48 ± 0.12 | 2.38 ± 0.16 | 2.27 ± 0.16 | a, b, c |
| Cingulate cortex thickness (mm) | ||||
| Rostral anterior cingulate | ||||
| Left | 2.70 ± 0.23 | 2.76 ± 0.23 | 2.66 ± 0.21 | |
| Right1 | 2.69 ± 0.17 | 2.61 ± 0.20 | 2.67 ± 0.14 | |
| Caudal anterior cingulate | ||||
| Left2, 3 | 2.61 ± 0.29 | 2.71 ± 0.29 | 2.53 ± 0.14 | |
| Right | 2.41 ± 0.23 | 2.42 ± 0.22 | 2.37 ± 0.30 | |
| Subcortical volume (mm3) | ||||
| Caudate nucleus | ||||
| Left1, 2, 3 | 3,436.7 ± 400.1 | 3,078.7 ± 282.6 | 2,268.4 ± 447.6 | a, b, c |
| Right1, 2, 3 | 3,522.8 ± 439.2 | 3,151.0 ± 354.5 | 2,411.0 ± 341.5 | a, b, c |
Post hoc testing indicates a significant difference (p ≤ 0.05) between a CAG− and preHD, b CAG− and HD, and c preHD and HD. Superscripts indicate significant effects (p ≤ 0.05) of covariates: 1age, 2gender, 3intracranial volume (ICV)
Structural-saccadic relationships in CAG+ individuals
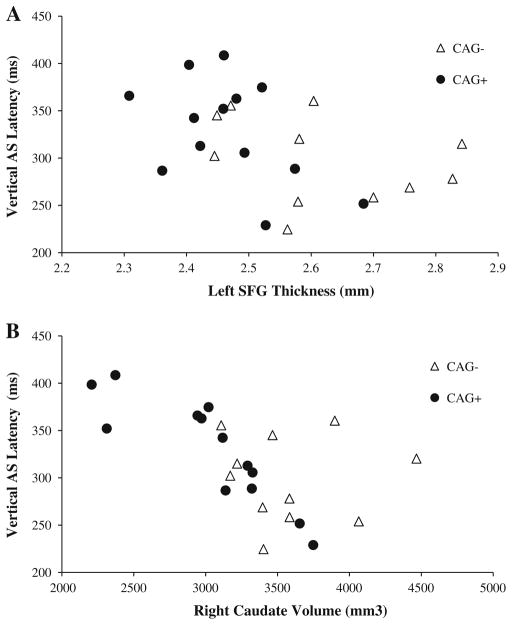
There was a significant negative association between vertical AS latency and left SFG thickness (p = 0.05, Fig. 1a, Table 5), left IPL thickness (p = 0.01), and right (Fig. 1b) and left caudate volume (p ≤ 0.0008). Percentage of vertical AS errors was positively associated with left SFG thickness (p = 0.04). Percentage of horizontal MG errors was negatively associated with left caudate volume (p = 0.01).
Fig. 1.
Plots of vertical AS latency and structural measures. Filled circles represent CAG+ individuals, and unfilled triangles represent CAG−. Plots show unadjusted values of vertical AS latency versus left SFG thickness (Spearman correlation coefficient −0.62, p = 0.05) (a) and right caudate volume (Spearman correlation coefficient −0.91, p = 0.0003) (b). Note: Only CAG+ individuals were included in the statistical analysis; CAG− individuals are shown for reference
Table 5.
Correlation coefficients (p values) of significant associations between gray matter measurements and saccade performance in CAG+ individuals
| AS
|
MG | ||
|---|---|---|---|
| % Errors (vertical) (n = 18) | Latency (vertical) (n = 13) | % Errors (horizontal) (n = 19) | |
| Frontal lobe | |||
| SFG | |||
| Left | 0.53 (0.04) | −0.62 (0.05) | |
| Parietal lobe | |||
| IPL | |||
| Left | −0.74 (0.01) | ||
| Subcortical | |||
| Caudate nucleus | |||
| Left | −0.88 (0.0008) | −0.61(0.01) | |
| Right | −0.91 (0.0003) | ||
AS Antisaccade, IPL inferior parietal lobule, MG memory guided, SFG superior frontal gyrus
We then tested for an association between these structural regions (left SFG, left IPL, left caudate. and right caudate). A significant association was found between left IPL thickness and the other three structures (p ≤ 0.01), as well as between right caudate and left caudate volume (p < 0.0001). However, left SFG thickness was not associated with right or left caudate volume (p ≥ 0.2).
Discussion
This study is the first to investigate the utility of including both horizontal and vertical saccades in the same sample of subjects with premanifest and early manifest HD. We found that both horizontal and vertical eye movements, particularly during the AS task, separated the Far and Near groups and the Near and HD groups in a non-redundant fashion. Since the largest effect sizes were found for vertical saccadic measures, we believe that future studies should also include measurements of vertical saccades that may prove more sensitive in identifying those individuals who are closer to disease onset. Only vertical AS latency was related to both cortical and subcortical gray matter loss, suggesting that vertical AS may more closely track disease-related cortical atrophy than either horizontal AS or MG measures.
Eye movement findings
We found significant increases in the AS error rate in both the horizontal and vertical directions. Error rate was sensitive enough to detect differences between the Far and Near groups as well as the Near and HD groups in the horizontal direction, while vertical AS error rate only differed between the Near and HD groups. However, the effect size for vertical AS error rate was the largest of any measure studied, suggesting that this measure may be particularly useful in quantifying disease progression in the late premanifest/early manifest stage.
Horizontal and vertical AS response latencies are also increased in the premanifest period, with significant differences between the Far and Near groups. As with AS error rate, the larger effect size for AS latency was found in the vertical direction. While we did not find a significant difference between the Near and HD groups, this could be explained by a ceiling effect as responses were excluded from the analysis when the latency was greater than 700 ms. Unfortunately no AS measures were able to detect differences between the CAG− and Far groups, though variability of latency in the vertical direction had a medium effect size, suggesting that a significant difference may emerge in a larger sample size. These results demonstrate that the AS task is quite useful for detecting changes in premanifest disease, that percentage of errors and latency are more likely to be informative than variability of latency, and that horizontal and vertical saccades are non-redundant sources of information.
For the MG task, only two post hoc tests were significant in this study. The Near group made significantly more horizontal errors than the Far group, and there was a significant increase in the variability of vertical MG latency in the HD group compared to the Near group. As was the case for AS measures, the largest effect size was found for a measure of vertical saccades. Once again there were no measures that could detect differences between the CAG− and Far groups, though a medium-sized effect between the CAG− versus far groups for vertical MG error rate suggests the possibility of detecting a difference in a larger sample. Overall, the MG task detects fewer group differences than the AS task and the effect sizes of the MG measures tend to be smaller. While more complicated versions [4, 10] of a MG task may be beneficial, it appears that the AS task is more useful than a simple MG task in evaluating disease progression.
Our results are largely consistent with findings from previous studies of AS and MG saccade performance in preHD [4, 10], although our study failed to find differences between CAG− and those furthest from estimated disease onset. A possible explanation for this is that previous studies classified groups based on motor symptoms while we used estimated TTO in this study. The latter classification tends to yield a far from onset group that is younger [33], and in this study the mean age of the Far group is 10 years younger than the CAG− group. This age difference could account for the lack of significant differences when comparing the CAG− and Far groups.
Association between atrophy and saccade impairment
The neural correlates of these saccade impairments are largely unknown in HD. In healthy individuals, FEF and SEF are activated to a greater extent during volitional saccades than during reflexive saccades [32]. These regions send projections to the superior colliculus directly and via the caudate [12, 34]. Reflexive saccades can be triggered in the parietal lobe via direct projections to the superior colliculus [12, 35–37], and there is considerable evidence that the parietal lobe also plays an important role in saccade inhibition and visuo-spatial attention during AS and MG saccades [32]. The one previous study examining the neural correlates of saccades in HD used diffusion tensor imaging to examine the relationship between white matter integrity and voluntary saccades [38]. The results suggested that fiber loss between the FEF and caudate could be the source of increased variability of latency in preHD.
In this study we examined the gray matter correlates of saccade function. We limited our analysis to regions of interest in saccade function, including FEF, SEF, IPL, DLPFC, and caudate. The most striking associations between cerebral degeneration and saccadic measures were with the latency of vertical AS. This was the only saccadic measure that was sensitive to both cortical and subcortical atrophy, and it explained more of the variation in size in left SFG, left IPL, and bilateral caudate than any of the other saccadic measures. The relationship among these regions is not entirely explained by inter-correlation between regional volume and thickness; left IPL thickness was significantly associated with the other structures, but left SFG thickness was not associated with caudate volume.
It was somewhat surprising to find strong anatomic associations with vertical AS but not horizontal AS latency. While brainstem control of vertical saccades has been studied extensively [39], cortical control is not well understood. Notwithstanding the differences between an acute focal lesion and more diffuse degenerative disease, Pflugshaupt and colleagues [40] described a patient with a unilateral lesion in the right FEF. Initially, this patient demonstrated hypometric horizontal saccades, which soon recovered. However, the patient continued to make significantly fewer exploratory vertical saccades, suggesting cortical control specific to vertical saccades and that loss of this control is not as readily compensated as are deficits in horizontal control. At the same time, our data also suggested that an increase in left SFG thickness is associated with more vertical AS errors. While others have shown increased thickness in the anterior cingulate in HD [20, 41, 42], similar increases have not been found in the SFG, and the exact mechanism for any putative increase in volume remains uncertain.
In contrast to AS, MG task performance was only related to subcortical atrophy. The caudate nucleus is a major site of cortical input to the basal ganglia [43], and appears to play a role in both the initiation [44] and inhibition [45] of saccades, consistent with its role as a relay between cortex and ocular motor output [12]. Our findings suggest that increased vertical AS latency most reflects caudate, SEF, and IPL degeneration, and that vertical AS latency may be one of the more sensitive markers of cortical and subcortical volume loss in premanifest and early manifest HD. While further studies, particularly longitudinal studies, will be necessary to establish the relationship between vertical AS latency and gray matter loss, the availability of portable saccade tracking systems raises the possibility that saccades can be used as an informative parameter in evaluating the clinical course of disease.
A major strength of this study was that all imaging and saccadic data were collected at the same study visit, avoiding any time-dependent discrepancies between the saccadic and structural measures. Also, all participants in the study had a parent diagnosed with HD, which better matched the groups with respect to environmental and related non-measurable influences. On the other hand, this study was limited by the relatively modest number of individuals who were imaged. This precluded whole brain analysis of the neural correlates of saccade function and reduced our power to detect weaker associations. We were also limited in our ability to conclude that there are no group differences in saccades between CAG− and individuals far from onset due to the age differences between these groups. Furthermore, we did not collect sufficient data regarding current medications to determine their possible effect on saccade measures. Finally, some results might not be anticipated, such as the largely unilateral (dominant hemisphere) correlations between function and anatomic measures and the lack of what might well be an intuitively hypothesized relationship between horizontal saccade errors and structural measures evaluated in this study. We anticipate that such ambiguities will be addressed in future research with larger samples.
To our knowledge, this is the first study to compare the use of horizontal and vertical saccadic measurements in premanifest and early manifest HD. While we recognize the preliminary nature of our findings, we believe they suggest that vertical saccadic measurements are an important and mostly un-utilized measure of impairment in HD. Additionally, the results suggest that cortical and subcortical gray matter loss are related to slowed vertical AS, while MG saccade measures are only related to subcortical volume loss. These findings suggest that future studies would be well-served to measure vertical AS, which may be an early clinical indication of disease manifestation that tracks gray matter integrity.
Acknowledgments
We gratefully acknowledge the individuals who participated in this study. We also thank Xabier Beristain, Jeanine Marshall, Marjorie Weaver, Michele Beal, Veronique Bragulat, and Courtney Robbins who provided expertise and technical support for the study. This work was supported by National Institute of Health Grants R01NS042659, R21NS060205, N01NS-3-2357, M01RR-00750, UL1RR025761, P30AG10133-18S1, a pilot Grant from the Center for Neuroimaging at Indiana University School of Medicine, a Research Support Fund Grant from Indiana University-Purdue University Indianapolis, and support from the CHDI Foundation, project 1214.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00415-011-6172-0) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Jason Rupp, Department of Medical and Molecular Genetics, Indiana University School of Medicine, 410 W. 10th Street (HS 4021), Indianapolis, IN 46202-5251, USA.
Mario Dzemidzic, Department of Neurology, Indiana University School of Medicine, 541 Clinical Drive (CL 285), Indianapolis, IN 46202, USA. Division of Imaging Sciences, Department of Radiology and Imaging Science, Center for Neuroimaging, Indiana University School of Medicine, Indianapolis, IN, USA.
Tanya Blekher, Department of Ophthalmology, Indiana University School of Medicine, Indianapolis, IN, USA.
John West, Division of Imaging Sciences, Department of Radiology and Imaging Science, Center for Neuroimaging, Indiana University School of Medicine, Indianapolis, IN, USA.
Siu Hui, Division of Biostatistics, Department of Medicine, Regenstrief Institute, Indiana University School of Medicine, Indianapolis, IN, USA.
Joanne Wojcieszek, Department of Neurology, Indiana University School of Medicine, 545 Barnhill Drive (EH 125), Indianapolis, IN 46202, USA.
Andrew J. Saykin, Division of Imaging Sciences, Department of Radiology and Imaging Science, Center for Neuroimaging, Indiana University School of Medicine, Indianapolis, IN, USA. Department of Medical and Molecular Genetics, Indiana University School of Medicine, 950 Walnut Street (R2 E124), Indianapolis, IN 46202, USA
David A. Kareken, Email: dkareken@iupui.edu, Department of Neurology, Indiana University School of Medicine, 541 Clinical Drive (CL 285), Indianapolis, IN 46202, USA. Division of Imaging Sciences, Department of Radiology and Imaging Science, Center for Neuroimaging, Indiana University School of Medicine, Indianapolis, IN, USA
Tatiana Foroud, Email: tforoud@iupui.edu, Department of Medical and Molecular Genetics, Indiana University School of Medicine, 410 W. 10th Street (HS 4021), Indianapolis, IN 46202-5251, USA.
References
- 1.Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood SC, Siemers E, Hodes ME, Conneally PM, Christian JC, Foroud T. Subtle changes among presymptomatic carriers of the Huntington’s disease gene. J Neurol Neurosurg Psychiatry. 2000;69:773–779. doi: 10.1136/jnnp.69.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA, Guttman M, Nance M, Kieburtz K, Oakes D, Shoulson I, Kayson E, Johnson S, Penziner E. Preparing for preventive clinical trials: the predict-HD study. Arch Neurol. 2006;63:883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 4.Blekher T, Johnson SA, Marshall J, White K, Hui S, Weaver M, Gray J, Yee R, Stout JC, Beristain X, Wojcieszek J, Foroud T. Saccades in presymptomatic and early stages of Huntington disease. Neurology. 2006;67:394–399. doi: 10.1212/01.wnl.0000227890.87398.c1. [DOI] [PubMed] [Google Scholar]
- 5.Golding CV, Danchaivijitr C, Hodgson TL, Tabrizi SJ, Kennard C. Identification of an oculomotor biomarker of preclinical Huntington disease. Neurology. 2006;67:485–487. doi: 10.1212/01.wnl.0000218215.43328.88. [DOI] [PubMed] [Google Scholar]
- 6.Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, Brandt J, Gourley LM, Liang K, Zhou H, Margolis RL, Ross CA. Onset and rate of striatal atrophy in pre-clinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 7.Paulsen JS, Nopoulos PC, Aylward E, Ross CA, Johnson H, Magnotta VA, Juhl A, Pierson RK, Mills J, Langbehn D, Nance M. Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Res Bull. 2010;82:201–207. doi: 10.1016/j.brainresbull.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leigh RJ, Newman SA, Folstein SE, Lasker AG, Jensen BA. Abnormal ocular motor control in Huntington’s disease. Neurology. 1983;33:1268–1275. doi: 10.1212/wnl.33.10.1268. [DOI] [PubMed] [Google Scholar]
- 9.Antoniades CA, Altham PM, Mason SL, Barker RA, Carpenter R. Saccadometry: a new tool for evaluating presymptomatic Huntington patients. Neuroreport. 2007;18:1133–1136. doi: 10.1097/WNR.0b013e32821c560d. [DOI] [PubMed] [Google Scholar]
- 10.Blekher TM, Yee RD, Kirkwood SC, Hake AM, Stout JC, Weaver MR, Foroud TM. Oculomotor control in asymptomatic and recently diagnosed individuals with the genetic marker for Huntington’s disease. Vision Res. 2004;44:2729–2736. doi: 10.1016/j.visres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Hicks SL, Robert MP, Golding CV, Tabrizi SJ, Kennard C. Oculomotor deficits indicate the progression of Huntington’s disease. Prog Brain Res. 2008;171:555–558. doi: 10.1016/S0079-6123(08)00678-X. [DOI] [PubMed] [Google Scholar]
- 12.Lasker AG, Zee DS. Ocular motor abnormalities in Huntington’s disease. Vision Res. 1997;37:3639–3645. doi: 10.1016/S0042-6989(96)00169-1. [DOI] [PubMed] [Google Scholar]
- 13.Rupp J, Blekher TM, Jackson JG, Beristain X, Marshall J, Hui SL, Wojcieszek JM, Foroud T. Progression in prediagnostic Huntington disease. J Neurol Neurosurg Psychiatry. 2010;81:379–384. doi: 10.1136/jnnp.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoniades CA, Xu Z, Mason SL, Carpenter RH, Barker RA. Huntington’s disease: changes in saccades and hand-tapping over 3 years. J Neurol. 2010;257:1890–1898. doi: 10.1007/s00415-010-5632-2. [DOI] [PubMed] [Google Scholar]
- 15.Bohanna I, Georgiou-Karistianis N, Hannan AJ, Egan GF. Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington’s disease. Brain Res Rev. 2008;58:209–225. doi: 10.1016/j.brainresrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, Como P, Zimmerman C, Lin M, Zhang L, Ulug AM, Beal MF, Matson W, Bogdanov M, Ebbel E, Zaleta A, Kaneko Y, Jenkins B, Hevelone N, Zhang H, Yu H, Schoenfeld D, Ferrante R, Rosas HD. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology. 2006;66:250–252. doi: 10.1212/01.wnl.0000194318.74946.b6. [DOI] [PubMed] [Google Scholar]
- 17.Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8:791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosas HD, Salat DH, Lee SY, Zaleta AK, Hevelone N, Hersch SM. Complexity and heterogeneity: what drives the ever-changing brain in Huntington’s disease? Ann N Y Acad Sci. 2008;1147:196–205. doi: 10.1196/annals.1427.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 20.Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain. 2008;131:1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blekher T, Weaver MR, Cai X, Hui S, Marshall J, Jackson JG, Wojcieszek J, Yee RD, Foroud TM. Test–retest reliability of saccadic measures in subjects at risk for Huntington disease. Invest Ophthalmol Vis Sci. 2009;50:5707–5711. doi: 10.1167/iovs.09-3538. [DOI] [PubMed] [Google Scholar]
- 22.Bond CE, Hodes ME. Direct amplification of the CAG repeat of huntingtin without amplification of CCG. Clin Chem. 1996;42:773–774. [PubMed] [Google Scholar]
- 23.Huntington Study Group. Unified Huntington’s Disease Rating Scale-99. Huntington Study Group; Rochester: 1999. [Google Scholar]
- 24.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M. Detection of Huntingtons disease decades before diagnosis: the predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, Whitwell L, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe KA, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 32.McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stout JC, Weaver M, Solomon AC, Queller S, Hui S, Johnson SA, Gray J, Beristain X, Wojcieszek J, Foroud T. Are cognitive changes progressive in prediagnostic HD? Cogn Behav Neurol. 2007;20:212–218. doi: 10.1097/WNN.0b013e31815cfef8. [DOI] [PubMed] [Google Scholar]
- 34.Dias EC, Kiesau M, Segraves MA. Acute activation and inactivation of macaque frontal eye field with GABA-related drugs. J Neurophysiol. 1995;74:2744–2748. doi: 10.1152/jn.1995.74.6.2744. [DOI] [PubMed] [Google Scholar]
- 35.Keating EG, Gooley SG, Pratt SE, Kelsey JE. Removing the superior colliculus silences eye movements normally evoked from stimulation of the parietal and occipital eye fields. Brain Res. 1983;269:145–148. doi: 10.1016/0006-8993(83)90971-x. [DOI] [PubMed] [Google Scholar]
- 36.Lynch JC, Graybiel AM, Lobeck LJ. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol. 1985;235:241–254. doi: 10.1002/cne.902350207. [DOI] [PubMed] [Google Scholar]
- 37.Pare M, Wurtz RH. Progression in neuronal processing for saccadic eye movements from parietal cortex area lip to superior colliculus. J Neurophysiol. 2001;85:2545–2562. doi: 10.1152/jn.2001.85.6.2545. [DOI] [PubMed] [Google Scholar]
- 38.Kloppel S, Draganski B, Golding CV, Chu C, Nagy Z, Cook PA, Hicks SL, Kennard C, Alexander DC, Parker GJ, Tabrizi SJ, Frackowiak RS. White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington’s disease. Brain. 2008;131:196–204. doi: 10.1093/brain/awm275. [DOI] [PubMed] [Google Scholar]
- 39.Bhidayasiri R, Plant GT, Leigh RJ. A hypothetical scheme for the brainstem control of vertical gaze. Neurology. 2000;54:1985–1993. doi: 10.1212/wnl.54.10.1985. [DOI] [PubMed] [Google Scholar]
- 40.Pflugshaupt T, Nyffeler T, von Wartburg R, Hess CW, Muri RM. Loss of exploratory vertical saccades after unilateral frontal eye field damage. J Neurol Neurosurg Psychiatry. 2008;79:474–477. doi: 10.1136/jnnp.2007.132290. [DOI] [PubMed] [Google Scholar]
- 41.Nopoulos PC, Aylward EH, Ross CA, Johnson HJ, Magnotta VA, Juhl AR, Pierson RK, Mills J, Langbehn DR, Paulsen JS. Cerebral cortex structure in prodromal Huntington disease. Neurobiol Dis. 2010;40:544–554. doi: 10.1016/j.nbd.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavese N, Gerhard A, Tai YF, Ho AK, Turkheimer F, Barker RA, Brooks DJ, Piccini P. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology. 2006;66:1638–1643. doi: 10.1212/01.wnl.0000222734.56412.17. [DOI] [PubMed] [Google Scholar]
- 43.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe K, Lauwereyns J, Hikosaka O. Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus. J Neurosci. 2003;23:10052–10057. doi: 10.1523/JNEUROSCI.23-31-10052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]