Abstract
Purpose
To determine if early restraint of axial elongation in response to plus lenses increases the subsequent response to interrupted hyperopia.
Methods
The normal, interrupted hyperopia group (n=5) had normal visual exposure until 24 days of visual experience (VE). Then, from 24 to 45 days of VE, the animals wore binocular −4 D lenses which shifted the refractive state of the eyes in the direction of hyperopia. Interrupted hyperopia was produced by removing the lenses for 2 hours per day. The early restraint, interrupted hyperopia group (n=5) wore binocular +4 D lenses continuously from 11 to 24 days of VE, becoming emmetropic with the lenses in place and hyperopic when they were removed. Then, from 24 to 45 days of VE, the lenses were removed 22 hours per day and replaced for 2 hours per day. This created the same initial regimen of interrupted hyperopia as in the normal, interrupted hyperopia group. A plus-lens control group wore binocular +4 D lenses (n=5) continuously from 11 to 45 Days of VE to assess the stability of the refractive compensation.
Results
In the normal, interrupted hyperopia animals, 2 hours of relief from the imposed hyperopia was sufficient to prevent myopia development. In the early restraint, interrupted hyperopia animals, 2 hours of relief from the hyperopia did not prevent myopia development; the eyes became myopic while wearing the lens. The control animals compensated for the +4 D lenses and maintained a stable with-the-lens emmetropia through 45 days of VE, demonstrating that the myopic shift in the early-restraint group was due to the interrupted hyperopia.
Conclusions
Compensation for plus lenses, involving slowed axial elongation, increases the response to subsequent interrupted hyperopia. Similar to previous reports of an eye-size factor in elongated eyes, these data provide evidence for an eye-size mechanism operating, in this case, in eyes that have restrained their axial length.
Keywords: myopia, animal models, refractive error, emmetropization, susceptibility, axial elongation, eye-size mechanism
At birth, animal and human eyes have a broad distribution of refractive errors. Typically, but not universally, they are hyperopic.1-8 This broad distribution may reflect normal variability between the genetically-determined focal plane and the (at birth) genetically-determined axial length.9 In most individuals, a visually-guided emmetropization mechanism10-14 rapidly reduces the initial refractive error and interocular differences; the majority of eyes achieve near-emmetropia (typically, a slight hyperopia) within weeks (chick, tree shrew, marmoset, guinea pig) or months (macaque monkey, human).1-8,15-18
This emmetropization mechanism corrects the mismatch between the focal plane and the axial length by modulating axial growth above, or below, that which would occur solely from genetically-determined growth (the “unadjusted” axial elongation rate).9 The eyes of human infants who are hyperopic at 3 months of age grow axially more rapidly during the next six months than do the eyes that are emmetropic.16 In animals, hyperopia, produced by wearing a minus lens, similarly produces an increase in axial elongation rate (vitreous chamber depth) so that the eyes become emmetropic while the lens is in place.19-23 With the lens removed, the eyes are myopic and also are elongated compared with untreated fellow control (or normal) eyes.
It is well established that, if minus lens wear or form deprivation is discontinued, the elongated eyes in juvenile animals experience myopic refractive error and typically will recover from this myopia by slowing their elongation rate.24,25 The recovery is visually-guided and results in the eyes regaining emmetropia with an axial length that matches that of untreated fellow control eyes or normal eyes.19,25-29
Previous studies have found that an “eye-size factor” or “shape factor” interacts with the emmetropization mechanism in elongated eyes.13,30,31 When exposed to the same refractive conditions, elongated eyes respond differently than do normal-sized eyes. For example, Nickla et al.30 found, in chicks that were myopic after form deprivation, that removing the diffuser 2 hours per day was sufficient to produce slowed eye growth and refractive recovery despite being form-deprived the rest of the time. In contrast, normal chicks that experienced the same 2 hours of refractive myopia, produced with a plus lens, and also form deprived the rest of the time, did not respond with slowed eye growth or refractive compensation. The two groups of animals experienced a similar degree of initial myopia, but the elongated eyes responded to the myopia much more strongly than did the plus-lens treated normal-sized eyes. A similar difference was found in tree shrews when recovery from minus lens wear was compared with continuous plus-lens wear in age matched juvenile tree shrews.32 In both these studies, the eye-size factor appeared to act in concert with the myopic refractive error; the elongated eyes responded more powerfully, with slowed elongation, to the same myopic refractive stimulus.
In the present study, we investigated whether an eye-size factor may also exist in shortened eyes that have restrained their elongation. Our hypothesis was that eyes that have restrained their axial growth in response to plus-lens wear, and have become shorter than normal, will respond more strongly to hyperopia than will age-matched normal eyes. To examine this, we compared a group of normal tree shrews with a group that had compensated for plus lenses, exposing each group to the same hyperopic conditions. Note that throughout this report, we describe the refractive state of the eyes, but because we did not control for viewing distance or make direct measures, we do not speculate on the amount of myopic or hyperopic defocus that the eyes may have experienced.
METHODS
Subjects and Experimental Groups
Maternally reared tree shrews (Tupaia glis belangeri), housed in a breeding colony on a 14 h light ON/10 h light OFF schedule, were the subjects in this study. The animals were housed in individual cages with illuminance of 500-1000 lux, measured at the top of the cages. All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
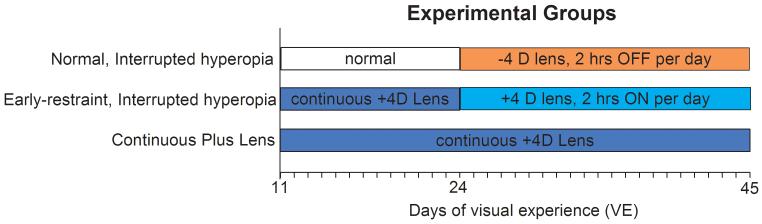
As illustrated in Fig. 1, there were three experimental groups. The normal, interrupted hyperopia group (n=5), had normal visual experience until 24 days of VE (days of visual experience; days after natural eyelid opening at approximately 20 postnatal days).2 From 24 to 45 days of VE they then wore binocular −4 D lenses, which shifted the refraction in the hyperopic direction. The lenses were removed for two hours per day from approximately 9:30 to 11:30 AM, providing relief from the lens-induced hyperopia.
Figure 1.
Experimental groups. A color version of this figure is available online at www.optvissci.com.
The early restraint, interrupted hyperopia animals (n=5) wore binocular +4 D lenses from 11 to 24 days of VE and became emmetropic while wearing the lenses. Starting at 24 days of VE the lenses were removed for 22 hours per day, which made the eyes hyperopic, and replaced for two hours per day from approximately 9:30 to 11:30 AM. Both the normal and the early-restraint interrupted-hyperopia animals initially experienced a hyperopic refractive state (of approximately 4 D) starting at 24 days of VE for 22 hours per day (12 hours while the colony lights were on) and had relief from the hyperopia for 2 hours per day. In the normal group the relief was achieved by removing the −4 D lens for 2 hours per day, while in the early restraint group it was achieved by wearing the +4 D lens for 2 hours per day.
We did not use continuous hyperopia for either the normal or early-restraint group because it is such a powerful stimulus that it would be expected to produce elongation in all eyes and would overwhelm any eye-size effect.19,20,23,33 Rather, we compared the early restraint animals vs. normal animals using a well-established temporal non-linearity that has been observed in the response of the eyes of several species to lens-imposed hyperopia: if the imposed hyperopia is removed for 2 hours per day, allowing the eyes relief from the hyperopia, normal eyes do not elongate to compensate for the hyperopia, even though it is present the rest of the day.34-36 Our prediction was that the eye-size factor would cause the early-restraint eyes, when exposed to interrupted hyperopia, to respond differently from the normal eyes and to develop a myopic shift in refraction that was associated with a return toward age-normal axial length.
Finally, a plus-lens control group (n=5) wore binocular +4 D lenses continuously for 34 days, from 11 to 45 days of VE, to verify that continuous plus-lens wear would result not only in attaining with-the-lens emmetropia but that this refractive condition would remain stable throughout the time-period when interrupted hyperopia was experienced by other groups. At 45 days of VE, lens wear was discontinued and the refractions measured over a period of 25 days to monitor the expected post-treatment refractive recovery produced by continuous hyperopia. Four of the animals had A-scan ultrasound measures of ocular component dimensions, using similar procedures as in previous studies2 at 10, 45, and 70 days of VE. The data from these animals were previously reported as part of the “young +4 D lens” group by Siegwart and Norton.32
Goggle Procedure and Ocular Measurements
A lightweight aluminum goggle that clipped onto a dental acrylic pedestal attached to the animal’s head was used to hold clear PMMA lenses (12 mm diameter, no edge bevels to give a maximum optical zone, Conforma Contact Lenses) in front of the eyes.37 The dental acrylic pedestal was installed at 10 ± 1 days of VE as previously described.38 Immediately before the pedestal was installed, A-scan ultrasonography was performed2 under anesthesia (Ketamine 17.5 mg; Xylazine 1.2 mg) to ensure that there were no abnormal axial differences between the eyes and to establish a pre-treatment axial length.
Lens treatment was begun by clipping the goggle onto the dental acrylic pedestal at the appropriate age (24 days of VE for the normal, interrupted-hyperopia group or 11 days of VE for the early-restraint interrupted-hyperopia and the plus-lens control groups). The lenses in all groups were cleaned twice each day at approximately 9:00 AM and 4:30 PM.
From 11 days of VE, refractive measures were made each day at approximately 9:00 AM. In lens-wearing groups the measures were made both with the lens removed and also with the lens in place.19,32 Comparison of with and without the lens measurements in the tree shrews achieved two purposes: 1) it allowed us to measure the refractive state that the eyes experienced while wearing the plus or minus lenses; 2) it showed that the eyes, even in the case of the binocular minus lenses, did not use accommodation to alter the effective power of the lenses, at least while the measurements were taken. All refractive measures were made in awake animals with an autorefractor (Nidek, Gamagori, Japan).
Because an emmetropic refractive state in these small eyes yields an autorefractor measure of approximately +4 D,39-41 this amount has been subtracted from all refractive values. Previous comparisons of cycloplegic vs. non-cycloplegic refractive measures with this instrument in tree shrews found a slight (~0.8 D) hyperopic shift under atropine cycloplegia and similar differences in refraction between treated and control eyes.42
Statistics
Paired t-tests were used to test for differences between the right and left eyes within each group at the end of treatment. No statistically significant right-left eye differences were found in any binocular treatment group (paired t-test, p > 0.05). Therefore, the mean spherical equivalent value of the right and left eye for each animal was used for further data analysis. Unpaired t-tests were used to examine differences between groups. Examination of the data with non-parametric statistics showed the same significant differences.
RESULTS
Normal, Interrupted-Hyperopia Group
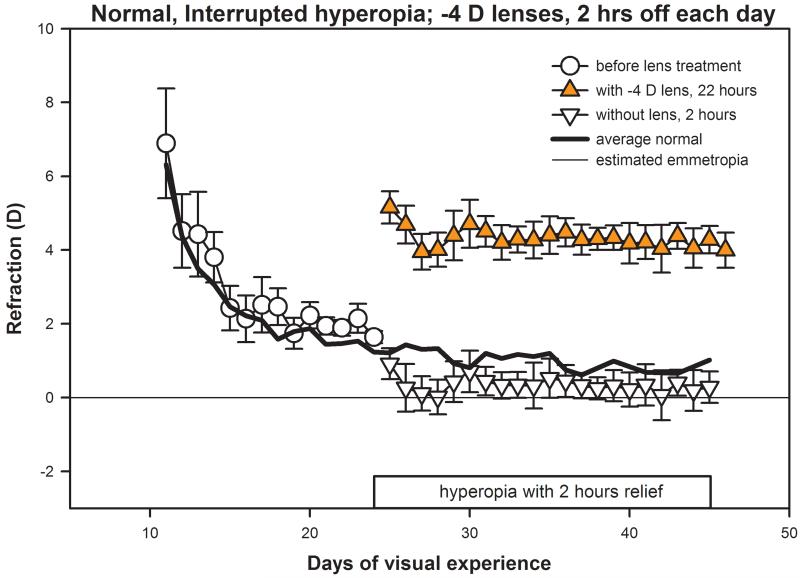
The daily non-cycloplegic refractions of the normal animals that wore binocular −4 D lenses with 2 hours relief per day are shown in Fig. 2. Before lens wear began at 24 days of VE, the refractive state of the eyes moved from an initial (11 days of VE) hyperopia of 6.9 ± 1.5 D (mean ± SEM), relative to estimated emmetropia, to a refraction of 1.6 ± 0.2 D at 24 days of VE, following the pattern seen in normal animals.2,15,19 When interrupted −4 D lens wear began at 24 days of VE, as shown in Fig. 2, a 3-4 day myopic shift was noted in the refractions of all five animals, both in measures made with and without the lenses in place over the eyes. During the period of interrupted −4 D lens wear, the mean normal daily refraction from 24 to 45 days of VE was 1.0 ± 0.1 D while the mean value in the treated group was 0.4 ± 0.1 D. This difference was significant (unpaired t-test, p < 0.05). The early myopic shift appeared to be transient. After 21 days of treatment (45 days of VE), the eyes measured without the lenses in place (0.3 ± 0.4 D) were not significantly lower than those of age-matched normal eyes. When the eyes were measured with the −4 D lenses in place at the end of treatment (45 VE), they were 4.3 ± 0.4 D hyperopic. This refractive stability when exposed to 22 hr per day of hyperopia is a similar result to that published previously with animals that were treated monocularly.34 In both studies, 2 hours of relief each day from hyperopia counteracted the myopiagenic effect of the negative lenses which, if left in place 24 hours per day, produce refractive compensation so that the eyes are emmetropic wearing the lens and myopic when it is removed.19
Figure 2.
Daily refractive measures (spherical equivalent, mean of both eyes) of the normal, interrupted-hyperopia animals that had normal visual experience until 24 days of VE. They then wore binocular −4 D lenses from 24 to 45 days of VE with two hours of relief per day, produced by removing the lenses. Circles indicate measures made before lens-wear began. Triangles indicate measures made with (filled triangles), and without (open triangles) the lenses. In this and subsequent figures, error bars indicate SEM. The solid black line represents the mean refraction of normal animals reported previously.19 A color version of this figure is available online at www.optvissci.com.
Early-restraint, Interrupted-Hyperopia Group
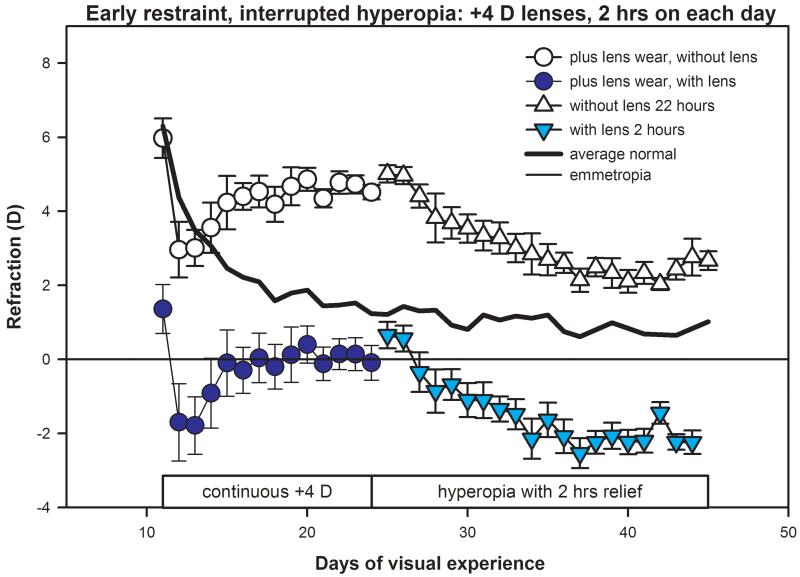
The daily non-cycloplegic refractions of the early-restraint animals that wore +4 D lenses continuously from 11 to 24 days of VE, followed by interrupted hyperopia, produced by lens removal for 22 hours per day from 24 to 45 days of VE are shown in Fig. 3. At the onset of plus lens wear, the refractions of this group were 6.0 ± 0.5 D, a value similar to that found in previous studies.32 For the first 1 – 2 days after the onset of plus-lens treatment, the eyes continued the normal rapid decrease in refractive state and all eyes became myopic with the lenses in place (filled circles in Fig. 3). The eyes then gradually compensated for the myopia produced by the lenses and, by 24 days of VE, were approximately emmetropic (−0.1 ± 0.5 D) with the lenses in place, but significantly less hyperopic (p < 0.05, unpaired t-test) than normal eyes which were 1.2 ± 0.1 D at 24 days of VE. With the +4 D lenses removed, the eyes were 4.5 ± 0.2 D hyperopic. Starting at 24 days of VE, the animals were exposed to this hyperopia by removing the lenses. Relief from this hyperopia was provided for 2 hours per day by replacing the +4 D lenses. In contrast to the normal, interrupted hyperopia animals, the refractions gradually moved in a myopic direction and, at the end of treatment, the eyes were, on average, −2.2 ± 0.3 D myopic with the lenses in place and still slightly hyperopic (2.7 ± 0.3 D) with the lenses removed.
Figure 3.
Daily refractions of the early-restraint, interrupted-hyperopia animals that wore binocular +4 D lenses continuously from 11 to 24 days of VE. Then, from 24 to 45 days of VE, the lenses were removed for 22 hours per day (12 hours while room lights were on), making the eyes optically hyperopic. Relief from the hyperopia was produced by replacing the lenses for 2 hours each day. Interrupted hyperopia produced a myopic shift in refractive state. A color version of this figure is available online at www.optvissci.com.
Continuous +4 D Lens Group
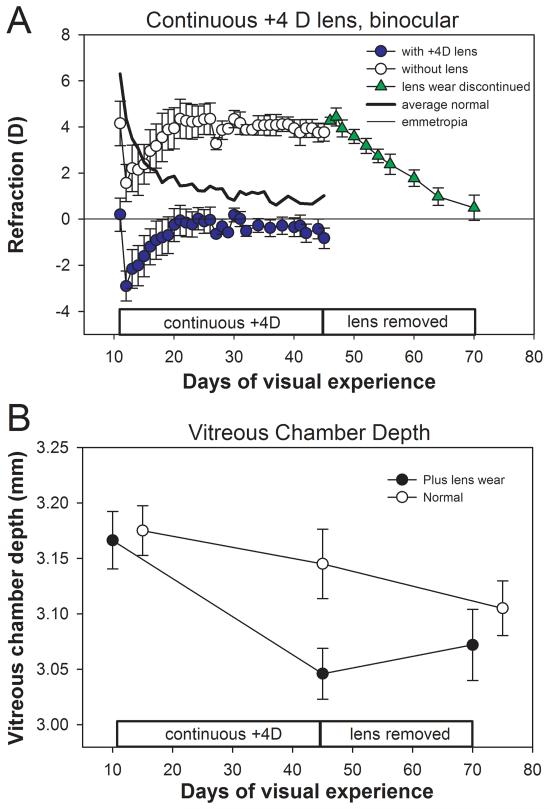
The average refractive state of the continuous plus-lens animals at the start of daily +4 D lens wear (11 days of VE) was 4.2 ± 1.0 D of hyperopia relative to estimated emmetropia (open circles in Fig. 4A). This starting value was lower than the other groups at 11 days of VE, but the difference between the groups was not significant (ANOVA, p = 0.17). The plus lenses shifted the refraction to near-emmetropia. The eyes continued the normal rapid decrease in refractive state for 1 – 2 days and all eyes became myopic with the lenses in place (filled circles in Fig. 4A). The eyes then gradually compensated for the myopia produced by the lenses and, by 24 days of VE, were emmetropic (0.0 ± 0.5 D) with the lenses in place. After three more weeks of lens wear (45 days of VE), the eyes were still nearly emmetropic with the lenses in place (−0.8 ± 0.4 D), and with the lenses removed, were hyperopic 3.8 ± 0.5 D. However, the refractive state of these eyes (1.0 ± 0.1D), was significantly lower than the age-normal hyperopia of normal eyes (unpaired t-test, p<0.01). Importantly, the with-the-lens emmetropia experienced continuously was sufficient to maintain the eyes stable refractions during the period from 24 to 45 days of VE. When the plus lenses were removed at 45 days of VE, exposing the eyes to a continuous hyperopic refractive state, the eyes responded fully and had returned to emmetropia by 70 days of VE.
Figure 4.
A. Daily refractions of the animals that wore binocular +4 D lenses continuously from 11 to 45 days of VE. The figure is modified from Siegwart & Norton.32 B. Vitreous chamber depth for four of the five animals, compared with normal animals.2 During the age range studied, the normal vitreous chamber depth is decreasing because the overall axial length increases more slowly than the increase in lens thickness. When measured at 45 days of VE, after the eyes had compensated for the +4 D lenses, the vitreous chamber was shorter than normal, indicating plus-lens wear had slowed overall axial elongation. When measured after the plus lenses had been removed for 25 days, and the eyes had compensated for the hyperopic shift produced by removing the lenses, vitreous chamber depth had increased to nearly a normal value. A color version of this figure is available online at www.optvissci.com.
A-scan ultrasound measures were made at 10, 45, and 70 days of VE in 4 of the 5 binocular plus-lens animals (Fig. 4B). The average vitreous chamber depth decreased below normal (Fig. 4B, 3.05 ± 0.02 mm vs. 3.14 ± 0.03 mm, unpaired t-test, p < 0.05) during lens treatment. A similar slowing of the elongation rate during plus lens treatment in tree shrews was found by Metlapally and McBrien.43 When plus lens wear was discontinued, the vitreous chamber depth increased toward age-normal values.
It must be noted that axial ocular component dimensions were not measured at the start of interrupted hyperopia in the early-restraint, interrupted hyperopia animals because to do so would have required anesthesia that might have in some way interfered with the ability of the emmetropization mechanism to control the axial elongation rate. However, the measures made on the continuous plus-lens group, showed that plus-lens wear caused a slowing of axial elongation and removal of the plus lenses produced increased axial elongation and the myopic shift in refraction. By extrapolation, it is reasonable to assume that similar axial changes occurred in the early-restraint, interrupted hyperopia animals. For similar reasons, axial ocular component dimensions were not measured in the normal interrupted hyperopia group. Given the lack of refractive changes in the group, it is unlikely that there were any significant changes in ocular component dimensions.
DISCUSSION
Previous studies provide clear evidence that an emmetropization mechanism guides eyes toward emmetropia in the early “infantile”44 postnatal period6,7,10-14,16 and remains active throughout the slower growth “juvenile”44 period.19,24,25,45 In some instances it continues to function into adulthood.19,46,47 This mechanism is able to guide eyes toward emmetropia from both hyperopia and from myopia by increasing or decreasing the axial growth of the eye.
The existence of a visually guided emmetropization mechanism is generally accepted although the details of the underlying biological mechanism are not fully known. The results of this, and previous studies, demonstrate that visually-guided deviations of axial length from that which would occur without visual guidance (the “unadjusted axial length”9) modify the eye’s response to future visual guidance. These data demonstrate an eye-size effect on the performance of the emmetropization mechanism and provide evidence for an eye-size mechanism that in essence, competes with the emmetropization mechanism for control of axial length. While the goal of the emmetropization mechanism is to eliminate refractive error by driving the eye away from the unadjusted axial length if that length does not, by itself, match the optical power of the eye, the goal of the putative eye-size mechanism appears to be to return the eye to the unadjusted axial length.
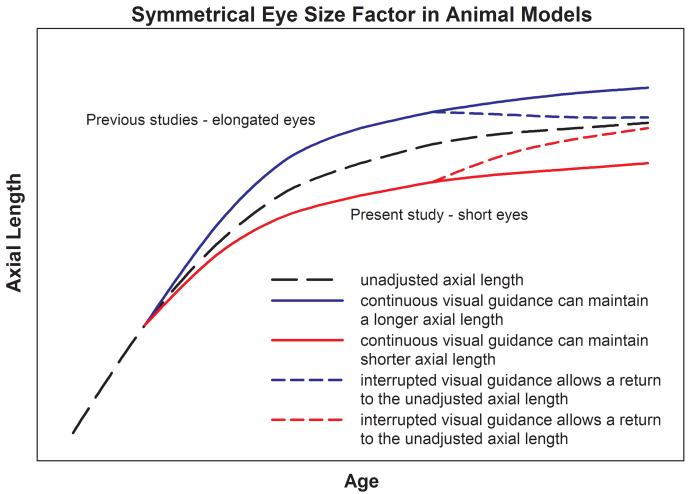
The Eye-Size Effect is Symmetrical
The results of this study, in combination with previous data, provide evidence that the effects of visually guided adjustment of axial length on the emmetropization mechanism are symmetrical. Examples of eye-size effects in elongated eyes, described in the Introduction30,32 and in other studies,13,31 show that essentially continuous, consistent refractive guidance is necessary to maintain eyes in an elongated state. The primary finding of the present study, as summarized in Fig. 5, is that continuous, consistent refractive guidance is also necessary to maintain eyes in a shortened axial condition. Thus, visually-guided deviation from the unadjusted axial length in either direction appears to produce a heightened sensitivity to visual stimuli (myopia in elongated eyes, hyperopia in the shortened eyes of the present study) that would return the eye to the unadjusted axial length if they were present continuously. The most stable axial length appears to be the unadjusted axial length. Zhu et al.48 reached a similar conclusion regarding the choroid: “Eyes in which the visual and shape-factors were in the same direction (removal of spectacle lenses) showed greater changes … than those in which lens compensation required further deviations from normal eye dimensions.”
Figure 5.
Interaction of the eye-size mechanism with visual guidance. Minus lens wear or form deprivation produces an increased axial length, relative to normal. If treatment is continuous, the emmetropization mechanism maintains the increased length. If the visual input to the emmetropization mechanism is interrupted by even brief periods of unrestricted vision, the eye-size factor causes a return toward the normal axial length. Continuous plus-lens wear produces, and maintains, a shortened axial length. If the visual guidance is interrupted, the eye-size mechanism causes a return toward the normal axial length. A color version of this figure is available online at www.optvissci.com.
Possible Mechanism
If an eye-size mechanism does exist, some form of information storage must occur – a form of homeostatic memory27,31 or “chalone”49 – so that eyes that have increased, or slowed, their axial elongation rate retain knowledge of the unadjusted axial length. This memory could reside in the retina, RPE, or choroid where altered eye length might somehow alter the temporal integration of “go” and “stop” visual stimuli in a way that moves the eye back toward the unadjusted axial length. However, based on our studies of sclera50-53, we speculate that the symmetrical eye-size effect could have its basis in tree shrews in the macro-structure of the sclera (the waviness of the collagen lamellae) and the alterations to that structure that occur when axial length is decreased or increased.
The nature of the information “storage” may differ across species. In chicks, eye enlargement in response to form deprivation or minus lenses involves increased growth of the cartilaginous inner scleral layer. In eutherian mammals, like tree shrews, there is no inner cartilage layer; the sclera is a fibrous extracellular matrix comprised of layers (lamellae) of type I collagen, proteoglycans and other materials that are remodeled under the control of a signaling cascade originating in the retina.53,54 Like collagen in other structures,55 the bundles of collagen within the flattened scleral layers (lamellae) are wavy as they are laid down by the scleral fibroblasts under normal intraocular pressure. This wavy pattern may constitute the normal, or baseline, collagen pattern. When scleral remodeling is increased during minus lens wear or form deprivation, the loss of material and attachments between the lamellae53,56 may allow the layers to slip across each other and redistribute stress to the collagen lamellae that decreases the waviness. This straightening of the wavy collagen could create potential energy and motive force for slowed axial elongation and a return to normal waviness during recovery from minus-lens treatment or form deprivation.57 The effects of plus-lens wear on sclera have not been measured, but might include increased waviness (collagen “crimping”)55,58 that would facilitate expansion of the sclera to return to the normal, less-wavy pattern.
Research clearly indicates that the emmetropization mechanism is not a single discrete biological mechanism, but rather a series of linked biological mechanisms in the retina, RPE, choroid, and sclera. The same may be true for the putative mechanism that produces the observed eye-size effects. While further research is needed to uncover the biological mechanisms that produce the symmetrical eye-size effect, it is clear that, in experimental animals, visually guided adjustment of axial length modifies the performance of the emmetropization mechanism compared to an eye with unadjusted axial length. The act of responding to refractive error modifies the future response to refractive error.
Analogy with Children Born Myopic?
Although the direct evidence for a symmetrical eye-size effect is from animal models, a potential implication of an eye-size mechanism for human myopia should be discussed. In the animals, the emmetropization mechanism produced slowed axial elongation in order to achieve refractive emmetropia. Potentially, the eyes of at least some children that are born myopic, and that initially achieve emmetropia,59,60 may undergo a similar restraint of elongation; in order to achieve emmetropia, the emmetropization mechanism reduces growth below the rate that would have occurred if there were no intervention by the emmetropization mechanism. If the data from the continuous plus-lens tree shrews pertains to humans, more-or-less continuous emmetropic guidance can prevent the eye-size mechanism from moving the eyes back to a longer length which, in these children, would make the eyes myopic.
Like our early-restraint tree shrews, these eyes may be more responsive to interrupted hyperopic defocus associated with near work61 than are eyes in which the emmetropization mechanism has not needed to slow the elongation rate to achieve emmetropia. As shown in previous studies, children born myopic are more likely to develop myopia later in life than are children who are born hyperopic59,60 perhaps, at least in some cases, because of the action of an eye-size mechanism.
Summary
The results of the present study show that early restraint of the axial elongation rate predisposes the eyes of juvenile tree shrews to respond more strongly to a myopiagenic visual condition (interrupted hyperopia) than do eyes that have not similarly restrained their axial growth rate. This situation does not involve a genetic abnormality, nor does it require an abnormality or alteration in the functioning of the emmetropization mechanism. The prior restraint of axial elongation may place the emmetropization mechanism at a disadvantage in its efforts to oppose a putative eye-size mechanism when exposed to visual conditions that include interrupted hyperopia. Whether or not a similar mechanism occurs generally in vertebrates remains to be examined as does the question of how long a period of relief from hyperopia (more than the 2 hours used in this study) is needed after early restraint to counteract the actions of the eye-size mechanism.
ACKNOWLEDGMENTS
Grant support: RO1 EY005922, P30 EY003909 (CORE).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40:214–29. [PubMed] [Google Scholar]
- 2.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–42. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 3.Andison ME, Sivak JG, Bird DM. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. J Comp Physiol (A) 1992;170:565–74. doi: 10.1007/BF00199333. [DOI] [PubMed] [Google Scholar]
- 4.Pickett-Seltner RL, Sivak JG, Pasternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Res. 1988;28:323–8. doi: 10.1016/0042-6989(88)90160-5. [DOI] [PubMed] [Google Scholar]
- 5.Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–13. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldschmidt E. Refraction in the newborn. Acta Ophthalmol (Copenh) 1969;47:570–8. doi: 10.1111/j.1755-3768.1969.tb08143.x. [DOI] [PubMed] [Google Scholar]
- 7.Howlett MH, McFadden SA. Emmetropization and schematic eye models in developing pigmented guinea pigs. Vision Res. 2007;47:1178–90. doi: 10.1016/j.visres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–24. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- 9.Siegwart JT, Jr., Norton TT. Perspective: how might emmetropization and genetic factors produce myopia in normal eyes? Optom Vis Sci. 2011;88:365–72. doi: 10.1097/OPX.0b013e31820b053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norton TT. Animal models of myopia: learning how vision controls the size of the eye. ILAR J. 1999;40:59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 11.Wildsoet CF. Active emmetropization—evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–90. [PubMed] [Google Scholar]
- 12.Smith EL, 3rd, Hung LF, Harwerth RS. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic Physiol Opt. 1999;19:90–102. [PubMed] [Google Scholar]
- 13.Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–34. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- 14.Troilo D. Neonatal eye growth and emmetropisation—a literature review. Eye. 1992;6:154–60. doi: 10.1038/eye.1992.31. [DOI] [PubMed] [Google Scholar]
- 15.Norton TT, Amedo AO, Siegwart JT., Jr. Darkness causes myopia in visually experienced tree shrews. Invest Ophthalmol Vis Sci. 2006;47:4700–7. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–80. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- 17.Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–8. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich DL, Atkinson J, Braddick O, Bobier W, Durden K. Reduction of infant myopia: a longitudinal cycloplegic study. Vision Res. 1995;35:1313–24. doi: 10.1016/0042-6989(94)00228-e. [DOI] [PubMed] [Google Scholar]
- 19.Norton TT, Amedo AO, Siegwart JT., Jr. The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50:564–76. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irving EL, Callender MG, Sivak JG. Inducing myopia, hyperopia, and astigmatism in chicks. Optom Vis Sci. 1991;68:364–8. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12:448–56. [PubMed] [Google Scholar]
- 22.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 23.Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–35. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 24.Siegwart JT, Jr., Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–15. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 25.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–63. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 26.Amedo AO, Norton TT. Visual guidance of recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Ophthalmic Physiol Opt. 2012;32:89–99. doi: 10.1111/j.1475-1313.2011.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Smith EL, 3rd, Hung LF, Harwerth RS. Effects of optically induced blur on the refractive status of young monkeys. Vision Res. 1994;34:293–301. doi: 10.1016/0042-6989(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 29.Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus) Vision Res. 2006;46:267–83. doi: 10.1016/j.visres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Nickla DL, Sharda V, Troilo D. Temporal integration characteristics of the axial and choroidal responses to myopic defocus induced by prior form deprivation versus positive spectacle lens wear in chickens. Optom Vis Sci. 2005;82:318–27. doi: 10.1097/01.opx.0000159368.31481.de. [DOI] [PubMed] [Google Scholar]
- 31.Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–50. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- 32.Siegwart JT, Jr., Norton TT. Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91:660–9. doi: 10.1016/j.exer.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–27. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Shaikh AW, Siegwart JT, Jr., Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–15. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Smith EL, 3rd, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43:291–9. [PubMed] [Google Scholar]
- 36.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–36. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 37.Norton TT, Siegwart JT, Jr., Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–99. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegwart JT, Jr., Norton TT. Goggles for controlling the visual environment of small animals. Lab Anim Sci. 1994;44:292–4. [PubMed] [Google Scholar]
- 39.Ramamirtham R, Norton TT, Siegwart JT, Roorda A. Wave aberrations of tree shrew eyes. Invest Ophthalmol Vis Sci. 2003;44 E-Abstract 1986. [Google Scholar]
- 40.Norton TT, Wu WW, Siegwart JT., Jr. Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80:623–31. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–6. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 42.Norton TT, Siegwart JT, German AJ, Robertson J, Wu WW. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia. Invest Ophthalmol Vis Sci. 2000;41:S563. [Google Scholar]
- 43.Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8:1 1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- 44.Sorsby A, Leary GA. A longitudinal study of refraction and its components during growth. Spec Rep Ser Med Res Counc. 1969;309:1–41. [PubMed] [Google Scholar]
- 45.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 46.Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial eye elongation and myopic refractive shift in one-year-old chickens. Vision Res. 1998;38:1883–8. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- 47.Smith EL, 3rd, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom Vis Sci. 1999;76:428–32. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, Sidhu A, Cernota NR, Wallman J. The effect of eye size on lens-compensation in chicks. Invest Ophthalmol Vis Sci. 2012;53 E-Abstract 3441. [Google Scholar]
- 49.Bullough WS. Mitotic and functional homeostasis: a speculative review. Cancer Res. 1965;25:1683–727. [PubMed] [Google Scholar]
- 50.Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–81. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 51.Siegwart JT, Jr., Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 52.Siegwart JT, Jr., Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46:3484–92. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frost MR, Norton TT. Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest Ophthalmol Vis Sci. 2012;53:322–36. doi: 10.1167/iovs.11-8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82:185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Diamant J, Keller A, Baer E, Litt M, Arridge RG. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci. 1972;180:293–315. doi: 10.1098/rspb.1972.0019. [DOI] [PubMed] [Google Scholar]
- 56.Gao H, Frost MR, Siegwart JT, Jr., Norton TT. Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis. 2011;17:903–19. [PMC free article] [PubMed] [Google Scholar]
- 57.Franchi M, Trire A, Quaranta M, Orsini E, Ottani V. Collagen structure of tendon relates to function. ScientificWorldJournal. 2007;7:404–20. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foolen J, van Donkelaar CC, Soekhradj-Soechit S, Ito K. European Society of Biomechanics S.M. Perren Award 2010: an adaptation mechanism for fibrous tissue to sustained shortening. J Biomech. 2010;43:3168–76. doi: 10.1016/j.jbiomech.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 59.Howland HC, Waite S, Peck L. Noninvasive Assessment of the Visual System, vol. 3 OSA Technical Digest Series. Optical Society of America; Washington, DC: 1993. Early focusing history predicts later refractive state: a longitudinal photorefractive study; pp. 210–3. [Google Scholar]
- 60.Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clin Vision Sci. 1993;8:337–44. [Google Scholar]
- 61.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–4. [PubMed] [Google Scholar]