Abstract
Appropriate acute treatment with plasminogen activators (PAs) can significantly increase the probability of minimal or no disability in selected ischemic stroke patients. There is a great deal of evidence showing that intravenous recombinant tissue PAs (rt-PA) infusion accomplishes this goal, recanalization with other PAs has also been demonstrated in the development of this treatment. Recanalization of symptomatic, documented carotid or vertebrobasilar arterial territory occlusions have also been achieved by local intra-arterial PA delivery, although only a single prospective double-blinded randomized placebo-controlled study has been reported. The increase in intracerebral hemorrhage with these agents by either delivery approach underscores the need for careful patient selection, dose-appropriate safety and efficacy, proper clinical trial design, and an understanding of the evolution of cerebral tissue injury due to focal ischemia. Principles underlying the evolution of focal ischemia have been expanded by experience with acute PA intervention. Several questions remain open that concern the manner in which PAs can be applied acutely in ischemic stroke and how injury development can be limited.
Keywords: stroke, plasminogen activators, acute intervention, thrombolysis, hemorrhage, intra-arterial, intravenous, thrombosis
Plasminogen activators (PAs) have found a firm niche in the acute therapeutic management of thrombotic/thromboembolic ischemic stroke. Despite inherent risk, conditions have been developed for successful treatment with improvement in outcome of a population of patients that had heretofore faced disregard and limited therapeutic options. With the evolution of the use of PAs in this setting, there has been the parallel evolution of new concepts of focal ischemic brain injury. Important to the outcomes of thrombus lysis in this setting has been the concept of the “penumbra” and its interrelationship to the protection of the targeted “territories-at-risk” by the collateral and vascular anastomoses of those territories. The demonstration that thrombi occlude the arteries supplying the symptomatic territory has been essential to proving the concept that PAs can be developed for this indication. Further evidence has come from the growing experience with other antithrombotic agents (i.e., antiplatelet agents and anticoagulants) that support the thrombotic features of the focal cerebral ischemic events. It is also evident that focal ischemia generates thrombotic occlusion of the microvasculature.
Essential to the successful outcomes of thrombus lysis is the timing of the intervention relative to the duration of ischemia. Acute intervention was defined by modern PA application in this setting. Imaging techniques have also played significant roles. The impact of both timing and ischemia duration on hemorrhagic transformation is central to the safety concerns of the acute use of PAs in ischemic stroke. Finally, off-target effects of thrombolytic agents have raised questions about the pathophysiology of the ischemic event. In this article, many of these features are discussed, as is the historical basis for the development of PAs in the setting of acute intervention and the outcomes of level 1 clinical studies. Though far from exhaustive, this presentation will consider some of the questions of the field that could serve as issues for future research.
Ischemic stroke represents a group of disorders of the central nervous system (CNS) vasculature, including occlusions of the arterial supply of the brain.1,2 It is now evident that removal of these symptomatic arterial occlusions in a timely manner limits the risk of residual neurologic deficit. Atherothrombotic stroke results from cerebral arterial occlusions due either to in situ arterial thrombosis or artery-to-artery emboli originating in intracranial arteries or the internal carotid artery (ICA), the aortic arch, or the vertebral-basilar (VB) artery system. Studies employing angiography within 6 hours of the onset of carotid artery territory ischemic stroke have shown a high frequency of atherothrombotic or thromboembolic arterial occlusions in the symptomatic territory.3–9 For instance, three prospective studies documented symptomatic occlusion of a brain-supplying artery within the carotid territory in 81% of patients within 8 hours of symptom onset.8 Separate studies have shown arterial occlusions in 59% of patients at 24 hours and in 41% at 1 week after symptom onset in patients with focal cerebral ischemia.3,4,6–8 Large-artery events in the carotid territory are primarily thrombotic or embolic in origin. VB ischemia results from in situ thrombosis on atheromata in the basilar or vertebral arteries or from emboli from more proximal sources.10
Generally, approximately 16 to 23% of ischemic stroke are due to thromboembolic events, 40 to 57% are due to in situ atherothrombotic strokes, 14% are due to lacunar strokes, and 10 to 15% of strokes are due to symptomatic hemorrhage. Emboli from left ventricular mural thrombi after myocardial infarction (MI), prosthetic valves, rheumatic valvular vegetations, and thromboemboli arising during nonvalvular atrial fibrillation (AF) can cause focal cerebral ischemia. The incidence of systemic thromboembolism, including stroke, after MI is 1 to 3% per year.11–16 In contrast, the risk of cerebral infarction after an episode of nonvalvular AF can be 20% per year, with a mortality rate from the initial infarct of 38% per year.17 This is in accordance with the experience of Hart et al, who reported a 34.8% incidence of symptomatic embolism (13% cerebral) in individuals not undergoing immediate anticoagulation.18
Ischemic stroke is still defined in terms of the duration, persistence, and severity of the symptoms within the syndrome. Transient ischemic attacks (TIAs) are events of short duration—usually less than 1 hour duration, or at most 24 hours—with clinical resolution.19 Nonetheless, persistent neuronal injury occurs. Events of extended duration have been termed minor strokes and reversible ischemic neurological deficits (RINDs). Completed strokes are those with permanent detectable injury. The depth of ischemia and the duration are relevant to the persistence of the defects and, potentially, their reversibility in the setting of re-establishment of local blood flow (see The Penumbra, below).
TIAs (e.g., amaurosis fugax)20–22 are often accompanied by subsequent stroke or other arterial disease. Completed stroke has been reported in 40 to 75% of individuals with one or more TIAs,23,24 with a prevalence of approximately 30% per year.23,24 Among individuals with a premonitory TIA, approximately 50% may have a stroke within the first year.25 Approximately 64% of individuals with TIAs showed evidence of cerebral infarction in their initial computerized tomography (CT) scan.26 The early risk of stroke is high, occurring in 3 to 5% of patients within 48 hours after a TIA and in 8 to 14% within 3 months.27 Stroke-related death and cardiac death occur in 28 and 37% of patients with TIAs, respectively, indicating that cardiovascular mortality is significant in the population of patients with stroke.25 A lower mortality is suggested from placebo groups in large trials of antithrombotic treatment. The combined outcome events of stroke, MI, and death occurred in 14% of individuals treated with the placebo for more than 2 years in the UK-TIA (United Kingdom transient ischemic attack) Study Group Trial.28
These thrombotic events are supported by migrating thromboemboli and refractile bodies observed in the retinal arteries of patients with focal cerebral ischemia29–31 and by thrombi in the cortical and carotid arteries during cerebral ischemia.32,33
Indirect evidence for the involvement of thrombi in cerebrovascular ischemia derives from observations of platelet, coagulation, and fibrinolytic system activation in the postictal setting.34–38 Platelet activation and evidence of platelet aggregation have been reported in patients with recent atherothrombotic and thromboembolic cerebral ischemia.34,37–44 Experimental studies in nonhuman primates confirm the deposition of fibrin and activated platelets in microvessels of the ischemic regions shortly after middle cerebral artery (MCA) occlusion.45–50 These observations implicate the local activation of hemostasis in the ischemic microvascular bed.
Patients presenting with an ischemic stroke are at risk for recurrence; the risk is highest within the first year. Following a first-time ischemic stroke, the rate of subsequent ischemic stroke, MI, death, or vascular-related death is 10 to 12% per year.51–53 The 5-year cumulative incidence of secondary stroke was 42% among men in one prospective study. Another study noted a 32% 7-year cumulative incidence for recurrent stroke.54
From the clinical standpoint, and relevant to clinical trial outcomes, improvements in neurologic outcome occur among patients who survive a stroke. Both neurologic presentation and outcome depend on stroke subtype.55 Patients with lacunar strokes fare better than do patients with thromboembolic events. The conditions under which stroke occurs can alter outcome. Female patients have a better 1-year survival and lower injury volume than male patients.56–58
Stroke-related mortality during the first 7 days after ictus was examined prospectively by Silver et al.59 Cerebral edema from large, hemispheric ischemic lesions led to transtentorial herniation and death in 78% of 46 patients who died in that interval. This is consistent with the number of fatal events (82%) in one retrospective pathology study.60 Often, the mortality results from failure to recanalize a major occluded brain-supplying artery (e.g., M1 segment of the MCA). This is evident in nonhuman primate models of MCA occlusion.45,61
Hemorrhagic Transformation
Hemorrhage accounts for approximately 10 to 15% of all strokes.1,5,6,8,62–67 However, hemorrhagic transformation of the ischemic lesion occurs commonly during thromboembolic stroke and is classified as hemorrhagic infarction (HI), parenchymal hematoma (PH), or both. HI are petechial or confluent petechial hemorrhages within the regions of ischemic injury.68–71 HI occurs in 50 to 70% of individuals in postmortem studies.68–70 HI are petechial or confluent petechial hemorrhages within the regions of ischemic injury.68–71 In clinical series, 10 to 43% of nonanticoagulated individuals with acute cerebral infarction in CT scan–based studies and 37.5% of patients with cardiogenic cerebral embolism demonstrate HI,72,73 compared with only 1.9% of patients with carotid territory thrombosis.74 Petechial hemorrhage can result from ischemia-related degradation of the microvessel basal lamina matrix components.75,76 Clinical deterioration is not usually associated with HI and is more likely due to the volume of injury.8,77
PH is a homogeneous mass of blood (coagulum), often associated with edema, that can displace brain tissue. Many reports of symptomatic hemorrhage in patients with cerebral embolism are associated with antithrombotic treatment.78,79 Antithrombotic agents, including PAs, characteristically increase the risk of hemorrhage within the cerebral ischemic beds.80
In addition, PH can result from the rupture of small penetrating arteries. An increased risk of intracerebral hemorrhage is also associated with advanced age (> 75 years) and the intensity of anticoagulation.64,65,80,81 Normal platelet function is necessary to maintain the integrity of cerebrovascular beds and to prevent clinically detectable hemorrhage.
The Penumbra
A basis for all reperfusion strategies rests on the observation of regions of metabolically active tissue within the ischemic territory. The evolving ischemic lesion stabilizes as an infarction by 24 hours after arterial occlusion of the supply artery.82–86 Astrup and colleagues depicted the development of the core of ischemic injury, destined for tissue infarction, with a circumferential region or “penumbra” of metabolically metastable tissue that has the potential for full recovery, if regional cerebral blood flow (rCBF) could be returned to normal levels.87–91 This concept encompasses characteristic electrophysiological changes, biochemical and molecular alterations, microvessel responses, metabolic changes, and regional differences in tissue perfusion and H2O diffusion as displayed by imaging studies (e.g., the DEFUSE study).91–93 By positron emission tomography (PET), infarction corresponds to rCBF below 12 mL/100 g/min and the cerebral metabolic rate for oxygen (CMRO2) below 65 µmol/100 g/min.94 The penumbra has been defined as rCBF decreased to 12 to 22 mL/ 100 g/min, CMRO2 above 65 µmol/100 g/min, and an oxygen extraction fraction (OEF) of 50 to 90%.94 Experimental data from vascular and molecular modeling studies, as well as recent imaging work, indicate that the penumbra is dynamic and also demonstrates that in the early minutes and hours after ischemia onset, the core contains pockets of injury surrounded by “mini-penumbrae.”91 It has been hypothesized that failure to resolve these mini-penumbrae into viable tissue leads to the homogenous injured tissue.91 These cell and microvessel events relate to local microvascular flow and regional perfusion, and they may underlie recoverability of the ischemic territory-at-risk when flow is re-established. Normal collateral arterial circuits protect the cerebral cortex and can contribute to the reversibility of the ischemic penumbra. Hence, acute reperfusion of the occluded artery (arteries) is expected to decrease the penumbra and recover normally functioning tissue.
Collateral Protection
Extraordinarily important to the maintenance of flow to brain regions, and protection of the territory-at-risk from ischemic injury, are anastomoses of the cerebral circulation. These include the common carotid arteries, the external carotid and the vertebral arteries, the ICA and the intracranial circulation through anastomoses of the ophthalmic artery, and the circle of Willis. Other meningeal anastomoses and secondary anastomoses are known.95 In the intracranial arterial system, the cerebral cortices are protected by a highly ordered, descending hexagonal array of penetrating arteries that terminate in microvessels and connect to the subcortical microvasculature.96,97 Cross-flow among the vascular “columns” is recognized. In the basal ganglia, a different and less well-understood set of anastomoses are achieved with the capillary branch points nearly every 30 µm.47 This reflects the subcortical structure and the lower rCBF seen in that territory. Both the cortical and basal ganglia microvasculature allow retrograde flow.
Transient Ischemic Attacks and Completed Stroke
TIAs and completed ischemic stroke often result from ongoing platelet and coagulation activation and from vascular injury to brain-supplying arteries. Changes that occur in plasma thrombin concentration and fibrin degradation product levels following ischemic stroke suggest that interruption of coagulation may be beneficial. Hence, there are roles for antiplatelet agents and anticoagulation.3–7,29,31,38,44,76,98 These should be considered, as patients who are candidates for PAs may be taking these agents.
Antiplatelet agents
Among accepted approaches for secondary prevention of ischemic cerebrovascular disease, aspirin (ASA) can reduce the incidence of TIAs and subsequent ischemic strokes.99,100 Dipyridamole (DP) when added to ASA reduces the incidence of stroke, MI, and death compared with what has been observed in patients who received placebo,101,102 or ASA alone.81,103,104 Generally, these antiplatelet regimens were started within months of the ictus; however, now there is reason to initiate oral treatment within 24 hours of the initial event. The thienopyridine ticlopidine has been shown to benefit patients with TIA/minor stroke105 and aid in the prevention of second strokes; it has been replaced by clopidogrel.106,107
ASA has been shown to reduce the incidence of secondary ischemic stroke after an initial completed stroke. The combination of ASA/DP in several formats appears to provide a significant reduction in second stroke risk. The European Stroke Prevention Study (ESPS) demonstrated a 33% reduction in the risk of stroke and death associated with the combination treatment.102 More recently, the ESPS-2 study compared the relative efficacy of extended-release (ER) DP (400 mg/day), ASA (50 mg/day), the combination of ASA/ER-DP, and placebo in limiting stroke, death, or both in individuals presenting with TIAs or stroke in a 2 × 2 factorial design.104 ASA/ER-DP (relative risk reduction [RRR], 36.7%) was superior to ASA or ER-DP alone (RRR for stroke, 15.8 and 17.7%, respectively), which were in turn superior to placebo at 2-year follow-up. For patients with a history of TIAs or recent stroke, the combination was superior to no treatment, but was not substantially different from ASA alone for the outcomes of stroke, MI, and mortality. The separate European/ Australian Stroke Prevention in Reversible Ischaemia Trial (ESPRIT) indicated that patients receiving the combination ASA/DP or ASA/ER-DP had a lower incidence of vascular demise, nonfatal stroke, nonfatal MI, or major hemorrhage, than did those receiving ASA alone (absolute risk reduction 1.0% per year).104
In comparison to ASA (325 mg per day) the Clopidogrel versus Aspirin in Individuals at Risk of Ischemic Events (CAPRIE) study demonstrated a significant 8.7% reduction in the combined risk of ischemic stroke, MI, or vascular-related death by clopidogrel (75 mg per day) in patients presenting with ischemic or lacunar stroke, MI less than 35 days old, or symptomatic atherosclerotic peripheral arterial disease.106 The overall benefit was driven by the peripheral arterial disease (PAD) outcome. However, a direct comparison for stroke-related outcomes is only now being investigated [http://clinicaltrials.gov/ct2/show/NCT00991029]. Regarding combination antiplatelet use, the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial compared combination ASA (75 mg)/clopidogrel (75 mg) with ASA (75 mg)/placebo for patients with multiple atherothrombotic risk factors and demonstrated no significant difference for the composite of MI, stroke, or cardiovascular mortality.108 The large (7,599 patients) Management of Atherothrombosis with Clopidogrel in high-risk patients (MATCH) trial compared the efficacy of ASA (75 mg)/clopidogrel (75 mg) to clopidogrel (75 mg) alone for ischemic events including stroke. No difference was seen in outcome between the two groups, though the ASA/clopidogrel combination was associated with significantly higher incidence of life-threatening hemorrhage (including intracranial hemorrhage).107
Anticoagulants
Some 30 years ago, uncontrolled reports suggested that heparin could decrease the incidence of basilar artery TIAs.109,110 Although two studies reported that long-term anticoagulation decreased the incidence of strokes and/ or vascular death among patients with recent TIAs, subsequent trials failed to show superiority of anticoagulation over its comparator for the incidence of stroke or death.111,112 Only the Cerebral Embolism Study Group trial indicated a benefit from immediate anticoagulation for cardiogenic embolism.113 The Warfarin-Aspirin Recurrent Stroke Study (WARSS) found no difference in outcomes of TIA and stroke patients treated with oral anticoagulation under tight control compared with patients treated with ASA.114,115
Despite uncontrolled studies suggesting little benefit of anticoagulants to prevent second stroke events,111,116–120 a more complex picture emerges from more recent reports. Data from the International Stroke Trial (IST) and the Chinese Acute Stroke Trial (CAST) demonstrated a significant reduction in total recurrent ischemic strokes with heparin exposure within 14 days of ictus.64,65,121,122 However, the increased incidence of intracerebral hemorrhage neutralized this advantage. Similarly, a series of studies with low-molecular-weight heparins (LMWHs) intended to alter the outcome of first-ever stroke were, with one exception,123 neutral— showing no apparent benefit or increase in hemorrhagic risk.124–126 However, none of the studies examined the impact of the LMWH prior to 24 hours after ictus, whereas the benefits shown in animal models occur when the LMWH (enoxaparin) is introduced acutely.127 At present, there is no evidence of an advantage to the use of anticoagulants for completed stroke.128,129 Notable is that none of those studies instituted anticoagulants in the acute phase.
Nonetheless, oral anticoagulation is effective in prevention of thromboembolic stroke from a cardiac source. A series of primary prevention trials for nonvalvular atrial fibrillation (AF)—including Copenhagen Atrial Fibrillation, Aspirin and Anticoagulant Therapy study, Stroke Prevention in Atrial Fibrillation I and II, European Atrial Fibrillation Trial, and the Boston Area Anticoagulation Trial for Atrial Fibrillation— have been seminal.130–136 Those outcomes have been supported by the demonstrated noninferiority of the oral antithrombin dabigatran and the oral anti-Xa inhibitors rivaroxaban and apixaban to warfarin in primary prevention.137–141 Dabigatran and apixaban are superior for efficacy. All such agents appear to have a lower incidence of intracerebral hemorrhage.
Clinically detectable systemic embolism from valvular cardiovascular disease (i.e., untreated rheumatic mitral stenosis and mitral insufficiency) occurs with a 3.7% (stenosis) and 1.9% (regurgitation) yearly incidence and may recur within 6 to 12 months after the signal embolism.142–144 The rate of systemic embolic events with valve prostheses is also increased,145–148 and cerebral embolism is greatly increased by concurrent AF.143,149,150
Oral anticoagulants generally reduce the incidence of thromboembolic events associated with mechanical prosthetic cardiac valves. Limited studies suggest that warfarin anticoagulation to a target international normalized ratio (INR) of 2.5 to 3.5 is efficacious151,152 for valves in the aortic position (bileaflet and tilting variety) compared with valves in the mitral position.146 The addition of antiplatelet agents increases protection but may increase the risk of hemorrhagic complications.153–156 Based on a tolerable safety profile, low-dose ASA is often added to warfarin.157 The combination of DP (400 mg/day) with warfarin anticoagulation (INR 2.0 to 2.5) further reduces the incidence of thromboembolic events.158,159 However, meta-analysis of trials combining DP with oral anticoagulation indicated a decrease in fatal and nonfatal thromboemboli.160,161
For bioprostheses, the greatest risk for systemic thromboembolic events occurs during the first 3 months after valve placement.162–164 The risk increases with concomitant AF.164,165 The possibility that ASA use might produce similar risk reduction in patients in sinus rhythm has not been rigorously tested.166–168 Hence, accepted practice requires anticoagulation for the initial 3 months following placement of the bioprosthesis.
Anticoagulation during the acute in-hospital and in the chronic posthospitalization phase following MI reduces the number of cerebrovascular events,11–16,169 whereas evidence for the effectiveness of antiplatelet agents continues to be scant.106 Two placebo-controlled trials have demonstrated a significant decrease in stroke incidence with anticoagulation.12,16 In both trials, hemorrhage was more common in the active treatment group. In the Warfarin Reinfarction Study (WARIS), the target INR was 2.8 to 4.8. The follow-on WARIS-II trial of warfarin (INR = 2.8 to 4.2), warfarin and ASA (75 mg per day), or ASA (160 mg per day) alone in patients with MI demonstrated that the combination warfarin/ASA was superior to the other regimens for the combined outcomes of death, nonfatal reinfarction, and cerebral stroke.170,171 Hemorrhagic risk was greatest in the warfarin/ASA cohort at the end of the study; however the long-term incidence of occult hemorrhage was not different among the three cohorts, suggesting overall benefit with INR of 2.0 to 3.0 in patients with a low or intermediate risk of hemorrhage.171,172
Plasminogen Activator Approaches to Cerebral Ischemia
The concept that PAs might limit the consequences of ischemic stroke first appeared in the 1960s.173 Based on the evolution in the understanding of the roles of plasmin generation in normal physiology and the development of PAs for systemic administration, interest in their use in ischemic stroke took hold.174
The use of PAs in the setting of TIAs and completed stroke has been problematic. This is in large part because of the difficulty in determining whether the studies selected patients on the basis of known pathology or stroke subtypes. Additionally, the time from symptom onset to treatment was often rather vague. Early PA trials in patients with completed stroke failed to demonstrate efficacy when symptomatic improvement and death were the primary outcomes. Clinical trials evaluating intravenous infusion of thrombolytic agents, usually streptokinase (SK) or urokinase-like plasminogen activator (u-PA), in completed stroke were inconclusive. The low-dose u-PA studies of Abe et al demonstrated safety but did not indicate efficacy.175,176 Ultimately, the report of Fletcher et al describing the lack of benefit in 31 patients treated with u-PA systemically within 10 to 12 hours of symptom onset, and the very troublesome frequency of symptomatic intracranial hemorrhage and related mortality, led to a general contraindication of the approach in the United States177
The long interval to treatment after stroke onset and the possibility that the stroke was caused by undiagnosed hemorrhage (scanning technologies were not widely available) were common concerns with those early studies (Table 1).178 A group of prospective, randomized, controlled, low-dose studies of intravenous u-PA or tissue plasminogen activator (t-PA) in patients with stable focal neurologic deficits of less than 5 days’ duration demonstrated no difference in clinical outcome or in the incidence of symptomatic intracerebral hemorrhage.179–182
Table 1.
Acute intervention
| Conditions | Contributor |
|---|---|
| Conceptual | The “penumbra” |
| Conceptual | Intervention within hours of ischemia onset would reduce subsequent injury and hemorrhage |
| Tissue imaging | Availability of CT technology to rule out cerebral hemorrhage as a cause for stroke; detection of hemorrhage as safety outcome |
| Vascular imaging | Angiographic demonstration of occlusion in symptomatic territories |
| Trial design | Adoption of appropriate trial design principles |
| Centers | Necessary involvement of multiple centers |
| Outcomes | Development of outcomes for recanalization and for clinical status |
Acute Interventions in Ischemic Stroke
A major advance in the approach to ischemic stroke occurred when the acute application of PAs was proposed (i.e., within 6 to 8 hours after symptom onset), based on the notion that rapid early recanalization of a thrombotic occlusion might restore territorial perfusion, restore cellular function, and reduce the ischemic injury and the evolution of parenchymal hemorrhage.178 It was suggested that rapid restoration of flow (perfusion) to the potentially reversible zones of neuron and tissue injury, the “penumbra,” could preserve intact tissue and improve regional function.87,183 If reperfusion were instituted early enough, before the evolving distribution was advanced, hemorrhagic transformation should not increase. The contributions of local vascular anatomy and collateral vascular protection to tissue perfusion, as well as the predominantly thrombotic basis for focal cerebral ischemia, provided conceptual support for attempts to achieve early recanalization with PAs. Recombinant t-PA (rt-PA) is now used in patients who present within 3 to 4.5 hours of symptom onset and do not have detectable cerebral hemorrhage to reduce injury or to improve clinical outcome. Other local infusion strategies and combined modality approaches are being considered.
Acute intervention with PAs in patients selected by strict CT scan and clinical criteria is associated with evidence of benefit.5,66 The development of direct intervention employs CT or magnetic resonance (MR) scanning for evidence of primary hemorrhage at baseline and in time for evidence of evolving brain injury. Initially, four-vessel angiography was employed to define the presence of arterial obstruction. Hence, both local intra-arterial and systemic infusion strategies of PAs evolved from the same experience and technical requirements. In early experience, recanalization of symptomatic carotid artery territory occlusions has approached 46 to 90% of symptomatic patients treated with direct intra-arterial infusion (of SK or u-PA within 8 hours).184–186 Similarly, recanalization of ICA occlusions by systemic PA infusion of occurs in < 25% patients,5,8,179,180,187,188 whereas partial or complete arterial recanalization has been reported in 34 to 59% of patients with more distal carotid artery territory occlusions.5,187–189
Systemic Infusion of Plasminogen Activators
Initial experience was gained in the direct intra-arterial delivery of SK or u-PA and provided proof of the concept that the acute intervention could be safe and result in arterial recanalization in a timely manner (Table 2). With the availability of the thrombus-selective t-PA derived from melanoma cells, three patients were treated acutely with varying results.190 Recanalization was achieved in two patients, but a fatal hemorrhage occurred in a patient who received t-PA by 12 hours.
Table 2.
Acute stroke: plasminogen activators
| Studya | Agent | Patients (n) | Δ(T-0)b (h) | Recanalization (%) | Hemorrhage (%) |
|---|---|---|---|---|---|
| Carotid territory: intra-arterial delivery | |||||
| del Zoppo et al185 | SK/u-PA | 20 | 1–24 | 90.0 | 20.0 |
| Mori et al184 | u-PA | 22 | 0.82–7 | 45.5 | 18.2 |
| Matsumoto and Satoh186 | u-PA | 40 | 1–24 | 60.0 | 32.5 |
| PROACT6 | scu-PA/h | 26 | <6.0 | 57.7 | 42.3 |
| C/h | 14 | <6.0 | 14.3 | 7.1 | |
| PROACT-2224 | scu-PA/h | 121 | <6.0 | 65.7 | 10.2 |
| —/h (iv) | 59 | <6.0 | 18.0 | 1.9 | |
| Carotid territory: intravenous delivery | |||||
| Yamaguchi188 | rt-PA | 52 | <6.0 | 38.5 | 28.6 |
| Von Kummer et al189 | rt-PA | 22 | <6.0 | 59.1 | 36.4 |
| del Zoppo et al8 | rt-PA | 93 (104)c | <8.0 | 34.4 | 30.8 |
| Mori et al187 | rt-PA | 19 | <6.0 | 47.4 | 52.6 |
| C | 12 | 16.7 | 41.7 | ||
| Yamaguchi205 | rt-PA | 47 (51)c | <6.0 | 21.3 | 47.1 |
| C | 46 (47)c | 4.4 | 46.8 | ||
Abbreviations: C, control; h, heparin; PROACT, Pro-urokinase in Acute Cerebral Thromboembolism; rt-PA, recombinant tissue-type plasminogen activator; scu-PA, single-chain urokinase plasminogen activator; SK, streptokinase.
Studies employing angiography.
Time from symptom onset to treatment.
Values within parentheses refer to the total number of patients treated in intention-to-treat group.
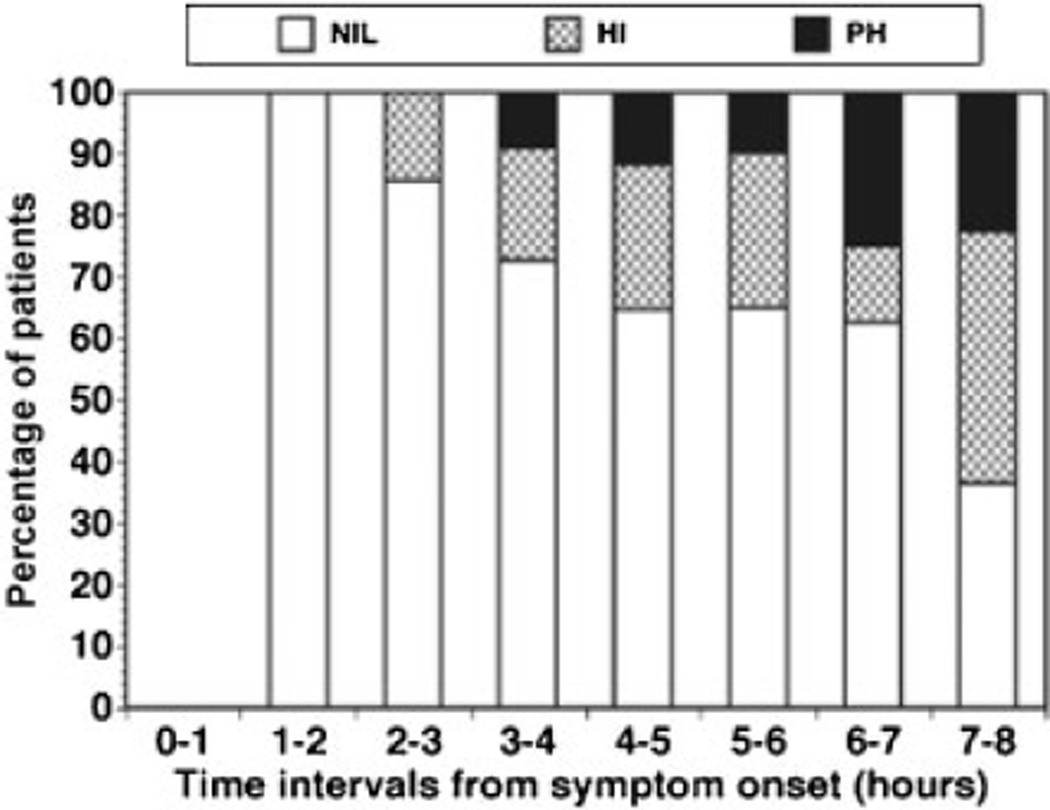
Two forms of rt-PA became available (single-chain and two-chain), allowing the conduct of well-conceived controlled clinical trials for recanalization efficacy and safety in the acute application in ischemic stroke.5,8 In preparation for a prospective phase III outcome trial of rt-PA, an open angiography-based dose-finding recanalization trial of two-chain rt-PA (duteplase) was undertaken.8 Of the 139 patients recruited, 80.6% had complete occlusion of the primary vessel at 5.4 ±1.7 hours after symptom onset. Although no dose-rate response of cerebral arterial recanalization was observed, division (M2) and branch (M3) occlusions were more likely to undergo recanalization by 60 minutes than M1 MCA or ICA occlusions with rt-PA (duteplase). Hemorrhagic transformation was within preset limits; however, the frequency became significant after 6 hours (Fig. 1).8
Fig. 1.
Incidence of hemorrhagic transformation relative to the time of initiation of treatment with recombinant plasminogen activator (duteplase) relative to symptom onset.8 The relative incidence of hemorrhage types is depicted. The absence of hemorrhage (NIL) detected by computed tomography scan at 24 hours after treatment was judged against hemorrhagic infarction (HI) and parenchymal hemorrhage (PH). Please see the text for definitions.
Mori et al were the first to demonstrate, in a blinded randomized controlled trial, that acute treatment of patients with 20 or 30 MIU of rt-PA (duteplase) significantly improved recanalization and clinical outcome at 30 days compared with patients treated with placebo.187 Patients demonstrating early recanalization had better neurologic outcome than did those not exhibiting recanalization. In those studies, detectable hemorrhage occurred in 29 to 53% of treated patients,5,187–189 which was similar to natural history.69
Three separate randomized controlled trials of acute intravenous delivery SK in patients with ischemic stroke were terminated because of excessive early mortality and symptomatic intracranial hemorrhage in the SK-treated groups.191–193 No preparatory dose-finding studies were performed in any of those trials. In the Multicentre Acute Stroke Trial-Italy (MAST-I) trial, the combination of ASA with SK produced excessive early case fatality.193 The potential utility of SK in ischemic stroke has not been pursued further. One possible interpretation, based on the immunology of SK, is that its presence in the ischemic territory activates a parenchymal response.
At the time of those studies, a contract program to determine the safety and efficacy of single chain rt-PA (alteplase) by intravenous delivery was undertaken by the National Institute of Neurological Disorders and Stroke (NINDS) and initiated in 1985 (Table 3).5 Patients were entered within 3 hours of symptom onset. In part 1 of that study, there was no difference in neurologic outcome at 24 hours between the rt-PA (0.9 mg/kg) and placebo-treated cohorts according to the National Institutes of Health Stroke Scale (NIHSS) score. In part 2, at 3 months following rt-PA treatment recipients displayed a statistically significant 11 to 13% absolute improvement over placebo in Barthel index, modified Rankin scale (mRS) score, Glasgow outcome scale score, and NIHSS for evidence of no or minimal disability/deficit. Symptomatic hemorrhage was significantly more frequent among patients treated with rt-PA (6.4%) than placebo (0.6%). Mortality was unchanged, but intracerebral hemorrhage significantly contributed to death. In follow-up, the outcome benefits in that population were sustained for 12 months and were most apparent in moderately severe strokes.194 Independent post-licensing use of rt-PA in general practice has confirmed the original report.195 Benefit was associated with age less than 75 years and the absence of “early signs” of ischemic injury.195 The responsive group of patients were those presenting with NIHSS scores of 10 to 14.194
Table 3.
Acute stroke: plasminogen activators
| Studya | Agent | Patients (n) | Δ(T-0)b(h) | Clinical improvement (%) | Hemorrhageg | |||
|---|---|---|---|---|---|---|---|---|
| Nil | HI | PH | % | |||||
| NINDS (part 1)5 | rt-PA | 144 | ≤1.5, ≤3.0 | 1.2c | — | — | 13 | 5.6 |
| C | 147 | — | — | 3 | 0.0 | |||
| NINDS (part 2)5 | rt-PA | 168 | ≤1.5, ≤3.0 | 50d 31e | — | — | 21 | 7.1 |
| C | 165 | 38d 20e | — | — | 8 | 2.1 | ||
| ECASS66 | rt-PA | 313 | <6.0 | 35.9 | 179 | 72 | 62 | 19.8 |
| C | 307 | 29.3 | 184 | 93 | 30 | 6.5 | ||
| ECASS-2196 | rt-PA | 409 | <6.0 | 40.3d | 217 | 142 | 48 | 11.8 |
| C | 391 | 36.6d | 233 | 141 | 12 | 3.1 | ||
| ECASS-3200 | rt-PA | 418 | 3.0–4.5 | 52.4d | 305f | 22f | 5.3 | |
| C | 403 | 45.2d | 332f | 9f | 2.2 | |||
Abbreviations: C, control; ECASS, European Cooperative Acute Stroke Study; HI, hemorrhagic infarction; NINDS, National Institute of Neurologic Disorders and Stroke; PH, parenchymatous hematoma; rt-PA, recombinant tissue-type plasminogen activator; SK, streptokinase.
Randomized studies without vascular diagnosis.
Time from symptom onset to treatment.
Relative risk reduction.
Modified Rankin scale (mRS) score 0 and 1.
National Institutes of Health Stroke Scale (NIHSS).
According to the ECASS II definition.
Any intracerebral hemorrhage.
Three other phase III prospective randomized safety and efficacy studies of intravenous rt-PA (alteplase) have been undertaken that emphasize limitations to the acute use of rt-PA and raise intriguing questions about the generalizability of this strategy (Table 3). The European Cooperative Acute Stroke Study (ECASS) compared parenteral infusion rt-PA (1.1 mg/kg, to a maximum of 100 mg) to placebo in patients within 6 hours of symptom onset. No significant difference was observed between the two groups for 90-day median mRS.66 A subgroup of patients with evidence of ischemia on the entry CT scan, who should have been excluded (“protocol violators”), displayed increased mortality and hemorrhage when treated with rt-PA. A post hoc analysis limited to the “target population” suggested an 11 to 12% absolute improvement over placebo in mRS 0 to 1 (no or minimal disability) in the rt-PA-treated cohort, when the protocol violator data was excluded. However, a significantly higher proportion of rt-PA patients (6.1%) had intracerebral hemorrhage causing neurological deterioration or death (PH2) than did patients in the placebo group (2.6%). ECASS-2, the follow-on randomized double-blind nonangiographic study, required a careful review of the baseline CT scans for “early signs of ischemia” to exclude patients displaying apparent defects exceeding 33% of the MCA territory.196,197 Here, 40.3% of patients who received rt-PA within 6 hours of symptom onset had a favorable outcome (mRS = 0 to 1), an insignificant 3.2% improvement over placebo. Severe symptomatic parenchymal hemorrhage (PH2) was significantly more frequent in the rt-PA group (11.7%) than in the placebo group (3.1%).
In Europe, two registries and a phase III trial were undertaken to secure the approval for the acute use of rt-PA in stroke patients. In preparation for a formal retest of this indication for rt-PA, prospective acute experience of patients was tallied in the two registries, SITS-MOST and SITS-ISTR.198,199 These allowed the design of ECASS-3, a multicenter, prospective, randomized, placebo-controlled trial comparing patients receiving best medical treatment together with either rt-PA (n = 418) or placebo (n = 403) between 3 and 4.5 hours from symptom onset.200 The primary efficacy outcome in ECASS-3 (mRS = 0 to 1 at 90 days) was significantly greater with rt-PA (52.4%) than placebo (45.2%). Symptomatic intracranial hemorrhage, as defined by the criteria used in the NINDS study, was diagnosed in 33 subjects treated with rt-PA (7.9%) and in 14 subjects given placebo (3.5%). The significantly increased incidence of symptomatic intracranial hemorrhage with the use of thrombolytic agents is consistent with the experience with rt-PA in other clinical trials testing the agent acutely.2,5,8,9,23–25,27,187,200–203 In ECASS-3, the incidence of intracerebral hemorrhage was not increased greatly despite the parenteral administration of anticoagulants for prevention of deep vein thrombosis within the first 24 hours following rt-PA treatment. Some implications of these findings have been reviewed recently.204 The results, consistent with the results in this time window from previous studies and pooled analyses of previous trials,187,205,206 suggest that intravenous rt-PA can be given safely to carefully selected patients treated 3 to 4.5 hours after stroke and that intravenous rt-PA given in this time period can improve outcomes after stroke in a selected group of patients.
The studies of intravenous rt-PA so far underscore the major contributors to the risk of intracerebral hemorrhage in ischemic stroke. These include increased time from symptom onset to treatment, low body mass, diastolic hypertension, older age, and the use of rt-PA.8,207,208 The appearance of “early signs of ischemia,” marked by low attenuation and/or sulcal effacement on the initial CT scan, is associated with an increased risk of death and hemorrhage (Table 4).66,208–211
Table 4.
Contributors to hemorrhagic transformation
| Factors | Agent |
|---|---|
| Time from symptom onset | rt-PA (duteplase) |
| Diastolic hypertension | rt-PA |
| Low body mass | rt-PA |
| Age | rt-PA |
| Atrial fibrillation | rt-PA |
| “Early” signs of ischemia | rt-PA rscu-PA |
| Plasminogen activator (e.g., rt-PA) | rt-PA |
Abbreviations: rscu-PA, recombinant single-chain urokinase plasminogen activator; rt-PA, recombinant tissue-type plasminogen activator.
Viewed together, the results of the NINDS and ECASS studies draw attention to the enormous importance of strict individual patient selection to beneficial outcome and in reducing the hemorrhagic risk accompanying the acute use of rt-PA in ischemic stroke.5,66,67 Heterogeneity among clinical studies of acute intervention with rt-PA is well recognized.212 Unfortunately, no documentation of the presence of arterial occlusions and their fate took place in those studies.
A search for the acute use of alternate PAs to rt-PA has been predicated on the notion that the thrombus-selective agents could have better safety profiles if applied differently. A theoretical advantage of a longer half-life variant of t-PA, tenecteplase (TNK), could be ease of administration with similar efficacy and safety to rt-PA in ischemic stroke. One open-label dose-escalation study of TNK in 88 patents achieved a dose of 0.4 mg/kg without symptomatic intracerebral hemorrhage.213 A follow-on randomized study was halted by the sponsor because of limited recruitment. A separate study of three cohorts of 25 patients each that compared TNK (at 0.1 mg/kg or 0.25 mg/kg) with rt-PA (alteplase) indicated significantly greater reperfusion at 24 hours and better mRS = 0 to 1 at 90 days with TNK (0.25 mg/ kg) compared with alteplase.214 Further trials are expected.
Another naturally occurring PA with a prolonged half-life and thrombus selectivity is derived from the common vampire bat (Desmodus rotundus), rαDS-PA (desmoteplase). A series of acute delivery studies in ischemic stroke patients within 3 to 9 hours of symptom onset, employing an MR for stratification of initial injury, have been undertaken with varying results. An early intravenous dose-finding study, Desmoteplase in Acute Ischemic Stroke Trial (DIAS), generated excess and unacceptable cerebral hemorrhage with the first dose and was halted.215 That study was revised to provide a dose-finding test, and it found that 125 µg/kg rαDS-PA was safe, with additional evidence of a clinical response.216 In the subsequent DIAS-2 trial, 125 µg/kg desmoteplase delivered within 3 to 9 hours from symptom onset failed to show benefit over placebo.217 With a re-examination of subgroup responses, two parallel phase III controlled blinded studies of desmoteplase in this time frame have been undertaken, DIAS-3 and DIAS-4, to test the hypothesis that rαDS-PA can result in increased benefit in treated patients compared with placebo.
Direct Local Infusion of Plasminogen Activators
Refinements of early intra-arterial guidewire-directed microcatheter techniques have taken place in parallel with the development of systemic infusion studies. Advantages of the interventional approaches are the higher local concentrations of PAs delivered to the thrombus face in arterial segments where blood flow is stagnant, the inherent definition of vascular anatomy, and the documentation of recanalization. Furthermore, the frequency of recanalization is likely to be greater than that achieved by systemic infusion. In early series, detectable hemorrhagic transformation in the ischemia territory occurred in 18 to 33% of treated patients (Table 2).184–186
Obstructions of the vertebral and/or basilar arteries and the subsequent ischemia can produce considerable disability.218,219 Nenci et al first demonstrated clinical improvement after local intra-arterial treatment of symptomatic basilar artery thrombosis.220 One retrospective comparison of clinical outcome in 43 patients who received intra-arterial u-PA or SK with 22 patients who received conventional therapy (i.e., heparin) suggested considerable survival benefit in those demonstrating recanalization.221 Subsequent reports have supported recanalization efficacy with these agents.186,222,223 However, significant hemorrhagic transformation also occurs in this setting. Current practice is approached on a case-by-case basis with the option for instrumentation in some. There have been no level 1 studies of the several techniques now available or in evolution with regard to clinical benefit superiority.
In the early 1990s, A. Sasahara expressed interest in developing an indication for recombinant scu-PA (rscu-PA, rpro-UK) in the acute direct intra-arterial dissolution of thrombi in the cerebral artery territories in ischemic stroke. The Prourokinase in Acute Cerebral Thromboembolism (PRO-ACT) study was the first and only randomized double-blinded placebo-controlled examination of PAs by intra-arterial infusion to demonstrate benefit.6 In that phase II study, direct arterial infusion of rscu-PA (6 mg) into the thrombus face was compared with placebo also delivered en face for recanalization of M1 and M2 MCA occlusions and safety outcomes.6 Overall, rscu-PA produced a significant increase in MCA recanalization. Hemorrhagic transformation also increased but was within the prescribed safety limits. Heparin infusion in both cohorts was required to prevent catheter thrombosis, with dosing based on contemporary interventional practice.6 However, it was determined that both recanalization and hemorrhage risk were heparin dependent.
A follow-on open study, PROACT-2, randomized patients with symptomatic proximal MCA occlusions to rscu-PA (9mg) or to catheter placement only (without agent infusion) for recanalization and for 3-month disability outcome.224 Both groups also received heparin. Recanalization was significantly greater with rscu-PA (65.7%) than no intervention (18.0%), as was the frequency of symptomatic intracerebral hemorrhage. The proportion of patients with no or minimal disability (mRS = 0 to 1) was not significantly different between the two groups, whereas rscu-PA was associated with a marginally significant improvement in outcome measured as mRS = 0 to 2 at 90 days. The development of rscu-PA has terminated, and it is not available for clinical use.
Currently, PROACT is the only level 1 study of the intraarterial direct infusion of a PA to demonstrate improved recanalization frequency, whereas PROACT-2 is the only to study to provide a hint of clinical efficacy. Importantly, co-infusion with an anticoagulant modulates both recanalization and hemorrhagic risk. There have been no subsequent placebo-controlled studies; however, a comparison of intravenous with intra-arterial infusion rt-PA has been completed.
Defibrinogenating Agents
Reduction of fibrinogen to 100 mg/dL by the defibrinogenating agent ancrod has been considered safe in patients with ischemic stroke.225,226 The decrease in plasma fibrinogen results in decreased viscosity, relative increased flow, and—potentially— limitation of thrombus formation. In New Zealand white rabbits, ancrod has been shown to increase circulating PA activity acutely.227
In one prospective randomized study (the Stroke Treatment with Ancrod Trial [STAT]), favorable outcome was more frequent in the ancrod-treated group (42.2%) than in the placebo-treated group (34.4%) in a prespecified covariate-adjusted analysis (taking into account baseline stroke severity).226,228 Intracranial hemorrhage was moderately more frequent in the ancrod group. A parallel study (the European Stroke Treatment with Ancrod Trial [ESTAT]) showed no advantage to ancrod.229 Differences in features between the two studies suggested conditions for two new trials to test the potential benefit of rapid defibrinogenation in the treatment of ischemic stroke within 6 hours of symptom onset. However, no difference in the efficacy outcome (“positive responder status”) was observed in a combined 500 patients randomized to multiple-day dosing with ancrod or placebo.230 Treatment levels intended for fibrinogen were reached in > 90% of the ancrod patients. Hence, no further work with this agent in stroke has been undertaken.
Thrombotic Events Associated with CNS Injury
Sagittal Sinus Thrombosis
Cortical vein thrombosis can occur in neonates, pediatric patients, and adults. In adults, sagittal sinus thrombosis can accompany local inflammation, thrombophilic states (including anticardiolipin or antiphospholipid antibodies), sickle cell disease, pregnancy, or birth control agents; it can also be idiopathic. A recent epidemiologic report underscores the relative lower morbidity of this condition compared with ischemic stroke.231 Generally, the treatment of choice for cortical venous thrombosis includes heparin anticoagulation followed by warfarin oral anticoagulation to a target INR of 2.5. Petechial hemorrhage is a common finding accompanying sagittal sinus thrombosis. Reports to date with PAs are anecdotal. Recovery in patients receiving u-PA232 or rt-PA233–235 by local direct infusion have been presented. Wasay et al suggested, about a nonrandomized comparison, that neurological outcome was superior in patients with sagittal sinus thrombosis who received local infusion of u-PA with heparin compared with heparin alone.236 There has been limited reported experience with u-PA in neonates and children with cerebral venous sinus thrombosis, with various outcomes.237 Those reports suggest the need for a controlled study of directed PA infusion against anticoagulation.
Retinal Vascular Thrombosis
Retinal vascular occlusive disorders include central retina artery thrombosis and retinal vein thrombosis. Retinal vascular occlusions are commonly related to carotid artery stenosis238 but may also be associated with evidence of circulating anticardiolipin antibodies,239 as in retinal vein occlusion. Fibrinolytic agents have been used successfully in some patients with retinal artery and retinal vein occlusion.240,241 These have often been followed by oral anticoagulation. Partial recovery of form vision in some patients with acute central retinal artery occlusion has been reported.242,243 More recently, limited prospective series and trials of systemic rt-PA have been undertaken.244,245 A trial of rt-PA is also planned.246 Access to the occluded retinal circulation by interventional methods has been proposed for local delivery of a PA.247,248
Pertinent Queries
The experience with PAs in CNS ischemic disorders has raised questions concerning patient care. Several variables in the treatment of patients presenting with ischemic stroke affect both the safety and efficacy of specific PAs. Many of these issues have been addressed in successive acute intervention trials. For these, specific conditions are better defined for the intravenous application of rt-PA than the intra-arterial delivery of specific PAs available at the time. Major concerns, including the dose and effect of rt-PA or other PAs, the appropriate time to treatment, and the adjunctive use of antithrombotic agents, have sufficient information available with which to provide a reasonable response.
Dose-Rates and Efficacies of PAs
Based on earlier work with rt-PA in the setting of MI, researchers investigated whether doses of rt-PA (alteplase) exceeding 1.0 mg/kg were associated with an increased frequency of intracranial hemorrhage in patients unselected for CNS disease.249,250 The run-up to the NINDS-sponsored trial included a dose-escalation for safety (CNS hemorrhage) phase.5 This underscored the doses chosen for usage (total 0.9 mg/kg, with 10% of the total dose as bolus) in the NINDS trial and 1.1 mg/kg in the ECASS trial.66 Concerns that these dose rates could be excessive has led to a careful re-examination by Mori in Japanese populations.251 Currently, in Japan and other Asian countries, dosing is 0.6 mg/kg,252,253 which has shown near equivalent outcomes to the higher dose rates used in the United States, Europe, and select other regions.251 Ironically, this lower dose rate is now being tested in predominantly Caucasian populations.
An important component of the strategy to develop PAs for acute use in ischemic stroke has been the careful dose escalation of the PA.5,8 An exception was the early development of rαDS-PA, in which dosing was assumed—and proved excessive —resulting in unacceptable intracerebral hemorrhage.215 The dosing was not based on thrombolytic efficacy. Subsequent studies refined the dosing of rαDS-PA on a per-weight basis.
Time to Treatment (Acute Intervention)
With regard to the outside time for treatment (“treatment window”), the early (1980s) work took two paths: (1) time limits were based on anecdotal evidence from intra-arterial PA infusion studies in which some patients presenting within 6 hours after symptom onset demonstrated recanalization and improvement, the impact on vertebrobasilar ischemia, and the requirement for time to complete CT and angiographic studies in each patient; and (2) the shorter limit (3 hours) chosen to reflect the need for rapid intervention, based on presumed efficacy, where angiographic studies were not employed. Both strategies have provided data supporting safety and efficacy with intravenous rt-PA, for instance.5,8 The current trend is to prospectively seek evidence that a longer time from symptom onset could be suitable for patients with carotid artery territory ischemia. This approach refers to the early anecdotal experience and seeks to make systemic infusion rt-PA available for more patients. Additionally, the approach seems reasonable given various meta-analyses based on published data, the known variability in outcomes among prospective trials, and the potential variability in collateral protection among treatment populations. Meta-analyses reported to date are redundant; however, they reflect the known variability among prospective trials with respect to study populations. To date no per-patient analyses have been performed, which would be particularly revealing about the nature of the neurologic deficit severity and outcome potential relative to the time to treatment. Nonetheless, there is unanimous agreement that patients must be brought to treatment within the shortest time possible.
Hemorrhagic Transformation
Among the antithrombotic agents, PAs produce the largest increase in detectable intracerebral hemorrhage when applied to ischemic stroke patients compared with no intervention.5,66,196,200 This increase is in part dependent on the time to intervention from the onset of ictus (Fig. 1),8 implying that the duration of ischemic injury evolution is central. This has been suggested by experimental models.254 The mechanisms are not worked out but are likely to involve the integrity of the microvasculature.75,76 Additionally, other clinical attributes associated with hemorrhage in the setting of rt-PA are listed in Table 4.
Notably, there has been considerable variation observed in the frequency of symptomatic hemorrhagic transformation among the four level 1 trials of acute intravenous rt-PA treatment in ischemic stroke.5,6,66,196,200,224 Although one would expect that the frequency of symptomatic hemorrhagic transformation of the placebo group should not vary from study to study, there has been considerable variation observed, with the lowest frequency associated with earliest treatment.5 This, however, suggests considerable population variation among the studies, even though three studies were conducted with the same group of collaborators.66,196,200 Importantly, the frequency of symptomatic hemorrhage (PH1 and PH2) among the acute rt-PA cohorts in the same studies also varies, and in a linear fashion relative to the placebo patients. Hence, the risk of hemorrhagic transformation in patients receiving rt-PA within 6 hours of symptom onset depends on the risk of hemorrhagic transformation in the population as a whole and varies among studies rather than being static. This indicates that the risk of hemorrhage is population dependent, and that undisclosed attributes of these populations are at play within and among the studies. There is little insight currently into the relevant biological characteristics of those patients that might be at play; however, these could include variations in hypertension, microvessel structure, permeability barrier stability, and collateral circuits, among others factors. The range in injury evolution suggested by the breadth in entry window, as well as genetic factors, may also play a role.
Important to this observation is the definition of hemorrhagic transformation, which is best seen in the ECASS III study.200 Here the frequency of PH2 varied linearly with the definition over a range of 1.9 to 7.9% among patients treated with rt-PA. This does not change the contribution of the population composition to this risk.
Collateral Preservation versus Primary Arterial Occlusion
Development of the use of PAs for acute intervention in ischemic stroke focuses on thrombotic/thromboembolic occlusion of the primary symptomatic brain-supplying artery. It is assumed that arterial recanalization and returned perfusion to the territory-at-risk is responsible for the clinical improvement. An alternate, or parallel, hypothesis is that preservation or opening of latent collateral circuits, and/or restitution of flow through the affected microvasculature, could improve tissue preservation/recovery. The clinical evidence for these elements is sparse; however, experimental model studies are consistent with the latter.255
Baseline Perfusion Characteristics
The possibility that the injury evolution in its early stages can be aborted by arterial recanalization, and the variability in outcome, have emphasized the need to better understand the natural history of injury evolution in the territory-at-risk and its contributions to selection of patients who could benefit from thrombus lysis. The hypothesis states that baseline clinical status can be a predictor of outcome. Employing 1.5T MR with rapid analysis, subgroups of stroke patients who received rt-PA treatment 3 to 6 hours after symptom onset could be identified with a “target PWI/DWI mismatch” at baseline who displayed a “favorable response” following early reperfusion.92 In the follow-on DEFUSE 2 study, high–field strength MR examination of the “target mismatch” group displayed a significantly greater clinical response (mRS = 0 to 2) associated with reperfusion than was seen in the “no target mismatch” group, a benefit that extended to 90 days.256 No impact on PH was observed.
Adjunctive Use of Antithrombotic Agents
Independent studies have examined the impact of antiplatelet agents and anticoagulants in the treatment of patients for secondary prevention of ischemic stroke on the background of a primary event. The notion is that an additional antithrombotic agent should reduce the PA requirement, improve the downstream benefit, and have less hemorrhagic risk. It is clear that all antithrombotic agents carry a risk of increasing the intracerebral hemorrhagic risk. Antiplatelet agents, generally, have a low risk of inducing hemorrhagic transformation, whereas anticoagulants have a significantly higher associative risk. The contribution of these agents to the risk of hemorrhage in the setting of the acute use of rt-PA in ischemic stroke patients is, therefore, of some interest. In the case of anticoagulants, the acute management of patients with PAs depends on the mode of delivery.
Antiplatelet agents
The potential effects of antiplatelet agents on the outcome of acute PA use in ischemic stroke depend on the point of view. A rather paradoxical situation attends the use of antiplatelet agents; for instance, whether antiplatelet agents (most commonly, ASA) (1) can produce an increased risk of hemorrhagic transformation by the acute use of rt-PA or (2) can, through controlled use, decrease the requirement for the PA (i.e., rt-PA) and thereby decrease the risk of hemorrhage while maintaining efficacy. In the latter case, a potent antiplatelet agent could be used to maintain or increase microvessel patency46 or to enhance the dissolution of the arterial occlusions and/or prevent reocclusion.
Studies of rt-PA (duteplase) did not stratify for the use of aspirin.8 However, in one study, there appeared to be no difference in the incidence of hemorrhage in patients who received at least 325 mg/day ASA. Similarly, in the NINDS-sponsored study, there did not appear to be, overall, an increased risk of hemorrhagic transformation in the setting of ASA. In general, patients who have received aspirin recently are not excluded from the use of rt-PA in the acute setting.
Agents that interfere with the binding of platelets to fibrinogen via the integrin αIIbβ3 receptor promote an increased risk of hemorrhage. Both rodent and nonhuman primate models of focal cerebral ischemia displayed a significant increase in debilitating hemorrhagic transformation during early focal ischemia when organic inhibitors have been applied.46,50 Dose rates of two agents were realized that did not substantially increase the risk of hemorrhage and, in one instance, supported efficacy.46,50 However, the therapeutic window was relatively narrow for this behavior in both cases. The use of the anti-integrin αIIbβ3 antibody construct abciximab was tested clinically in both phase II study and phase III outcome studies. No substantial increase in efficacy was seen in the phase II study, though the hemorrhagic transformation did not appear to be increased.257 However, the subsequent phase III study was terminated because a significant increase in symptomatic hemorrhage was seen (in the setting of the use of rt-PA).
To test the possibility that an antiplatelet agent could improve outcome and, therefore, allow reduction of the dosing of the PA, a prospective randomized trial of rt-PA with or without the αIIbβ3 antagonist eptifibatide (the Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke [CLEAR Stroke Trial]) was undertaken. Eptifibatide at low doses provided a safe adjunct to decreased doses of rt-PA.258 That study showed the safety of the applied dosing of eptifibatide. Recently, the Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke - Enhanced Regimen (CLEAR-ER) has begun comparing rt-PA (0.6 mg/kg total) and eptifibatide (bolus 135 µg/kg and 2-hour infusion at 0.75 µg/kg/min) compared with 0.9 mg/kg rt-PA in patients with ischemic stroke that can have the rt-PA initiated within 3 hours of symptom onset.
Anticoagulation
The use of anticoagulants in ischemic stroke has several features, including their use adjunctively in intra-arterial PA infusion formats, and their potential to increase the hemorrhagic risk of PAs.
In the intra-arterial infusion studies of rscu-PA (PROACT), heparin was employed to maintain sheath patency for intraarterial catheter PA delivery. That study was the first indicator in the clinical setting that an anticoagulant could modify both safety and efficacy of the PA.6 In that instance, an increasing (albeit small) frequency of intracerebral hemorrhagic events dictated a review of the dosing pattern such that when the heparin dose rate was decreased, there was a consequent reduction in hemorrhagic transformation paralleled by a decrease in recanalization efficacy.6 Overall, the study demonstrated no particular increase in the instance of symptomatic intracerebral hemorrhage. The heparin dosing scheme applied here was used in a subsequent open study of pro-urokinase by intra-arterial delivery. The incidence of hemorrhage in that setting was 10.2% compared with 1.9% (control).224
Limitations on the use of oral anticoagulants for patients who are candidates to receive rt-PA intravenously are part of the exclusion criteria.5 For instance, the current licensing in North America requires an INR < 1.5 for the patient to receive rt-PA in the acute setting. Other guidelines have proposed a threshold of INR < 1.7. The more conservative INR threshold is appropriate for instrumentation and surgical procedures. Patients receiving warfarin anticoagulation must have their INR adjusted to the appropriate threshold.
Oral antithrombins and anti–factor Xa inhibitors
The new oral antithrombotics dabigatran, rivaroxaban, and apixaban have recently come available for primary prevention of non-valvular AF due to their noninferiority to warfarin in this setting. Their use has been associated with a significant reduction in the incidence of hemorrhage in patients presenting with stroke who have been treated for nonvalvular AF.137,138,140,141,259 The mechanisms for the apparent safety benefits in this setting have not been determined.139 However, it has been suggested that, because these agents each target a single coagulation factor interaction, and oral anticoagulation with warfarin targets multiple areas, including Factor VIIa activity, the more specific inhibitors would be expected to have a lower incidence of hemorrhagic risk.
An associated concern with the use of both classes of oral anticoagulants is the absence of a commercially available neutralizing agent and a test to determine the circulating concentration in real time. Without a convenient ability to reverse the anticoagulant effects of dabigatran (except for dialysis), the potential additional risk of hemorrhage achieved with acute rt-PA use when dabigatran is not removed makes patients receiving dabigatran unsuitable for rt-PA treatment for many centers. A similar situation holds for rivaroxaban and apixaban, where reversal of activity could promote thrombosis.
Adjunctive Use of Intra-Arterial and Mechanical Thrombus Extraction Techniques
In part because of the lower frequency of thrombus dissolution with systemic delivery of rt-PA, combination with thrombus extraction methods has been contemplated. A series of studies have attempted to test strategies that could increase recanalization of the symptomatic brain-supplying artery to enhance tissue perfusion and improve outcome. So far, there has been little evidence of additional benefit. The Interventional Management of Stroke (IMS) study, a phase II trial, examined 80 patients with a median NIHSS score of at least 10 who received rt-PA (0.6 mg/kg over 30 minutes) within 3 hours and had additional rt-PA administered directly by microcatheter.260 The combined approach was found to be as safe as rt-PA alone. IMS II examined the impact of rt-PA infusion via a specific microcatheter after intravenous rt-PA infusion (0.6 mg/kg over 30 minutes) and demonstrated comparable safety with rt-PA alone.261 The follow-on phase III randomized open-label IMS III study of the same design (in a projected 900 patients) has been halted as of this writing. Hence, it is unclear whether the combination of systemic and intra-arterial delivery of rt-PA will have any advantage in this format.
Reocclusion
Rethrombosis of a documented occlusive lesion previously lysed in a brain-supplying cerebral artery has been observed, although the incidence is small. Several anecdotal reports indicate reocclusion of a thrombosed artery subject to local intra-arterial rt-PA infusion or instrumentation with successful re-establishment of flow.262,263 Cessation of flow following successful intravenous rt-PA infusion has been reported.264 On the basis of those reports, an open-label prospective treatment of patients receiving rt-PA within 4.5 hours with either intravenous ASA (300 mg) or placebo within 24 hours of rt-PA treatment was undertaken, with clinical (not angiographic) outcome.265 That study was terminated for futility of the clinical outcome, but it also demonstrated a significantly higher incidence of symptomatic intracerebral hemorrhage in the ASA arm (4.4%) compared with the placebo arm (1.6%). Here the assumption was made that reocclusion would lead to neurological deterioration, although no proof of thrombus lysis or reocclusion was made. Although reocclusion is apparent in intra-arterial thrombus lysis, its contribution to outcome is not defined.
Outcomes Measures
Currently the mRS score measured at 90 days after symptom onset is taken as a useful measure of outcome, often dichotomized to mRS of 0 to 1, and the remainder. Reliance on an mRS of 0 to 1 provides a consistent appreciation that the residual deficit has resolved completely and/or that the patient does not recognize a residual deficit. An mRS of 0 to 2 is more problematic in that it allows a residual deficit that is more severe than baseline, suggesting a perceptual deficit, moderate motor deficit, or inability to gauge the deficit. This uncertainty about mRS = 2 deficits undermines the purpose of such scales to quantify outcome. Therefore, it is uncertain what is meant when an outcome between two treatments is not significant for an mRS of 0 to 1 but is of marginal significance for an mRS of 0 to 2, particularly if the analysis is post hoc.5,66,194
The NIHSS score, developed during the NINDS sponsored rt-PA trial, has provided a measure of neurological status weighted for motor defects.5 All neurological scales appear to provide related results and relatively agreeable determinants of neurological deficit.266 Because the scales employed so far have a nonlinear relation to the actual deficits and are ordinal in nature, appropriate statistical methods are required. Nonetheless, all of the neurological scales depend on changes in motor ability in the affected territory. Examination of data from the rt-PA (duteplase) dose-escalation trial demonstrated that simple distal upper extremity movement correlated well with recanalization.8,267 The durability of the outcome following acute intervention was shown by the benefit seen at 12 months.194
Limitations of current scales and quantitative methods are inherent in the variability in deficits observed at baseline in clinical trials and the overall tendency for deficits to improve even in the absence of intervention.55 Wityk et al demonstrated a standard deviation of two NIHSS scale points at each determination, suggesting that improvements based on of a transit of 4 NIHSS scale points is significant while encompassing two SDs.55 Other scale systems, including the Oxford handicap scale (OHS), the Glasgow outcome scale, and the Barthel index have been employed. Congruence of multiple scale outcomes has been offered as demonstration of the strength of the treatment effect.5 Little work has been performed on the issue of the relation between neurological and clinical outcome and the degree of recanalization.267
Future Efforts
Several issues concerning the acute use of PAs in the setting of thrombotic and thromboembolic stroke remain unexplored. Pursuit of these may be helpful to the refinement of their clinical use in this setting and to understanding the development of ischemic injury. Plasmin plays as-yet poorly understood roles in the CNS. Among these are the role of PAs in dendritic development and the separate roles in the antithrombotic milieu of the microvasculature. If and how both settings might modulate neuron injury is still poorly understood. Furthermore, there is so far little evidence that the events pursued in model systems impact the clinical outcome of patients.
Relevant to the clinical use of rt-PA and other PAs are the still poorly understood mechanisms of alterations in microvessel and hemorrhagic transformation. Reduction in hemorrhagic risk is necessary to improve clinical outcome. Knowing the specifics of cerebral tissue injury is required, as is knowing how PAs can alter these. The processes involved in the loss of microvessel integrity are also poorly understood—in particular, the manner in which the extracellular matrix is altered during focal ischemia and how astrocyte and endothelial cell function can be preserved. Central to these investigations are the cell-cell and cell-matrix interactions that determine the integrity of the neurovascular unit.
Acknowledgments
This work was supported by grants PO1 HL31950, RO1 NS26945, RO1 NS38710, and RO1 NS053715 from the National Institutes of Health. The contributions of Ms. Greta Berg to the development of this article through many climes are gratefully acknowledged.
References
- 1.Mohr JP, Caplan LR, Melski JW, et al. The Harvard Cooperative Stroke Registry: a prospective registry of patients hospitalized with stroke. Neurology. 1978;28(8):754–762. doi: 10.1212/wnl.28.8.754. [DOI] [PubMed] [Google Scholar]
- 2.Mohr JP, Barnett HJM. Classification of ischemic strokes. In: Barnett HJM, Stein BM, Mohr JP, Yatsu FM, editors. Stroke: Pathophysiology, Diagnosis and Management. Vol. 1. New York: Churchill Livingstone; 1986. pp. 281–291. [Google Scholar]
- 3.Solis OJ, Roberson GR, Taveras JM, Mohr J, Pessin MS. Cerebral angiography in acute cerebral infarction. Rev Interam Radiol. 1977;2(1):19–25. [PubMed] [Google Scholar]
- 4.Fieschi C, Argentino C, Lenzi GL, Sacchetti ML, Toni D, Bozzao L. Clinical and instrumental evaluation of patients with ischemic stroke within the first six hours. J Neurol Sci. 1989;91(3):311–321. doi: 10.1016/0022-510x(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 5.The National Institutes of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 6.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 7.Irino T, Taneda M, Minami T. Angiographic manifestations in postrecanalized cerebral infarction. Neurology. 1977;27(5):471–475. doi: 10.1212/wnl.27.5.471. [DOI] [PubMed] [Google Scholar]
- 8.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32(1):78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 9.Fieschi C, Bozzao L. Transient embolic occlusion of the middle cerebral and internal carotid arteries in cerebral apoplexy. J Neurol Neurosurg Psychiatry. 1969;32(3):236–240. doi: 10.1136/jnnp.32.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Wood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303(16):897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 11.Kligman AM. Long-term anticoagulant therapy after myocardial infarction A study of 747 patients in 15 hospitals. JAMA. 1965;193:929–934. [PubMed] [Google Scholar]
- 12.Harvald B, Hilden T, Lund E. Long-term anticoagulant therapy after myocardial infarction. Lancet. 1962;2(7257):626–630. doi: 10.1016/s0140-6736(62)92540-0. [DOI] [PubMed] [Google Scholar]
- 13.Loeliger EA, Hensen A, Kroes F, et al. A double-blind trial of long-term anticoagulant treatment after myocardial infarction. Acta Med Scand. 1967;182(5):549–566. doi: 10.1111/j.0954-6820.1967.tb10881.x. [DOI] [PubMed] [Google Scholar]
- 14.Breddin K, Loew D, Lechner K, Oberla K, Walter E. The German-Austrian Aspirin Trial: a comparison of acetylsalicyclic acid, placebo, and phenprocoumon in secondary prevention of myocardial infarction. Circulation. 1980;62(Suppl):V63–V72. [PubMed] [Google Scholar]
- 15.Report of the Sixty Plus Reinfarction Study Research Group. A double-blind trial to assess long-term oral anticoagulant therapy in elderly patients after myocardial infarction. Lancet. 1980;2(8202):989–994. [PubMed] [Google Scholar]
- 16.Smith P, Arnesen H, Holme I. The effect of warfarin on mortality and reinfarction after myocardial infarction. N Engl J Med. 1990;323(3):147–152. doi: 10.1056/NEJM199007193230302. [DOI] [PubMed] [Google Scholar]
- 17.Sage JI, Van Uitert RL. Risk of recurrent stroke in patients with atrial fibrillation and non-valvular heart disease. Stroke. 1983;14(4):537–540. doi: 10.1161/01.str.14.4.537. [DOI] [PubMed] [Google Scholar]
- 18.Hart RG, Coull BM, Hart D. Early recurrent embolism associated with nonvalvular atrial fibrillation: a retrospective study. Stroke. 1983;14(5):688–693. doi: 10.1161/01.str.14.5.688. [DOI] [PubMed] [Google Scholar]
- 19.del Zoppo GJ. Prevention and treatment of acute stroke. In: Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ, editors. Hemostasis and Thrombosis. 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2006. pp. 1477–1496. [Google Scholar]
- 20.Mundall J, Quintero P, Von Kaulla KN, Harmon R, Austin J. Transient monocular blindness and increased platelet aggregability treated with aspirin A case report. Neurology. 1972;22(3):280–285. doi: 10.1212/wnl.22.3.280. [DOI] [PubMed] [Google Scholar]
- 21.Harrison MJG, Marshall J, Meadows JC, Russell RW. Effect of aspirin in amaurosis fugax. Lancet. 1971;2(7727):743–744. doi: 10.1016/s0140-6736(71)92108-8. [DOI] [PubMed] [Google Scholar]
- 22.Dyken ML, Kolar OJ, Jones FH. Differences in the occurrence of carotid transient ischemic attacks associated with antiplatelet aggregation therapy. Stroke. 1973;4(5):732–736. doi: 10.1161/01.str.4.5.732. [DOI] [PubMed] [Google Scholar]
- 23.Marshall J. The natural history of transient ischemic cerebrovascular attacks. Q J Med. 1964;33:309–324. [PubMed] [Google Scholar]
- 24.Wolf PA, Kannel WB, McGee DL, Meeks SL, Bharucha NE, McNamara PM. Duration of atrial fibrillation and imminence of stroke: the Framingham study. Stroke. 1983;14(5):664–667. doi: 10.1161/01.str.14.5.664. [DOI] [PubMed] [Google Scholar]
- 25.Whisnant JP, Matsumoto N, Elveback LR. Transient cerebral ischemic attacks in a community. Rochester, Minnesota, 1955 through 1969. Mayo Clin Proc. 1973;48(3):194–198. [PubMed] [Google Scholar]
- 26.Caplan LR. Are terms such as completed stroke or RIND of continued usefulness? Stroke. 1983;14(3):431–433. doi: 10.1161/01.str.14.3.431. [DOI] [PubMed] [Google Scholar]
- 27.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 28.UK-TIA Study Group. United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: interim results. Br Med J (Clin Res Ed) 1988;296(6618):316–320. [PMC free article] [PubMed] [Google Scholar]
- 29.Denny-Brown D. Recurrent cerebrovascular episodes. Arch Neurol. 1960;2:194–210. doi: 10.1001/archneur.1960.03840080080013. [DOI] [PubMed] [Google Scholar]
- 30.Russell RWR. Atheromatous retinal embolism. Lancet. 1963;2(7322):1354–1356. doi: 10.1016/s0140-6736(63)90737-2. [DOI] [PubMed] [Google Scholar]
- 31.Hollenhorst RW. Vascular status of patients who have cholesterol emboli in the retina. Am J Ophthalmol. 1966;61(5 Pt 2):1159–1165. doi: 10.1016/0002-9394(66)90238-8. [DOI] [PubMed] [Google Scholar]
- 32.Barnett HJ. The pathophysiology of transient cerebral ischemic attacks. Med Clin North Am. 1979;63(4):649–679. doi: 10.1016/s0025-7125(16)31667-4. [DOI] [PubMed] [Google Scholar]
- 33.Marshall J. The Management of Cerebrovascular Diseases. Oxford, England: Blackwell Scientific Publications; 1976. [Google Scholar]
- 34.Dougherty JH, Levy DE, Weksler BB. Platelet activation in acute cerebral ischemia. Lancet. 1977;3:821–824. doi: 10.1016/s0140-6736(77)92774-x. [DOI] [PubMed] [Google Scholar]
- 35.Mettinger KL, Nyman D, Kjellin K-G, Sedén Å, Söderström CE. Factor VIII related antigen, anti-thrombin III, spontaneous platelet aggregation and plasminogen activator in ischemic cerebrovascular disease. J Neurol Sci. 1979;41:31–38. doi: 10.1016/0022-510x(79)90137-0. [DOI] [PubMed] [Google Scholar]
- 36.de Boer AC, Turpie AG, Butt RW, Duke RJ, Bloch RF, Genton E. Plasma betathromboglobulin and serum fragment E in acute partial stroke. Br J Haematol. 1982;50(2):327–334. doi: 10.1111/j.1365-2141.1982.tb01923.x. [DOI] [PubMed] [Google Scholar]
- 37.Cella G, Zahavi J, de Haas HA, Kakkar VV. beta-Thromboglobulin, platelet production time and pletelet function in vascular disease. Br J Haematol. 1979;43(1):127–136. doi: 10.1111/j.1365-2141.1979.tb03727.x. [DOI] [PubMed] [Google Scholar]
- 38.Feinberg WM, Bruck DC, Ring ME, Corrigan JJ., Jr Hemostatic markers in acute stroke. Stroke. 1989;20(5):592–597. doi: 10.1161/01.str.20.5.592. [DOI] [PubMed] [Google Scholar]
- 39.van Kooten F, Ciabattoni G, Patrono C, Schmitz PI, van Gijn J, Koudstaal PJ. Evidence for episodic platelet activation in acute ischemic stroke. Stroke. 1994;25(2):278–281. doi: 10.1161/01.str.25.2.278. [DOI] [PubMed] [Google Scholar]
- 40.Feinberg WM, Pearce LA, Hart RG, et al. Markers of thrombin and platelet activity in patients with atrial fibrillation: correlation with stroke among 1531 participants in the stroke prevention in atrial fibrillation III study. Stroke. 1999;30(12):2547–2553. doi: 10.1161/01.str.30.12.2547. [DOI] [PubMed] [Google Scholar]
- 41.Fisher M, Zipser R. Increased excretion of immunoreactive thromboxane B2 in cerebral ischemia. Stroke. 1985;16(1):10–14. doi: 10.1161/01.str.16.1.10. [DOI] [PubMed] [Google Scholar]
- 42.van Kooten F, Ciabattoni G, Koudstaal PJ, Dippel DW, Patrono C. Increased platelet activation in the chronic phase after cerebral ischemia and intracerebral hemorrhage. Stroke. 1999;30(3):546–549. doi: 10.1161/01.str.30.3.546. [DOI] [PubMed] [Google Scholar]
- 43.Shah AB, Beamer N, Coull BM. Enhanced in vivo platelet activation in subtypes of ischemic stroke. Stroke. 1985;16(4):643–647. doi: 10.1161/01.str.16.4.643. [DOI] [PubMed] [Google Scholar]
- 44.Iwamoto T, Kubo H, Takasaki M. Platelet activation in the cerebral circulation in different subtypes of ischemic stroke and Binswanger’s disease. Stroke. 1995;26(1):52–56. doi: 10.1161/01.str.26.1.52. [DOI] [PubMed] [Google Scholar]
- 45.Del Zoppo GJ, Copeland BR, Harker LA, et al. Experimental acute thrombotic stroke in baboons. Stroke. 1986;17(6):1254–1265. doi: 10.1161/01.str.17.6.1254. [DOI] [PubMed] [Google Scholar]
- 46.Abumiya T, Fitridge R, Mazur C, et al. Integrin alpha(IIb)beta(3) inhibitor preserves microvascular patency in experimental acute focal cerebral ischemia. Stroke. 2000;31(6):1402–1409. doi: 10.1161/01.str.31.6.1402. discussion 1409–1410. [DOI] [PubMed] [Google Scholar]
- 47.del Zoppo GJ, Schmid-Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22(10):1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 48.Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat) Am J Pathol. 1994;144(1):188–199. [PMC free article] [PubMed] [Google Scholar]
- 49.Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25(9):1847–1853. doi: 10.1161/01.str.25.9.1847. discussion 1853–1854. [DOI] [PubMed] [Google Scholar]
- 50.Choudhri TF, Hoh BL, Zerwes HG, et al. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J Clin Invest. 1998;102(7):1301–1310. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gent M, Blakely JA, Easton JD, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Lancet. 1989;1(8649):1215–1220. doi: 10.1016/s0140-6736(89)92327-1. [DOI] [PubMed] [Google Scholar]
- 52.Britton M, Helmers C, Samuelsson KA. Swedish Cooperative Study. High-dose acetylsalicylic acid after cerebral infarction. Stroke. 1987;18:325–334. doi: 10.1161/01.str.18.2.325. [DOI] [PubMed] [Google Scholar]
- 53.Gent M, Blakely JA, Hachinski V, et al. A secondary prevention, randomized trial of suloctidil in patients with a recent history of thromboembolic stroke. Stroke. 1985;16(3):416–424. doi: 10.1161/01.str.16.3.416. [DOI] [PubMed] [Google Scholar]
- 54.Scmidt EV, Smirnov VE, Ryabova VS. Results of the seven-year prospective study of stroke patients. Stroke. 1988;19(8):942–949. doi: 10.1161/01.str.19.8.942. [DOI] [PubMed] [Google Scholar]
- 55.Wityk RJ, Pessin MS, Kaplan RF, Caplan LR. Serial assessment of acute stroke using the NIH Stroke Scale. Stroke. 1994;25(2):362–365. doi: 10.1161/01.str.25.2.362. [DOI] [PubMed] [Google Scholar]
- 56.Holroyd-Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke. 2000;31(8):1833–1837. doi: 10.1161/01.str.31.8.1833. [DOI] [PubMed] [Google Scholar]
- 57.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20(4):631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 58.McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32(3):796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- 59.Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke: a prospective review. Stroke. 1984;15(3):492–496. doi: 10.1161/01.str.15.3.492. [DOI] [PubMed] [Google Scholar]
- 60.Shaw C-M, Alvord EC, Jr, Berry RG. Swelling of the brain following ischemic infarction with arterial occlusion. Arch Neurol. 1959;1:161–177. doi: 10.1001/archneur.1959.03840020035006. [DOI] [PubMed] [Google Scholar]
- 61.Spetzler RF, Selman WR, Weinstein P, et al. Chronic reversible cerebral ischemia: evaluation of a new baboon model. Neurosurgery. 1980;7(3):257–261. doi: 10.1227/00006123-198009000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19(9):1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 63.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19(5):547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 64.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349(9065):1569–1581. [PubMed] [Google Scholar]
- 65.Chen ZM, Sandercock P, Pan HC, et al. Indications for early aspirin use in acute ischemic stroke : A combined analysis of 40 000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31(6):1240–1249. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- 66.Hacke W, Kaste M, Fieschi C, et al. The European Cooperative Acute Stroke Study (ECASS). Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. JAMA. 1995;274(13):1017–1025. [PubMed] [Google Scholar]
- 67.del Zoppo GJ. Acute stroke—on the threshold of a therapy? N Engl J Med. 1995;333(24):1632–1633. doi: 10.1056/NEJM199512143332410. [DOI] [PubMed] [Google Scholar]
- 68.Fisher CM, Adams RD. Observations on brain embolism with special reference to hemorrhage infarction. In: Furlan AJ, editor. The Heart and Stroke. Exploring Mutual Cerebrovascular and Cardiovascular Issues. 1st ed. New York: Springer-Verlag; 1987. pp. 17–36. [Google Scholar]
- 69.Fisher M, Adams RD. Observations on brain embolism with special reference to the mechanism of hemorrhagic infarction. J Neuropathol Exp Neurol. 1951;10(1):92–94. [PubMed] [Google Scholar]
- 70.Jörgensen L, Torvik A. Ischaemic cerebrovascular diseases in an autopsy series. 2. Prevalence, location, pathogenesis, and clinical course of cerebral infarcts. J Neurol Sci. 1969;9(2):285–320. doi: 10.1016/0022-510x(69)90078-1. [DOI] [PubMed] [Google Scholar]
- 71.Kwa VI, Franke CL, Verbeeten B., Jr Stam J; Amsterdam Vascular Medicine Group. Silent intracerebral microhemorrhages in patients with ischemic stroke. Ann Neurol. 1998;44(3):372–377. doi: 10.1002/ana.410440313. [DOI] [PubMed] [Google Scholar]
- 72.Okada Y, Yamaguchi T, Minematsu K, et al. Hemorrhagic transformation in cerebral embolism. Stroke. 1989;20(5):598–603. doi: 10.1161/01.str.20.5.598. [DOI] [PubMed] [Google Scholar]
- 73.Hornig CR, Dorndorf W, Agnoli AL. Hemorrhagic cerebral infarction—a prospective study. Stroke. 1986;17(2):179–185. doi: 10.1161/01.str.17.2.179. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi T, Minematsu K, Choki J, Ikeda M. Clinical and neuroradiological analysis of thrombotic and embolic cerebral infarction. Jpn Circ J. 1984;48(1):50–58. doi: 10.1253/jcj.48.50. [DOI] [PubMed] [Google Scholar]
- 75.Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26(11):2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- 76.Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/re-perfusion. J Cereb Blood Flow Metab. 1996;16(6):1373–1378. doi: 10.1097/00004647-199611000-00036. [DOI] [PubMed] [Google Scholar]
- 77.Pessin MS, Teal PA, Caplan LR. Hemorrhagic infarction: guilt by association? AJNR Am J Neuroradiol. 1991;12(6):1123–1126. [PMC free article] [PubMed] [Google Scholar]
- 78.Cerebral Embolism Study Group. Immediate anticoagulation of embolic stroke: brain hemorrhage and management options. Stroke. 1984;15(5):779–789. doi: 10.1161/01.str.15.5.779. [DOI] [PubMed] [Google Scholar]
- 79.Babikian VL, Kase CS, Pessin MS, Norrving B, Gorelick PB. Intracerebral hemorrhage in stroke patients anticoagulated with heparin. Stroke. 1989;20(11):1500–1503. doi: 10.1161/01.str.20.11.1500. [DOI] [PubMed] [Google Scholar]
- 80.Levine MN, Raskob G, Landefeld S, Kearon C. Hemorrhagic complications of anticoagulant treatment. Chest. 2001;119(1, Suppl):108S–121S. doi: 10.1378/chest.119.1_suppl.108s. [DOI] [PubMed] [Google Scholar]
- 81.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2 Dipyridamole and acetyl-salicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143(1–2):1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 82.Baron JC, von Kummer R, del Zoppo GJ. Treatment of acute ischemic stroke. Challenging the concept of a rigid and universal time window. (Editorial)Stroke. 1995;26(12):2219–2221. doi: 10.1161/01.str.26.12.2219. [DOI] [PubMed] [Google Scholar]
- 83.von Kummer R, Meyding-Lamadé U, Forsting M, et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol. 1994;15(1):9–15. discussion 16–18. [PMC free article] [PubMed] [Google Scholar]
- 84.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. 1995;37(2):231–241. doi: 10.1002/ana.410370214. [DOI] [PubMed] [Google Scholar]
- 85.Garcia JH, Liu KF, Ho KL. Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke. 1995;26(4):636–642. doi: 10.1161/01.str.26.4.636. discussion 643. [DOI] [PubMed] [Google Scholar]
- 86.Tagaya M, Liu KF, Copeland B, et al. DNA scission after focal brain ischemia. Temporal differences in two species. Stroke. 1997;28(6):1245–1254. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- 87.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12(6):723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 88.Branston NM, Symon L, Crockard HA, Pasztor E. Relationship between the cortical evoked potential and local cortical blood flow following acute middle cerebral artery occlusion in the baboon. Exp Neurol. 1974;45(2):195–208. doi: 10.1016/0014-4886(74)90112-5. [DOI] [PubMed] [Google Scholar]
- 89.Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8(1):51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- 90.Branston NM, Strong AJ, Symon L. Extracellular potassium activity, evoked potential and tissue blood flow. Relationships during progressive ischaemia in baboon cerebral cortex. J Neurol Sci. 1977;32(3):305–321. doi: 10.1016/0022-510x(77)90014-4. [DOI] [PubMed] [Google Scholar]
- 91.del Zoppo GJ, Sharp FR, Heiss W-D, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31(9):1836–1851. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Albers GW, Thijs VN, Wechsler L, et al. DEFUSE Investigators. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 93.Olivot JM, Mlynash M, Thijs VN, et al. Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke. 2009;40(5):1692–1697. doi: 10.1161/STROKEAHA.108.538082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heiss WD. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20(9):1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 95.Zülch K-J. The Cerebral Infarct: Pathology, Pathogenesis, and Computed Tomography. 1st ed. Berlin: Springer-Verlag; 1985. [Google Scholar]
- 96.Bär T. Morphometric evaluation of capillaries in different laminae of rat cerebral cortex by automatic image analysis: Changes during development and aging. In: Cervos-Navarro J, editor. Advances in Neurology. 20th ed. New York: Raven Press; 1978. pp. 1–9. [PubMed] [Google Scholar]
- 97.Bär T. The vascular system of the cerebral cortex. Adv Anat Embryol Cell Biol. 1980;59:I–VI. doi: 10.1007/978-3-642-67432-7. 1–62. [DOI] [PubMed] [Google Scholar]
- 98.Russell RWR. Observations on the retinal blood-vessels in monocular blindness. Lancet. 1961;2(7218):1422–1428. doi: 10.1016/s0140-6736(61)91247-8. [DOI] [PubMed] [Google Scholar]
- 99.Fields WS, Lemak NA, Frankowski RF, Hardy RJ. Controlled trial of aspirin in cerebral ischemia. Stroke. 1977;8(3):301–314. doi: 10.1161/01.str.8.3.301. [DOI] [PubMed] [Google Scholar]
- 100.The Canadian Cooperative Study Group. A randomized trial of aspirin and sulfinpyrazone in threatened stroke. N Engl J Med. 1978;299(2):53–59. doi: 10.1056/NEJM197807132990201. [DOI] [PubMed] [Google Scholar]
- 101.Bousser MG, Eschwege E, Haguenau M, et al. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke. 1983;14(1):5–14. doi: 10.1161/01.str.14.1.5. [DOI] [PubMed] [Google Scholar]
- 102.The ESPS Group. The European Stroke Prevention Study. (ESPS) Principal end-points. Lancet. 1987;2(8572):1351–1354. [PubMed] [Google Scholar]
- 103.ESPS-2 Working Group. Second European Stroke Prevention Study. ESPS-2 Working Group. J Neurol. 1992;239(6):299–301. doi: 10.1007/BF00867583. [DOI] [PubMed] [Google Scholar]
- 104.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367(9523):1665–1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 105.Hass WK, Easton JD, Adams HP, Jr, et al. Ticlopidine Aspirin Stroke Study Group A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. N Engl J Med. 1989;321(8):501–507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- 106.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348(9038):1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 107.Diener H-C, Bogousslavsky J, Brass LM, et al. MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 108.Bhatt DL, Fox KA, Hacke W, et al. CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 109.Campbell MH. Basilar artery syndrome. Can Med Assoc J. 1953;69(3):314–315. [PMC free article] [PubMed] [Google Scholar]
- 110.Millikan CH, Siekert RG, Shick RM. Studies in cerebrovascular disease. III. The use of anticoagulant drugs in the treatment of insufficiency or thrombosis within the basilar arterial system. Proc Staff Meet Mayo Clin. 1955;30(6):116–126. [PubMed] [Google Scholar]
- 111.Baker RN, Broward JA, Fang HC, et al. Anticoagulant therapy in cerebral infarction. Report on cooperative study. Neurology. 1962;12:823–835. doi: 10.1212/wnl.12.12.823. [DOI] [PubMed] [Google Scholar]
- 112.Baker RN, Ramseyer JC, Schwartz WS. Prognosis in patients with transient cerebral ischemic attacks. Neurology. 1968;18:1157–1165. doi: 10.1212/wnl.18.12.1157. [DOI] [PubMed] [Google Scholar]
- 113.Cerebral Embolism Study Group. Immediate anticoagulation of embolic stroke: a randomized trial. Stroke. 1983;14(5):668–676. doi: 10.1161/01.str.14.5.668. [DOI] [PubMed] [Google Scholar]
- 114.Albers GW. Antithrombotic agents in cerebral ischemia. Am J Cardiol. 1995;75(6):34B–38B. doi: 10.1016/0002-9149(95)80008-g. [DOI] [PubMed] [Google Scholar]
- 115.Mohr JP, Thompson JLP, Lazar RM, et al. Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 116.Fisher CM. Anticoagulant therapy in cerebral thrombosis and cerebral embolism. A national cooperative study, interim report. Neurology. 1961;11(4):119–131. doi: 10.1212/wnl.11.4_part_2.119. [DOI] [PubMed] [Google Scholar]
- 117.Hill AB, Marshall J, Shaw DA. Cerebrovascular disease: trial of long-term anticoagulant therapy. BMJ. 1962;2(5311):1003–1006. doi: 10.1136/bmj.2.5311.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDowell F, McDevitt E. Treatment of the completed stroke with long-term anticoagulant. Six and one-half years’ experience. In: Siekert RH, Whisnant JR, editors. Cerebral Vascular Diseases, Fourth Princeton Conference. New York, NY: Grune & Stratton; 1965. pp. 185–199. [Google Scholar]
- 119.Enger E, Boyesen S. Long-term anticoagulant therapy in patients with cerebral infarction: a controlled clinical study. Acta Med Scand. 1965;179(Suppl):1–61. [PubMed] [Google Scholar]
- 120.Baker RN. An evaluation of anticoagulant therapy in the treatment of cerebrovascular disease. Report of the Veterans Administration Cooperative Study of Atherosclerosis, Neruology Section. Neurology. 1961;11(4):132–138. doi: 10.1212/wnl.11.4_part_2.132. [DOI] [PubMed] [Google Scholar]
- 121.CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet. 1997;349(9066):1641–1649. [PubMed] [Google Scholar]
- 122.Chen ZM, Xie JX, Peto R, et al. Chinese Acute Stroke Trial (CAST): rationale, design and progress. Cerebrovasc Dis. 1996;6:23. [Google Scholar]
- 123.Kay R, Wong KS, Yu YL, et al. Low-molecular-weight heparin for the treatment of acute ischemic stroke. N Engl J Med. 1995;333(24):1588–1593. doi: 10.1056/NEJM199512143332402. [DOI] [PubMed] [Google Scholar]
- 124.Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study GroupHeparin in Acute Embolic Stroke Trial. Lancet. 2000;355(9211):1205–1210. doi: 10.1016/s0140-6736(00)02085-7. [DOI] [PubMed] [Google Scholar]
- 125.Diener HC, Ringelstein EB, von Kummer R, et al. Therapy of Patients With Acute Stroke (TOPAS) Investigators. Treatment of acute ischemic stroke with the low-molecular-weight heparin certoparin: results of the TOPAS trial. Stroke. 2001;32(1):22–29. doi: 10.1161/01.str.32.1.22. [DOI] [PubMed] [Google Scholar]
- 126.Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358(9283):702–710. doi: 10.1016/s0140-6736(01)05837-8. [DOI] [PubMed] [Google Scholar]
- 127.Stutzmann JM, Mary V, Wahl F, Grosjean-Piot O, Uzan A, Pratt J. Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review. CNS Drug Rev. 2002;8(1):1–30. doi: 10.1111/j.1527-3458.2002.tb00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu M, Counsell C, Sandercock P. Anticoagulants for preventing recurrence following ischaemic stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2000;(2):CD000248. doi: 10.1002/14651858.CD000248. [DOI] [PubMed] [Google Scholar]
- 129.Gubitz G, Counsell C, Sandercock P, Signorini D. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2000;(2):CD000024. doi: 10.1002/14651858.CD000024. [DOI] [PubMed] [Google Scholar]
- 130.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989;1(8631):175–179. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 131.Stroke Prevention in Atrial Fibrillation Study Group Investigators. Preliminary report of the Stroke Prevention in Atrial Fibrillation Study. N Engl J Med. 1990;322(12):863–868. doi: 10.1056/NEJM199003223221232. [DOI] [PubMed] [Google Scholar]
- 132.The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323(22):1505–1511. doi: 10.1056/NEJM199011293232201. [DOI] [PubMed] [Google Scholar]
- 133.Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348(9028):633–638. [PubMed] [Google Scholar]
- 134.The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 135.EAFT (European Atrial Fibrillation Trial) Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342(8882):1255–1262. [PubMed] [Google Scholar]
- 136.The Atrial Fibrillation Investigators. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. Arch Intern Med. 1997;157(11):1237–1240. [PubMed] [Google Scholar]
- 137.Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY Steering Committee, Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 138.Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 139.del Zoppo GJ, Eliasziw M. Newoptions in anticoagulation for atrial fibrillation. N Engl J Med. 2011;365(10):952–953. doi: 10.1056/NEJMe1107516. [DOI] [PubMed] [Google Scholar]
- 140.Connolly SJ, Eikelboom J, Joyner C, et al. AVERROES Steering Committee, Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 141.Granger CB, Alexander JH, McMurray JJ, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 142.Coulshed N, Epstein EJ, McKendrick CS, Galloway RW, Walker E. Systemic embolism in mitral valve disease. Br Heart J. 1970;32(1):26–34. doi: 10.1136/hrt.32.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Szekely P. Systemic embolism and anticoagulant prophylaxis in rheumatic heart disease. BMJ. 1964;1(5392):1209–1212. doi: 10.1136/bmj.1.5392.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daley R, Mattingly TW, Holt CL, Bland EF, White PD. Systemic arterial embolism in rheumatic heart disease. Am Heart J. 1951;42(4):566–581. doi: 10.1016/0002-8703(51)90152-4. [DOI] [PubMed] [Google Scholar]
- 145.Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briët E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333(1):11–17. doi: 10.1056/NEJM199507063330103. [DOI] [PubMed] [Google Scholar]
- 146.Stein PD, Alpert JS, Bussey HI, Dalen JE, Turpie AG. Antithrombotic therapy in patients with mechanical and biological prosthetic heart valves. Chest. 2001;119(1, Suppl):220S–227S. doi: 10.1378/chest.119.1_suppl.220s. [DOI] [PubMed] [Google Scholar]
- 147.Horstkotte D, Schulte H, Bircks W, Strauer B. Unexpected findings concerning thromboembolic complications and anticoagulation after complete 10. year follow up of patients with St. Jude Medical prostheses. J Heart Valve Dis. 1993;2(3):291–301. [PubMed] [Google Scholar]
- 148.Horstkotte D, Scharf RE, Schultheiss HP. Intracardiac thrombosis: patient-related and device-related factors. J Heart Valve Dis. 1995;4(2):114–120. [PubMed] [Google Scholar]
- 149.Levine HJ, Pauker SG, Salzman EW. Antithrombotic therapy in valvular heart adisease. Chest. 1989;95(2, Suppl):98S–106S. doi: 10.1378/chest.95.2_supplement.98s. [DOI] [PubMed] [Google Scholar]
- 150.Stein PD, Kantrowitz A. Antithrombotic therapy in mechanical and biological prosthetic heart valves and saphenous vein bypass grafts. Chest. 1989;95(2, Suppl):107S–117S. doi: 10.1378/chest.95.2_supplement.107s. [DOI] [PubMed] [Google Scholar]
- 151.Horstkotte D, Schulte HD, Bircks W, Strauer BE. Lower intensity anticoagulation therapy results in lower complication rates with the St. Jude Medical prosthesis. J Thorac Cardiovasc Surg. 1994;107(4):1136–1145. [PubMed] [Google Scholar]
- 152.Stein PD, Grandison D, Hua TA, et al. Therapeutic level of oral anticoagulation with warfarin in patients with mechanical prosthetic heart valves: review of literature and recommendations based on international normalized ratio. Postgrad Med J. 1994;70(Suppl 1):S72–S83. [PubMed] [Google Scholar]
- 153.Dale J, Myhre E, Storstein O, Stormorken H, Efskind L. Prevention of arterial thromboembolism with acetylsalicylic acid. A controlled clinical study in patients with aortic ball valves.AmHeart J. 1977;94(1):101–111. doi: 10.1016/s0002-8703(77)80351-7. [DOI] [PubMed] [Google Scholar]
- 154.Altman R, Boullon F, Rouvier J, Raca R, de la Fuente L, Favaloro R. Aspirin and prophylaxis of thromboembolic complications in patients with substitute heart valves. J Thorac Cardiovasc Surg. 1976;72(1):127–129. [PubMed] [Google Scholar]
- 155.Sullivan JM, Harken DE, Gorlin R. Effect of dipyridamole on the incidence of arterial emboli after cardiac valve replacement. Circulation. 1969;39(5) Suppl 1:I149–I153. doi: 10.1161/01.cir.39.5s1.i-149. [DOI] [PubMed] [Google Scholar]
- 156.Sullivan JM, Harken DE, Gorlin R. Pharmacologic control of thromboembolic complications of cardiac-valve replacement. N Engl J Med. 1971;284(25):1391–1394. doi: 10.1056/NEJM197106242842501. [DOI] [PubMed] [Google Scholar]
- 157.Altman R, Rouvier J, Gurfinkel E, Scazziota A, Turpie AG. Comparison of high-dose with low-dose aspirin in patients with mechanical heart valve replacement treated with oral anticoagulant. Circulation. 1996;94(9):2113–2116. doi: 10.1161/01.cir.94.9.2113. [DOI] [PubMed] [Google Scholar]
- 158.Kontozis L, Skudicky D, Hopley MJ, Sareli P. Long-term follow-up of St. Jude Medical prosthesis in a young rheumatic population using low-level warfarin anticoagulation: an analysis of the temporal distribution of causes of death. Am J Cardiol. 1998;81(6):736–739. doi: 10.1016/s0002-9149(97)01007-2. [DOI] [PubMed] [Google Scholar]
- 159.Chesebro JH, Fuster V, Elveback LR, et al. Trial of combined warfarin plus dipyridamole or aspirin therapy in prosthetic heart valve replacement: danger of aspirin compared with dipyridamole. Am J Cardiol. 1983;51(9):1537–1541. doi: 10.1016/0002-9149(83)90673-2. [DOI] [PubMed] [Google Scholar]
- 160.Pouleur H, Buyse M. Effects of dipyridamole in combination with anticoagulant therapy on survival and thromboembolic events in patients with prosthetic heart valves. A meta-analysis of the randomized trials. J Thorac Cardiovasc Surg. 1995;110(2):463–472. doi: 10.1016/S0022-5223(95)70243-1. [DOI] [PubMed] [Google Scholar]
- 161.Massel D, Little SH. Risks and benefits of adding anti-platelet therapy to warfarin among patients with prosthetic heart valves: a meta-analysis. Am Coll Cardiol. 2001;37:569–578. doi: 10.1016/s0735-1097(00)01135-9. [DOI] [PubMed] [Google Scholar]
- 162.Oyer PE, Stinson EB, Griepp RB, Shumway NE. Valve replacement with the Starr-Edwards and Hancock prostheses: comparative analysis of late morbidity and mortality. Ann Surg. 1977;186(3):301–309. doi: 10.1097/00000658-197709000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ionescu MI, Smith DR, Hasan SS, Chidambaram M, Tandon AP. Clinical durability of the pericardial xenograft valve: ten years experience with mitral replacement. Ann Thorac Surg. 1982;34(3):265–277. doi: 10.1016/s0003-4975(10)62496-4. [DOI] [PubMed] [Google Scholar]
- 164.Williams JB, Karp RB, Kirklin JW, et al. Considerations in selection and management of patients undergoing valve replacement with glutaraldehyde-fixed porcine bioprotheses. Ann Thorac Surg. 1980;30(3):247–258. doi: 10.1016/s0003-4975(10)61253-2. [DOI] [PubMed] [Google Scholar]
- 165.Cohn LH, Allred EN, DiSesa VJ, Sawtelle K, Shemin RJ, Collins JJ., Jr Early and late risk of aortic valve replacement. A 12. year concomitant comparison of the porcine bioprosthetic and tilting disc prosthetic aortic valves. J Thorac Cardiovasc Surg. 1984;88(5 Pt 1):695–705. [PubMed] [Google Scholar]
- 166.Kelton JG. Antiplatelet agents: rationale and results. Clin Haematol. 1983;12(1):311–354. [PubMed] [Google Scholar]
- 167.Nuñez L, Aguado MG, Celemín D, Iglesias A, Larrea JL. Aspirin or Coumadin as the drug of choice for valve replacement with porcine bioprosthesis. Ann Thorac Surg. 1982;33(4):354–358. doi: 10.1016/s0003-4975(10)63227-4. [DOI] [PubMed] [Google Scholar]
- 168.Nuñez L, Gil Aguado M, Larrea JL, Celemín D, Oliver J. Prevention of thromboembolism using aspirin after mitral valve replacement with porcine bioprosthesis. Ann Thorac Surg. 1984;37(1):84–87. doi: 10.1016/s0003-4975(10)60717-5. [DOI] [PubMed] [Google Scholar]
- 169.Breddin K, Loew D, Lechner K, Uberla K, Walter E. Secondary prevention of myocardial infarction: a comparison of acetylsalicylic acid, placebo and phenprocoumon. Haemostasis. 1980;9(6):325–344. doi: 10.1159/000214375. [DOI] [PubMed] [Google Scholar]
- 170.Hurlen M, Smith P, Arnesen H. Effects of warfarin, aspirin and the two combined, on mortality and thromboembolic morbidity after myocardial infarction. The WARIS-II (Warfarin-Aspirin Reinfarction Study) design. Scand Cardiovasc J. 2000;34(2):168–171. doi: 10.1080/14017430050142198. [DOI] [PubMed] [Google Scholar]
- 171.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969–974. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 172.Hurlen M, Eikvar L, Seljeflot I, Arnesen H. Occult bleeding in three different antithrombotic regimes after myocardial infarction. A WARIS-II subgroup analysis. Thromb Res. 2006;118(4):433–438. doi: 10.1016/j.thromres.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 173.Meyer JS, Herndon RM, Gotoh F, Tazaki Y, Nelson JN, Johnson JF. Therapeutic thrombolysis. In: Millikan CH, Siekert RG, Whisnant JP, editors. Cerebral Vascular Diseases, Third Princeton Conference. New York: Grune and Stratton; 1961. pp. 160–177. [Google Scholar]
- 174.Sherry S. Historical developments in fibrinolysis. In: Sawaya R, editor. Fibrinolysis and the Central Nervous System. Philadelphia: Hanley & Belfus, Inc; 1990. pp. 3–13. [Google Scholar]
- 175.Abe T, Kazawa M, Naito I, et al. Clinical effect of urokinase (60,000 units/day) on cerebral infarction–Comparative study by means of multiple center double-blind test. Blood Vessels. 1981;12:342–358. [Google Scholar]
- 176.Abe T, Kazama M, Naito I, et al. Clinical evaluation for efficacy of tissue culture urokinase (TCUK) on cerebral thrombosis by means of multicenter double-blind study. Blood Vessels. 1981;12:321–341. [Google Scholar]
- 177.Fletcher AP, Alkjaersig N, Lewis M, et al. A pilot study of urokinase therapy in cerebral infarction. Stroke. 1976;7(2):135–142. doi: 10.1161/01.str.7.2.135. [DOI] [PubMed] [Google Scholar]
- 178.del Zoppo GJ, Zeumer H, Harker LA. Thrombolytic therapy in stroke: possibilities and hazards. Stroke. 1986;17(4):595–607. doi: 10.1161/01.str.17.4.595. [DOI] [PubMed] [Google Scholar]
- 179.Atarashi J, Otomo E, Araki G, et al. Clinical utility of urokinase in the treatment of acute stage of cerebral thrombosis: multi-center double-blind study in comparison with placebo. Clin Eval. 1985;13:659–709. [Google Scholar]
- 180.Otomo E, Araki G, Itoh E, et al. Clinical efficacy of urokinase in the treatment of cerebral thrombosis. Clin Eval. 1985;13:711–751. [Google Scholar]
- 181.Abe T, Terashi A, Tohgi H, Sasoh S, Naito I. Clinical efficacy of intravenous administration of SM-9527 (t-PA) in cerebral thrombosis. Clin Eval. 1990;18:39–69. [Google Scholar]
- 182.Otomo E, Tohgi H, Hirai S, et al. Clinical efficacy of AK-124 (tissue plasminogen activator) in the treatment of cerebral thrombosis: study by means of multicenter double-blind comparison with urokinase. Yakuri To Chiryo. 1988;16:3775–3821. [Google Scholar]
- 183.Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11(Suppl 1):2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- 184.Mori E, Tabuchi M, Yoshida T, Yamadori A. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke. 1988;19(7):802–812. doi: 10.1161/01.str.19.7.802. [DOI] [PubMed] [Google Scholar]
- 185.del Zoppo GJ, Ferbert A, Otis S, et al. Local intra-arterial fibrinolytic therapy in acute carotid territory stroke. A pilot study. Stroke. 1988;19(3):307–313. doi: 10.1161/01.str.19.3.307. [DOI] [PubMed] [Google Scholar]
- 186.Matsumoto K, Satoh K. Topical intra-arterial urokinase infusion for acute stroke. In: Hacke W, del Zoppo GJ, Hirschberg M, editors. Thrombolytic Therapy in Acute Ischemic Stroke. Heidelberg, Germany: Springer-Verlag; 1991. pp. 207–212. [Google Scholar]
- 187.Mori E, Yoneda Y, Tabuchi M, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology. 1992;42(5):976–982. doi: 10.1212/wnl.42.5.976. [DOI] [PubMed] [Google Scholar]
- 188.Yamaguchi T. Intravenous rt-PA in acute embolic stroke. In: Hacke W, del Zoppo GJ, Hirschberg M, editors. Thrombolytic Therapy in Acute Ischemic Stroke. Heidelberg, Germany: Springer-Verlag; 1991. pp. 168–174. [Google Scholar]
- 189.von Kummer R, Forsting M, Sartor K, Hacke W. Intravenous recombinant tissue plasminogen activator in acute stroke. In: Hacke W, del Zoppo GJ, Hirschberg M, editors. Thrombolytic Therapy in Acute Ischemic Stroke. Heidelberg, Germany: Springer-Verlag; 1991. pp. 161–167. [Google Scholar]
- 190.del Zoppo GJ. Thrombolysis: New concepts in the treatment of stroke. In: Hennerici M, Sitzer G, Weger H-D, editors. Carotid Artery Plaques. Karger: Basel; 1988. pp. 247–272. [Google Scholar]
- 191.Hommel M, Boissel JP, Cornu C, et al. Termination of trial of streptokinase in severe acute ischemic stroke. Lancet. 1995;345:578–579. doi: 10.1016/s0140-6736(95)91179-0. [DOI] [PubMed] [Google Scholar]
- 192.Donnan GA, Davis SM, Chambers BR, et al. Trials of streptokinase in severe acute ischaemic stroke. Lancet. 1995;345(8949):578–579. [PubMed] [Google Scholar]
- 193.Multicenter Acute Stroke Trial-Italy (MAST-I) Group. Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischaemic stroke. Multicentre Acute Stroke Trial—Italy (MAST-I) Group. Lancet. 1995;346(8989):1509–1514. [PubMed] [Google Scholar]
- 194.Kwiatkowski TG, Libman RB, Frankel M, et al. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. Effects of tissue plasminogen activator for acute ischemic stroke at one year. N Engl J Med. 1999;340(23):1781–1787. doi: 10.1056/NEJM199906103402302. [DOI] [PubMed] [Google Scholar]
- 195.Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000;283(9):1145–1150. doi: 10.1001/jama.283.9.1145. [DOI] [PubMed] [Google Scholar]
- 196.Hacke W, Kaste M, Fieschi C, et al. Second European-Australasian Acute Stroke Study Investigators. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352(9136):1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 197.von Kummer R, Allen KL, Holle R, et al. Acute stroke: usefulness of early CT findings before thrombolytic therapy. Radiology. 1997;205(2):327–333. doi: 10.1148/radiology.205.2.9356611. [DOI] [PubMed] [Google Scholar]
- 198.Wahlgren N, Ahmed N, Dávalos A, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 199.Wahlgren N, Ahmed N, Dávalos A, et al. SITS investigators. Thrombolysis with alteplase 3–4.5. h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372(9646):1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- 200.Hacke W, Kaste M, Bluhmki E, et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 201.Hacke W, Kaste M, Fieschi C, et al. Second European-Australasian Acute Stroke Study Investigators. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352(9136):1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 202.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0-to 6-hour acute stroke trial, part A (A0276g) : results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke. 2000;31(4):811–816. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 203.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282(21):2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 204.del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yamaguchi T, Hayakawa T, Kikuchi H. for the Japanese Thrombolysis Study Group. Intravenous tissue plasminogen activator in acute thromboembolic stroke: A placebo-controlled, double-blind trial. In: del Zoppo GJ, Mori E, Hacke W, editors. Thrombolytic Therapy in Acute Ischemic Stroke II. Heidelberg, Germany: Springer-Verlag; 1993. pp. 59–65. [Google Scholar]
- 206.Hacke W, Donnan G, Fieschi C, et al. ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 207.Levy DE, Brott TG, Haley EC, Jr, et al. Factors related to intracranial hematoma formation in patients receiving tissue-type plasminogen activator for acute ischemic stroke. Stroke. 1994;25(2):291–297. doi: 10.1161/01.str.25.2.291. [DOI] [PubMed] [Google Scholar]
- 208.Larrue V, von Kummer R, del Zoppo GJ, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke. 1997;28(5):957–960. doi: 10.1161/01.str.28.5.957. [DOI] [PubMed] [Google Scholar]
- 209.Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32(2):438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 210.Dubey N, Bakshi R, Wasay M, Dmochowski J. Early computed tomography hypodensity predicts hemorrhage after intravenous tissue plasminogen activator in acute ischemic stroke. J Neuroimaging. 2001;11(2):184–188. doi: 10.1111/j.1552-6569.2001.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 211.Kalafut MA, Schriger DL, Saver JL, Starkman S. Detection of early CT signs of >1/3 middle cerebral artery infarctions : interrater reliability and sensitivity of CT interpretation by physicians involved in acute stroke care. Stroke. 2000;31(7):1667–1671. doi: 10.1161/01.str.31.7.1667. [DOI] [PubMed] [Google Scholar]
- 212.Wardlaw JM, Murray V, Berge E, Del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2009;(4):CD000213. doi: 10.1002/14651858.CD000213.pub2. [DOI] [PubMed] [Google Scholar]
- 213.Haley EC, Jr, Lyden PD, Johnston KC, Hemmen TM. TNK in Stroke Investigators. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;36(3):607–612. doi: 10.1161/01.STR.0000154872.73240.e9. [DOI] [PubMed] [Google Scholar]
- 214.Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366(12):1099–1107. doi: 10.1056/NEJMoa1109842. [DOI] [PubMed] [Google Scholar]
- 215.Hacke W, Albers G, Al-Rawi Y, et al. DIAS Study Group. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36(1):66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 216.Furlan AJ, Eyding D, Albers GW, et al. DEDAS Investigators. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37(5):1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 217.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8(2):141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Caplan LR. “Top of the basilar” syndrome. Neurology. 1980;30(1):72–79. doi: 10.1212/wnl.30.1.72. [DOI] [PubMed] [Google Scholar]
- 219.Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiological correlation. Stroke. 1977;8(3):383–390. doi: 10.1161/01.str.8.3.383. [DOI] [PubMed] [Google Scholar]
- 220.Nenci GG, Gresele P, Taramelli M, Agnelli G, Signorini E. Thrombolytic therapy for thromboembolism of vertebrobasilar artery. Angiology. 1983;34(9):561–571. doi: 10.1177/000331978303400901. [DOI] [PubMed] [Google Scholar]
- 221.Hacke W, Zeumer H, Ferbert A, Brückmann H, del Zoppo GJ. Intraarterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke. 1988;19(10):1216–1222. doi: 10.1161/01.str.19.10.1216. [DOI] [PubMed] [Google Scholar]
- 222.Zeumer H, Freitag HJ, Grzyska U, Neunzig HP. Local intraarterial fibrinolysis in acute vertebrobasilar occlusion. Technical developments and recent results. Neuroradiology. 1989;31(4):336–340. doi: 10.1007/BF00344178. [DOI] [PubMed] [Google Scholar]
- 223.Mobius E, Berg-Dammer E, Kuhne D, Aster HC. Local thrombolytic therapy in acute basilar artery occlusion. In: Hacke W, del Zoppo GJ, Hirschberg M, editors. Thrombolytic Therapy in Acute Ischemic Stroke. Berlin, Germany: Springer Verlag; 1991. pp. 213–215. [Google Scholar]
- 224.Furlan AJ, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282(21):2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 225.Bell WR, Jr, Bells WR., Jr Defibrinogenating enzymes. Drugs. 1997;54(Suppl 3):18–30. doi: 10.2165/00003495-199700543-00005. discussion 30–31. [DOI] [PubMed] [Google Scholar]
- 226.The Ancrod Stroke Study Investigators. Ancrod for the treatment of acute ischemic brain infarction. Stroke. 1994;25(9):1755–1759. doi: 10.1161/01.str.25.9.1755. [DOI] [PubMed] [Google Scholar]
- 227.Krishnamurti C, Barr CF, Hassett MA, Young GD, Alving BM. Plasminogen activator inhibitor: a regulator of ancrod-induced fibrin deposition in rabbits. Blood. 1987;69(3):798–803. [PubMed] [Google Scholar]
- 228.Sherman DG, Atkinson RP, Chippendale T, et al. Intravenous ancrod for treatment of acute ischemic stroke: the STAT study: a randomized controlled trial. Stroke Treatment with Ancrod Trial. JAMA. 2000;283(18):2395–2403. doi: 10.1001/jama.283.18.2395. [DOI] [PubMed] [Google Scholar]
- 229.Orgogozo JM, Verstraete M, Kay R, Hennerici M, Lenzi GL. Outcomes of ancrod in acute ischemic stroke. Independent Data and Safety Monitoring Board for ESTAT. Steering Committee for ESTAT. European Stroke Treatment with Ancrod Trial. JAMA. 2000;284(15):1926–1927. [PubMed] [Google Scholar]
- 230.Levy DE, del Zoppo GJ, Demaerschalk BM, et al. Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke. 2009;40(12):3796–3803. doi: 10.1161/STROKEAHA.109.565119. [DOI] [PubMed] [Google Scholar]
- 231.Borhani Haghighi A, Edgell RC, Cruz-Flores S, et al. Mortality of cerebral venous-sinus thrombosis in a large national sample. Stroke. 2012;43(1):262–264. doi: 10.1161/STROKEAHA.111.635664. [DOI] [PubMed] [Google Scholar]
- 232.Guo XB, Guan S, Fan Y, Song LJ. Local thrombolysis for severe cerebral venous sinus thrombosis. AJNR Am J Neuroradiol. 2012;33(6):1187–1190. doi: 10.3174/ajnr.A2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sidani CA, Ballourah W, El Dassouki M, et al. Venous sinus thrombosis leading to stroke in a patient with sickle cell disease on hydroxyurea and high hemoglobin levels: treatment with thrombolysis. Am J Hematol. 2008;83(10):818–820. doi: 10.1002/ajh.21261. [DOI] [PubMed] [Google Scholar]
- 234.Weatherby SJM, Edwards NC, West R, Heafield MTE. Good outcome in early pregnancy following direct thrombolysis for cerebral venous sinus thrombosis. J Neurol. 2003;250(11):1372–1373. doi: 10.1007/s00415-003-0173-6. [DOI] [PubMed] [Google Scholar]
- 235.Yamini B, Loch Macdonald R, Rosenblum J. Treatment of deep cerebral venous thrombosis by local infusion of tissue plasminogen activator. Surg Neurol. 2001;55(6):340–346. doi: 10.1016/s0090-3019(01)00471-2. [DOI] [PubMed] [Google Scholar]
- 236.Wasay M, Bakshi R, Kojan S, Bobustuc G, Dubey N, Unwin DH. Nonrandomized comparison of local urokinase thrombolysis versus systemic heparin anticoagulation for superior sagittal sinus thrombosis. Stroke. 2001;32(10):2310–2317. doi: 10.1161/hs1001.096192. [DOI] [PubMed] [Google Scholar]
- 237.Wasay M, Dai AI, Ansari M, Shaikh Z, Roach ES. Cerebral venous sinus thrombosis in children: a multicenter cohort from the United States. J Child Neurol. 2008;23(1):26–31. doi: 10.1177/0883073807307976. [DOI] [PubMed] [Google Scholar]
- 238.Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke. 2002;33(8):1963–1967. doi: 10.1161/01.str.0000023445.20454.a8. [DOI] [PubMed] [Google Scholar]
- 239.Bashshur ZF, Taher A, Masri AF, Najjar D, Arayssi TK, Noureddin BN. Anticardiolipin antibodies in patients with retinal vein occlusion and no risk factors: a prospective study. Retina. 2003;23(4):486–490. doi: 10.1097/00006982-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 240.Kwaan HC. Thromboembolic disorders of the eye. In: Comerota AC, editor. Thrombolytic Therapy. Orlando, FL: Grune & Stratton; 1988. pp. 153–163. [Google Scholar]
- 241.Hattenbach LO, Wellermann G, Steinkamp GW, Scharrer I, Koch FH, Ohrloff C. Visual outcome after treatment with low-dose recombinant tissue plasminogen activator or hemodilution in ischemic central retinal vein occlusion. Ophthalmologica. 1999;213(6):360–366. doi: 10.1159/000027455. [DOI] [PubMed] [Google Scholar]
- 242.Freitag H-J, Zeumer H, Knospe V. Acute central retinal artery occlusion and the role of thrombolysis. In: del Zoppo GJ, Mori E, Hacke W, editors. Thrombolytic Therapy in Acute Ischemic Stroke II. Heidelberg, Germany: Springer-Verlag; 1993. pp. 103–105. [Google Scholar]
- 243.Schmidt D, Schumacher M, Wakhloo AK. Microcatheter urokinase infusion in central retinal artery occlusion. Am J Ophthalmol. 1992;113(4):429–434. doi: 10.1016/s0002-9394(14)76167-7. [DOI] [PubMed] [Google Scholar]
- 244.Hattenbach LO, Friedrich Arndt C, Lerche R, et al. Retinal vein occlusion and low-dose fibrinolytic therapy (R.O.L.F.): a prospective, randomized, controlled multicenter study of low-dose recombinant tissue plasminogen activator versus hemodilution in retinal vein occlusion. Retina. 2009;29(7):932–940. doi: 10.1097/IAE.0b013e3181a3b870. [DOI] [PubMed] [Google Scholar]
- 245.Costen MT, Donaldson WB, Olson JA. Acute central retinal vein occlusion successfully treated with intravenous thrombolysis. Br J Ophthalmol. 1999;83(10):1196–1197. doi: 10.1136/bjo.83.10.1194c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Chen CS, Lee AW, Campbell B, et al. Study of the efficacy of intravenous tissue plasminogen activator in central retinal artery occlusion. Int J Stroke. 2011;6(1):87–89. doi: 10.1111/j.1747-4949.2010.00545.x. [DOI] [PubMed] [Google Scholar]
- 247.Hattenbach LO, Puchta J, Hilgenberg I. Experimental endoscopic endovascular cannulation: a novel approach to thrombolysis in retinal vessel occlusion. Invest Ophthalmol Vis Sci. 2012;53(1):42–46. doi: 10.1167/iovs.11-8486. [DOI] [PubMed] [Google Scholar]
- 248.Schwenn OK, Wüstenberg EG, Konerding MA, Hattenbach LO. Experimental percutaneous cannulation of the supraorbital arteries: implication for future therapy. Invest Ophthalmol Vis Sci. 2005;46(5):1557–1560. doi: 10.1167/iovs.04-1129. [DOI] [PubMed] [Google Scholar]
- 249.The TIMI Study Group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) phase II trial. N Engl J Med. 1989;320(10):618–627. doi: 10.1056/NEJM198903093201002. [DOI] [PubMed] [Google Scholar]
- 250.Rao AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial—phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11(1):1–11. doi: 10.1016/0735-1097(88)90158-1. [DOI] [PubMed] [Google Scholar]
- 251.Mori E. Safety and efficacy of 0.6 mg/kg rt-PA: optimum rt-PA dose revisited. Ann N Y Acad Sci. 2012;1268:108–112. doi: 10.1111/j.1749-6632.2012.06689.x. [DOI] [PubMed] [Google Scholar]
- 252.Yamaguchi T, Mori E, Minematsu K, et al. Japan Alteplase Clinical Trial (J-ACT) Group. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT) Stroke. 2006;37(7):1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3. [DOI] [PubMed] [Google Scholar]
- 253.Nakagawara J, Minematsu K, Okada Y, et al. J-MARS Investigators. Thrombolysis with 0.6 mg/kg intravenous alteplase for acute ischemic stroke in routine clinical practice: the Japan post-Marketing Alteplase Registration Study (J-MARS) Stroke. 2010;41(9):1984–1989. doi: 10.1161/STROKEAHA.110.589606. [DOI] [PubMed] [Google Scholar]
- 254.Slivka A, Pulsinelli WA. Hemorrhagic complications of thrombolytic therapy in experimental stroke. Stroke. 1987;18:1148–1156. doi: 10.1161/01.str.18.6.1148. [DOI] [PubMed] [Google Scholar]
- 255.Edvinsson L, MacKenzie ET, McCulloch J. General and comparative anatomy of the cerebral circulation. In: Edvinsson L, MacKenzie ET, McCulloch J, editors. Cerebral Blood Flow and Metabolism. 1 ed. New York: Raven Press; 1993. pp. 3–39. [Google Scholar]
- 256.Lansberg MG, Straka M, Kemp S, et al. DEFUSE 2 study investigators. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11(10):860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.The Abciximab in Ischemic Stroke Investigators. Abciximab in acute ischemic stroke: a randomized, double-blind, placebo-controlled, dose-escalation study. Stroke. 2000;31(3):601–609. doi: 10.1161/01.str.31.3.601. [DOI] [PubMed] [Google Scholar]
- 258.Pancioli AM, Broderick J, Brott T, et al. CLEAR Trial Investigators. The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke. 2008;39(12):3268–3276. doi: 10.1161/STROKEAHA.108.517656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study) Am J Cardiol. 2007;100(9):1419–1426. doi: 10.1016/j.amjcard.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 260.IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35(4):904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 261.IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38(7):2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 262.Qureshi AI, Hussein HM, Abdelmoula M, Georgiadis AL, Janjua N. Subacute recanalization and reocclusion in patients with acute ischemic stroke following endovascular treatment. Neurocrit Care. 2009;10(2):195–203. doi: 10.1007/s12028-008-9161-0. [DOI] [PubMed] [Google Scholar]
- 263.Janjua N, Alkawi A, Suri MF, Qureshi AI. Impact of arterial reocclusion and distal fragmentation during thrombolysis among patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2008;29(2):253–258. doi: 10.3174/ajnr.A0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59(6):862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 265.Zinkstok SM, Roos YB. ARTIS investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380(9843):731–737. doi: 10.1016/S0140-6736(12)60949-0. [DOI] [PubMed] [Google Scholar]
- 266.De Haan R, Horn J, Limburg M, Van Der Meulen J, Bossuyt P. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke. 1993;24(8):1178–1181. doi: 10.1161/01.str.24.8.1178. [DOI] [PubMed] [Google Scholar]
- 267.del Zoppo GJ, Koziol JA. Recanalization and stroke outcome. Circulation. 2007;115(20):2602–2605. doi: 10.1161/CIRCULATIONAHA.107.698225. [DOI] [PubMed] [Google Scholar]