Abstract
Oxidative stress has been implicated in the pathophysiology of retinopathy of prematurity (ROP) for decades. It is becoming increasingly understood that reactive oxygen species (ROS) can trigger signaling pathways that have beneficial or pathologic outcomes. Broad inhibition of ROS in the preterm infant may lead to unwanted consequences as has been experienced with vitamin E studies in the past. In this report, we will provide a current understanding of the role of oxidative stress in activating signaling pathways that cause pathologic features in severe ROP as it manifests currently in the time of oxygen regulation.
Oxidative stress has been implicated in the pathophysiology of retinopathy of prematurity (ROP) for several decades.1 Early thinking focused on tissue damage from excessive generated reactive oxygen species (ROS).2 potentially due to various oxygen stresses3;4;5;6, changes in photoreceptor metabolism during darkness7;8, and the high concentration of polyunsaturated fatty acids in photoreceptors9. In fact, studies were performed decades ago to test vitamin E as an antioxidant to reduce ROP, but these investigations were stopped because of complications of sepsis and necrotizing enterocolitis.10
It is now becoming increasingly recognized that ROS activate signaling pathways that result in either physiologic or pathologic effects. Particularly of interest in ROP are those involved in apoptosis and angiogenesis. Apoptosis is natural to the developing neural and vascular retina through loss of ganglion cells and vascular remodeling,11 as examples. ROS can cause apoptosis,12 which is associated with and, in some studies, causative of delayed retinal vascular development in models of ROP.12;13 Angiogenesis is essential to retinal vascular development but when aberrant or disordered, can lead to disoriented dividing endothelial cells in the form of intravitreal neovascularization.14
In addition, bursts of leukocyte-generated superoxide are important to fight off invading microorganisms and this may be particularly important in the immune-suppressed preterm infant. However, when uncontrolled, ROS may lead to chronic pathology. Therefore, it is helpful to study the signaling cascades activated by ROS that mediate pathologic features in ROP to find potential safer therapies, rather than broadly proposing antioxidants.
Two phases of oxygen-induced retinopathy (OIR) and the study of human ROP
The description of two phases of ROP was based on studies in the 1950’s by Ashton, who exposed newborn kittens to oxygen stresses similar to what preterm infants experienced at that time, prior to technology to regulate oxygen15;16. Kinsey, Patz and others later studied oxygen effects in human infants and found that time in oxygen and low birth weight were strong predictors of severe ROP.17 Today, a common model based on oxygen levels similar to those Ashton used is the mouse model of OIR, developed by Smith and D’Amore.18 In this model, newly developed capillaries regress leaving central areas of vaso-obliteration following exposure to constant, high oxygen (75% O2) from postnatal day (p)7 to p12. This oxygen level causes arterial oxygen greater than 300 mm Hg.19 Following return to room air, a relative hypoxia occurs in the vaso-obliterated retina, which stimulates the release of angiogenic factors, to cause vasoproliferation of blood vessels into the vitreous. This model portrays ROP of the 1950’s prior to the ability to regulate oxygen and may still potentially play a role in units today in which technology to regulate oxygen is not implemented or in which high oxygen must be used for other reasons.1 The mouse OIR model is extremely important in the study of genetic mechanisms of high oxygen induced vessel loss and in the recovery of lost vasculature,20 as well as in angiogenic processes.
Since Ashton’s time, there have been changes in oxygen exposure to preterm infants and recognition of earlier stages in the development of ROP. In ROP, rather than vaso-obliteration, there is delayed physiologic retinal vascular development that causes peripheral avascular retina. Animal models of OIR were developed that recreate features seen in human ROP. The rat model of fluctuating oxygen concentrations developed by Penn21 causes mainly a delay in physiologic retinal vascular development (PRVD) in the peripheral retina, and some central capillary constriction, following fourteen days of fluctuations between 50% and 10% inspired oxygen every 24 hours. The pups are brought into room air and then develop vasoproliferation at the junction of vascularized and avascular retina at p18. The “50/10” OIR model shares several similarities with human ROP. The model produces an appearance similar to stage 3 ROP that occurs in units in which oxygen is regulated, such as in the US, UK, Canada and Australia today.22 The rat pups are exposed to fluctuations in inspired oxygen, which cause arterial oxygen levels similar to transcutaneous oxygen measurements in human infants who developed severe ROP.23;24 The 50/10 OIR model is helpful to study delayed PRVD and vasoproliferation in severe ROP currently.
Role of oxygen in ROS generation
ROS are oxygen containing atoms, ions or molecules and include the hydroxyl radical, superoxide radical, and hydrogen peroxide, as examples. Therefore, it is helpful to review the role of oxygen concentration in the generation of ROS. Hyperoxia is generally accepted as a mechanism to increase ROS generation by increasing superoxide. Besides hyperoxia, hypoxia can, in theory, increase ROS generation by slowing upstream events in the electron transport chain and increase the concentration of oxygen donors that promote electron transfer to oxygen.25 Hypoxia can also lead to the activation of nitric oxide synthethase (NOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase,26 which are enzymes that generate ROS.13;27
ROS generators in the preterm infant
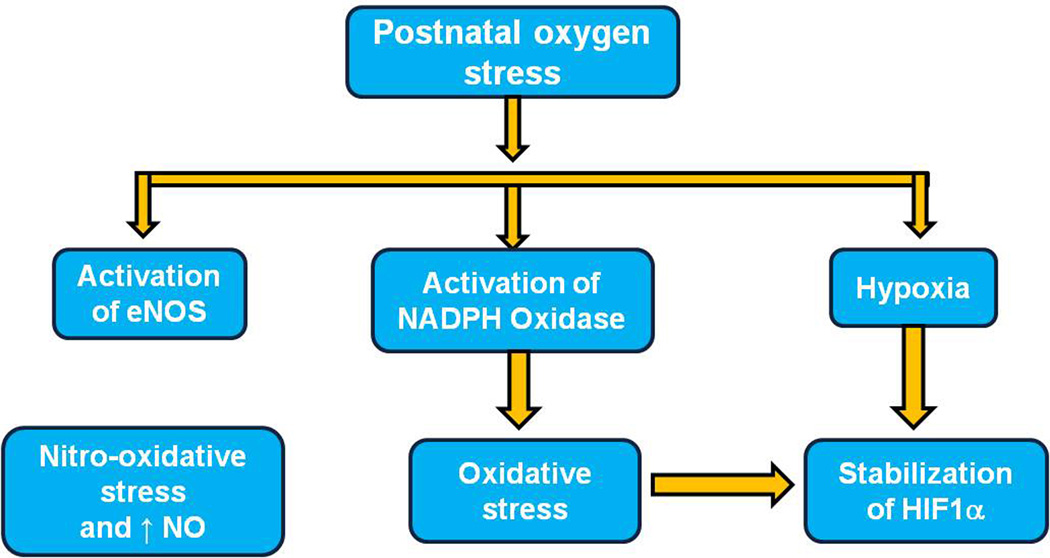
Within the cell, the mitochondrion is recognized as a key source of superoxide radical, but some enzymes are also involved in ROS generation, including NADPH oxidase and nitric oxide synthetase (Figure 1). Anti-oxidant enzymes, such as superoxide dismutases (SODs), glutathione peroxidase (GSH-Px), and catalase, quench oxidative products rendering them less reactive. A balance between generation and quenching of ROS is important for physiologic metabolism while minimizing pathologic processes.
Figure 1.
Postnatal oxygen stress induces oxidative stress and nitro-oxidative stress via activation of NADPH oxidase, and eNOS, and via hypoxia-or ROS stabilized HIF1α. (ENOS, nitric oxide synthetase; HIF1α, hypoxia-inducible factor-1 alpha; NADPH oxidase, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide)
In the events surrounding the preterm infant’s early life, increased oxygen content is postulated to generate ROS directly or perhaps from tissue hypoxia that develops when hyperoxia injures newly developed capillaries in vulnerable immature tissue beds. Besides increased ROS generation, the preterm infant has reduced ability to make antioxidant enzymes3 and to quench ROS.28
ROS Activated Signaling Causes Avascular Retina or Intravitreal Neovascularization: Evidence from Animal Models
Using several OIR models, studies showed that retinal ROS were generated in association with or had a causal role in avascular retina or intravitreal neovascularization.29;30 In the 50/10 OIR model, there was a trend toward increased retinal end-products of ROS, lipid hydroperoxides (LHP), in temporal association with the both delayed PRVD and with intravitreal neovascularization.12 However, neither intravitreal neovascularization nor delayed PRVD was affected by systemic administration of the broad antioxidant, n-acetyl cysteine (NAC), at a dose that significantly inhibited retinal LHP.12 However, in the mouse model of OIR, daily NAC by intraperitoneal injection given to pups exposed to hyperoxia inhibited central vaso-obliteration by 42% at p12. When pups were exposed to hyperoxia (75% O2) and then relative hypoxia (21% O2) received daily NAC from p7 to p17, there was a 62.1% reduction in intravitreal neovascularization at p17.31
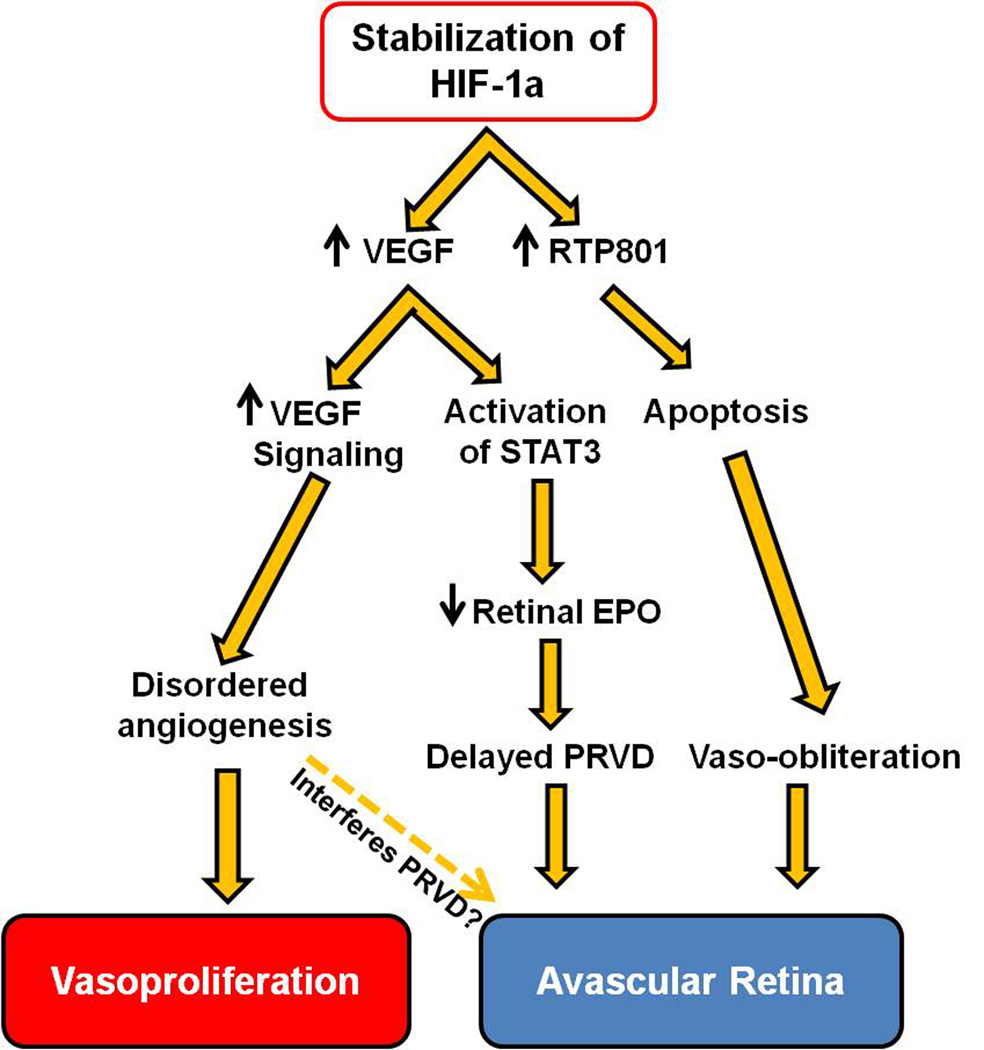
Treatment with vitamins C or E5;36 or liposomal superoxide dismutase32 improved PRVD and reduced vascular leakage compared to sham-injected controls but did not reduce pathologic intravitreal neovascularization in the 50/10 OIR model. NADPH oxidase-dependent ROS generation was found to result in apoptosis and delayed PRVD at p14 in the 50/10 OIR model.12 Treatment with apocynin, an inhibitor of NADPH oxidase, significantly improved PRVD by reducing activated caspase 3 in the retina. Repeated oxygen fluctuations led to increased retinal VEGF expression that activated Janus kinase (JAK)/signaling transducer and activator of transcription 3 (STAT3).33;34 Translocation of STAT3 into the nucleus downregulated Müller cell-derived erythropoietin, which contributed to delayed PRVD at p14 in the 50/10 OIR model (Figure 2). Exogenous erythropoietin given at p2, 4, and 6 significantly increased retinal vascularization approximately 40%,34 suggesting that increased retinal VEGF alone requires other angiogenic factors, such as erythropoietin, to support PRVD. However, exogenous erythropoietin given late at p12 was not able to improve PRVD in the 50/10 OIR model (unpublished data), indicating that timing of erythropoietin administration is critical.34
Figure 2.
Signaling events regulated by stabilized HIF-1a contribute to the pathogenesis of ROP: Avascular retina and Vasoproliferation. (HIF1α, Hypoxia-inducible factor-1 ALPHA; VEGF, vascular endothelial growth factor; STAT3, signaling transducer and activator of transcription 3; EPO, erythropoietin; PRVD, physiologic retinal vascular development)
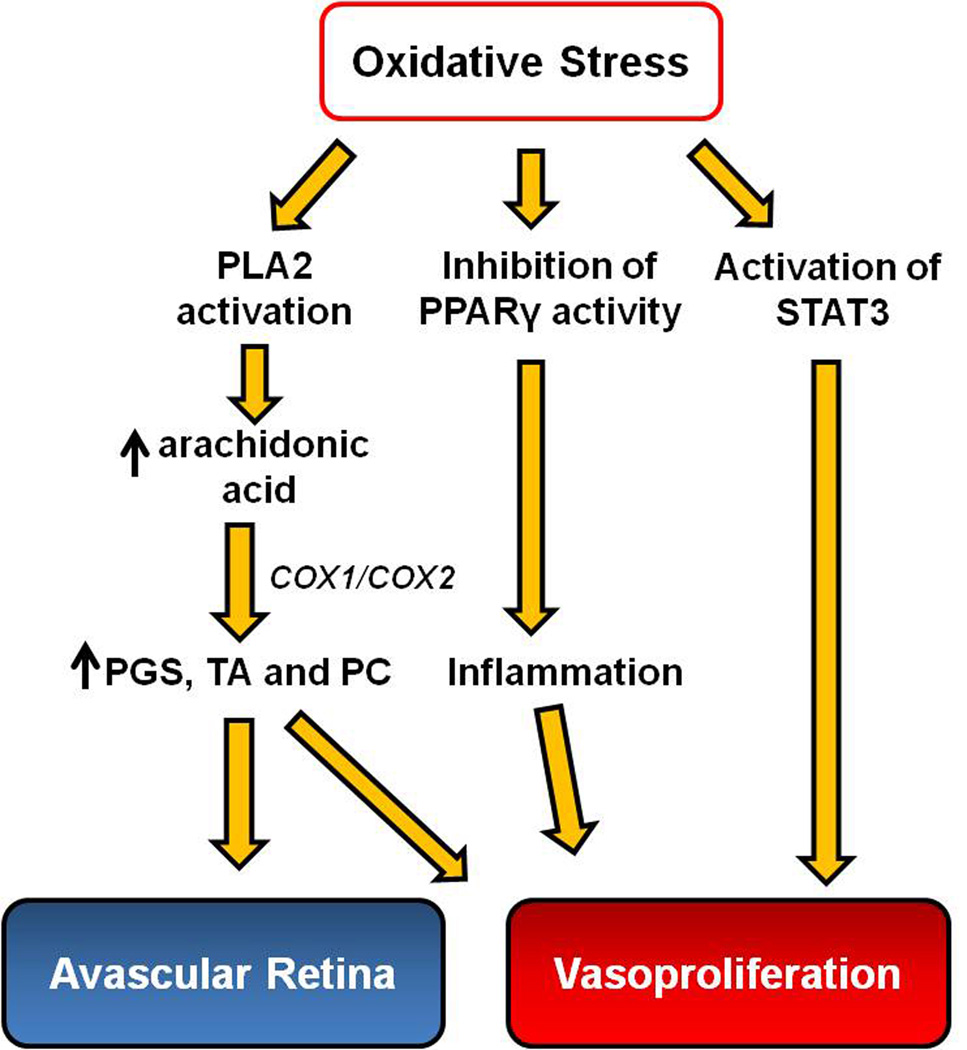
Several studies have measured the effect of the NADPH oxidase inhibitor, apocynin, on intravitreal neovascularization. In the mouse model of OIR, daily apocynin given to mice during relative hypoxia from p12 to p17 significantly reduced intravitreal neovascularization; however, treatment also increased the central vaso-obliterated area more than 2 fold compared to control. Apocynin also abolished the increase in retinal VEGF expression measured at p14.29 In another study, activated NADPH oxidase mediated VEGF expression and intravitreal neovascularization, in part, by inhibiting the anti-inflammatory effect of the transcription factor, peroxisome proliferator-activated receptor gamma (PPARγ) (Figure 3).35 In the 50/10 OIR model, in which rats were brought into supplemental oxygen (28% O2) instead of room air (21% O2), retinal NADPH oxidase activation was increased compared to the standard 50/10 OIR model (p=0.02).29 Apocynin given to pups exposed to 50/10 OIR and supplemental oxygen from p12 to p17 reduced the percent of intravitreal neovascular area to total retinal area at p18 from approximately 4.5% to 2.5%.29 Exacerbated NADPH oxidase activation caused by supplemental oxygen induced intravitreal neovascularization by mediating STAT3 activity.29 In these retinas, VEGF was decreased compared to the standard 50/10 OIR, suggesting that NADPH oxidase may also act independent of VEGF.
Figure 3.
Signaling events regulated by oxidative stress contribute to the pathogenesis of ROP: Avascular retina and Vasoproliferation. (PLA2, phospholipase A2; COX, cyclooxygenase; PPARγ, peroxisome proliferator-activated receptor gamma; PGS, prostaglandins; PC, prostacyclin; TA, thromboxanes; STAT3, signaling transducer and activator of transcription 3)
Together, these findings implicate NADPH oxidase in ROS generation, STAT3 activation, VEGF expression, and intravitreal neovascularization in ROP. Consistent with these findings, in-depth studies over the past decade have further dissected which NADPH oxidase (Nox) isoform(s) contribute to the regulation of VEGF expression, endothelial function and angiogenesis. The Nox family consists of the phagocyte NADPH oxidase (Nox2/ gp91phox – phox stands for phagocytic oxidase) and six homolog members identified in non-phagocytes: Nox1, Nox3, Nox4, Nox5, Duox1 and Duox2. In the vascular system, Nox1, Nox2, Nox4 and Nox5 are expressed, but Nox4 has the highest expression level in human retinal,36 umbilical37 and aorta endothelial cells.38 Unlike the other Nox family members, the activity of Nox4 does not require cytosolic subunits P47phox, P67phox and Rac1,39 but does require P22phox, which colocalizes with Nox4 in the plasma membrane. Another important feature of Nox4 is that it generates H2O2 instead of superoxide, and, therefore, does not produce damaging peroxynitrite in the presence of nitric oxide. Nox4 appears to play a role in hypoxia-induced HIF-1α stabilization and VEGF up-regulation.36 In addition, Nox4 was implicated in STAT3-mediated VEGF expression in endothelial cells36 and is required for endothelial cell proliferation and migration.40 These results indicate a potential role of Nox4 in hypoxia-elicited pro-angiogenic responses of endothelial cells.41 The evidence of its role in apoptosis is conflicting.42;43 Nevertheless, how Nox4 is implicated in the pathogenesis of avascular retina and intravitreal neovascularization in ROP is of great interest and is yet to be investigated in the future.
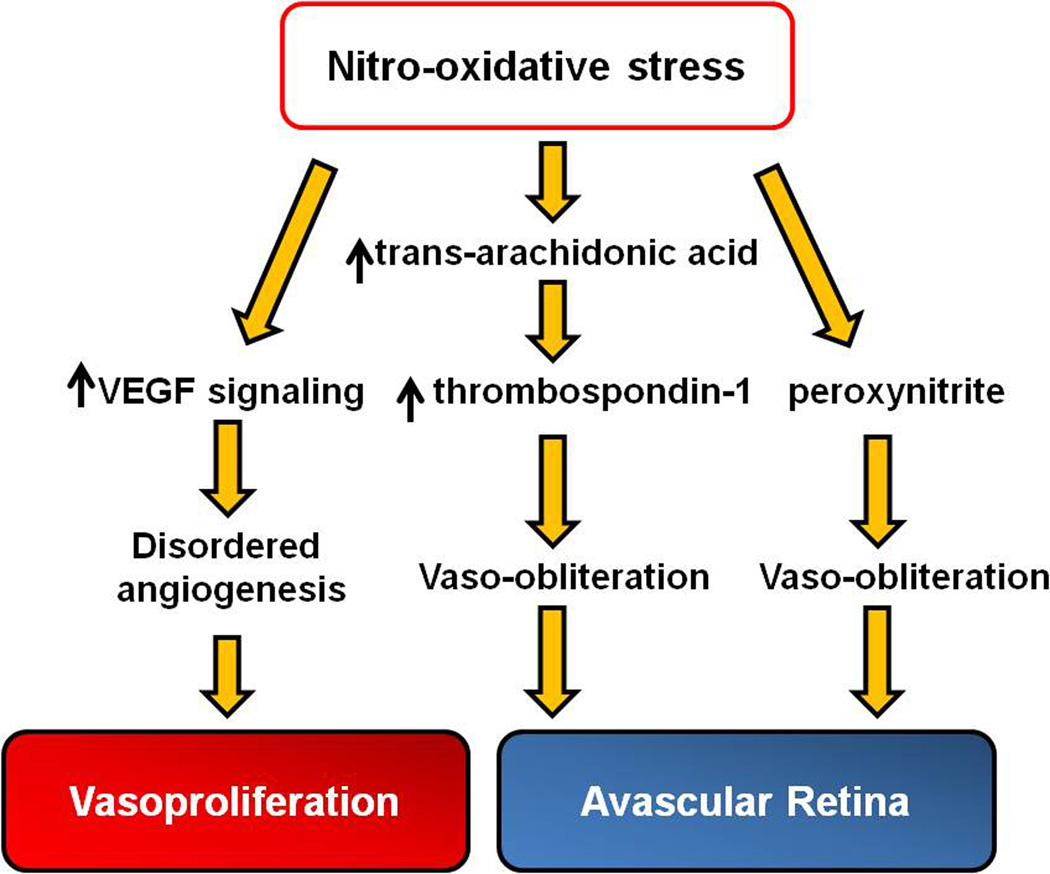
Other enzymes that catalyze ROS generation in endothelial cells and have been implicated in the phases of ROP include eNOS and cyclooxygenase (COX). eNOS catalyzes nitric oxide (NO) generation. NO is an important vasodilator and has both protective and proangiogenic properties in the eye that preserve endothelial cell barrier integrity by reducing apoptosis. However, NO can react with ROS and generate damaging compounds including nitrites, nitrates and peroxynitrite by processing nitro-oxidative stress.27;44 Retinal peroxynitrite was significantly elevated in the mouse model of OIR and caused vaso-obliteration and later vasoproliferation by enhancing VEGF-signaling.30 In the 50/10 OIR model, phosphorylated eNOS was found associated with increased arteriolar tortuosity, but evidence regarding the effect on VEGF was not conclusive.45;46 Reactive nitrogen species, such as ∙NO2, can isomerize arachidonic acid to trans-arachidonic acid, which contributed to vaso-obliteration in the mouse OIR model by increasing the expression of anti-angiogenic agent, thrombospondin-1.47 Retinal polyunsaturated fatty acids are susceptible to forming trans-arachidonic acids when arachidonic acid reacts with reactive nitrogen species (Figure 4). Therefore, evidence suggests that oxidative stress regulated signaling can cause pathologic features independent of and in association with VEGF signaling.48 However, technologic limitations force the need to use whole retinas to measure signal activation and, therefore, effects in individual cells can be missed.
Figure 4.
Signaling events regulated by nitro-oxidative stress contribute to the pathogenesis of ROP: Avascular retina and Vasoproliferation. (VEGF, vascular endothelial growth factor)
There is overlap in molecular signaling between oxidative and inflammatory compounds,49 in that complex networks of signaling pathways link oxidative agents and pro-inflammatory cytokines. Release and activation of phospholipid metabolites from cell membranes can potentiate or exacerbate inflammation and trigger signaling of angiogenic or apoptotic pathways.50 One family of metabolites includes the phospholipase A2 (PLA-2) enzymes that catalyze the hydrolysis of fatty acids from membrane phospholipids. PLA-2 is activated by oxidative stresses and hypoxia51 and can lead to the release of arachidonic acid, platelet activating factor (PAF), and lysophospholipids. From arachidonic acid, COX1 and COX2 can oxidize and catalyze arachidonic acid into the proangiogenic eicosanoids, which include the prostaglandins, prostacyclin, and thromboxanes.52;53 These effectors and their downstream signaling have been associated with both phases in models of ROP by inducing activation of VEGF signaling in vascular endothelial cells (Figure 3). Inhibition of PLA-2 significantly reduced proangiogenic prostaglandins and intravitreal neovascularization in 50/10 OIR model.51
The HIFs are important in retinal vascular development, in part, by increasing transcription of important angiogenic factors, such as vascular endothelial growth factor (VEGF) during physiologic hypoxia,54; 55 and also by increasing apoptotic effects caused by RTP801.56 ROS can also regulate HIFs by increasing transcriptional activity even in normoxia (Figure 1),57 whereas HIF1α is usually stabilized and transcriptionally activated in hypoxia. The von Hippel Lindau protein regulates HIF1α stability via the formation of an ubiquitin ligase complex58 and prolyl hydroxylases are the enzymes that enable the interaction of HIF-1α and von Hippel Lindau protein. Using the mouse OIR model, inhibiting prolyl hydroxylase chemically during the hyperoxic phase reduced the breakdown of HIF1α and permitted intraretinal vascularization and angiogenesis.60 By promoting this physiologic retinal vascularization even in the presence of high oxygen, intravitreal endothelial budding during room air and relative hypoxia in phase 2 were also reduced.59; 74 RTP801 is a HIF-1 responsive gene that is strongly upregulated in ischemic cells of neuronal origin or in conditions associated with increased oxidative compounds such as cigarette smoke,60 leading to neuronal59;64 and alveolar septal cell apoptosis (Figure 2).56;60–62 RTP801 also can lead to inflammation and oxidative stress by promoting nuclear factor-κB activation in cultured cells and in mouse lung.60 Retinal RTP801 expression was increased during relative hypoxia at p17 in wild type mice exposed to the OIR model, whereas RTP801 deficient mice had reduced intravitreal neovascularization in association with reduced apoptotic cells in the inner nuclear layer of the retina.57
There is also evidence that some cytokines and growth factors can lead to increased generation of ROS. For example, VEGF, itself, can induce NADPH oxidase activation via activation of its subunit, Rac1, in endothelial cells63 to increase ROS generation.64
Summary
It is useful to understand the signaling pathways that can be activated by ROS and relate these to the pathologic features seen in the phases of ROP. The first phase of delayed PRVD and avascular retina occurs in part because of increased apoptosis supported by data from animal models implicating NADPH oxidase, eNOS, and HIF-1alpha-induced RTP801. In addition, signaling through JAK/STAT can cause downregulation of the angiogenic factor, erythropoietin, in Müller cells, which contributes to delayed PRVD in phase 1. In phase 2, intravitreal neovascularization can be affected by increased generation of ROS caused by supplemental oxygen and mediated through the activation of STAT3. ROS also trigger the activation of inflammatory pathways including those involving PLA-2, arachidonic acid and angiogenic growth factors. Hypoxia or ROS can stabilize HIF-1 that increases transcription of angiogenic factors, including VEGF.
Broad inhibition of ROS generation as a therapy for ROP may not have been effective or safe, because ROS can have beneficial effects as transcription factors in physiologic processes and are also a first line offense to invading microorganisms in the immune suppressed preterm infant. Future therapies may target pathologic downstream effectors activated by ROS.
Reference List
- 1.Katz ML, Robison WG., Jr Autoxidative damage to the retina: Potential role in retinopathy of prematurity. Birth Defects. 1988;24:237–248. [PubMed] [Google Scholar]
- 2.Penn JS. Oxygen-induced retinopathy in the rat: possible contribution of peroxidation reactions. Doc Ophthalmol. 1990;74:179–186. doi: 10.1007/BF02482607. [DOI] [PubMed] [Google Scholar]
- 3.Buonocore G, Perrone S, Bracci R. Free radicals and brain damage in the newborn. Biol Neonate. 2001;79(3–4):180–186. doi: 10.1159/000047088. [DOI] [PubMed] [Google Scholar]
- 4.Patz A. The role of oxygen in retrolental fibroplasia. Am J Ophthalmol. 1982;94:715–743. [Google Scholar]
- 5.Michaelson IC. The mode of development of the vascular system of the retina. With some observations on its significance for certain retinal diseases. Trans Ophthal Soc UK. 1948;68:137–180. [Google Scholar]
- 6.Ashton N, Blach R. Studies on developing retinal vessels: effect of oxygen on the retinal vessels of the ratling. Br J Ophthalmol. 1961;45:321–340. doi: 10.1136/bjo.45.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88(4):521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprara C, Grimm C. From oxygen to erythropoietin: Relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012;31:89–119. doi: 10.1016/j.preteyeres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Birch EE, Birch DG, Hoffman DR, Uauy R. Dietary essential fatty acid supply and visual acuity development. Investigative Ophthalmology Visual Science. 1992;33:3242–3253. [PubMed] [Google Scholar]
- 10.Johnson L, Bowen FW, Jr, Abbasi S, Herrmann N, Weston M, Sacks L, et al. Relationship of prolonged pharmacologic serum levels of vitamin E to incidence of sepsis and necrotizing enterocolitis in infants with birth weight 1,500 grams or less. Pediatrics. 1985;75(4):619–638. [PubMed] [Google Scholar]
- 11.Ishida S, Yamashiro K, Usui T, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9:781–789. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 12.Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis. 2007;13:840–853. [PMC free article] [PubMed] [Google Scholar]
- 13.Gu X, El Remessy AB, Brooks SE, Al Shabrawey M, Tsai NT, Caldwell RB. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. AJP - Cell Physiology. 2003;285:C546–C554. doi: 10.1152/ajpcell.00424.2002. [DOI] [PubMed] [Google Scholar]
- 14.Zeng G, Taylor SM, McColm JR, et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007;109:1345–1352. doi: 10.1182/blood-2006-07-037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–430. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsey VE. Cooperative study of retrolental fibroplasia and the use of oxygen. Arch Ophthalmol. 1956;56:481–543. [PubMed] [Google Scholar]
- 17.Kinsey VE, Arnold HJ, Kalina RE, et al. PaO2 levels and retrolental fibroplasia: A report of the cooperative study. Pediatrics. 1977;60:655–668. [PubMed] [Google Scholar]
- 18.Smith LEH, Wesolowski E, McLellan A, et al. Oxygen induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 19.Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci. 1995;36:2063–2070. [PubMed] [Google Scholar]
- 20.Kondo T, Vicent D, Suzuma K, et al. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest. 2003;111:1835. doi: 10.1172/JCI17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Develop. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 23.York JR, Landers S, Kirby RS, Arbogast PG, Penn JS. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24:82–87. doi: 10.1038/sj.jp.7211040. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham S, Fleck BW, Elton RA, Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 25.Ward JPT. Oxygen sensors in context. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2008;1777:1–14. doi: 10.1016/j.bbabio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Al Shabrawey M, Bartoli M, El Remessy AB, et al. Inhibition of NAD(P)H Oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Path. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks SE, Gu X, Samuel S, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:222–228. [PubMed] [Google Scholar]
- 28.Buhimschi IA, Buhimschi CS, Pupkin M, Weiner CP. Beneficial impact of term labor: Nonenzymatic antioxidant reserve in the human fetus. Am J Obstet Gynecol. 2003;189:181–188. doi: 10.1067/mob.2003.357. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H Oxidase from Supplemental Oxygen Induces Neovascularization Independent of VEGF in Retinopathy of Prematurity Model. Investigative Ophthalmology Visual Science. 2008;49:1591–1598. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Remessy AB, Al-Shabrawey M, Platt DH, et al. Peroxynitrite mediates VEGF-Æs angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J. 2007;21:2528–2539. doi: 10.1096/fj.06-7854com. [DOI] [PubMed] [Google Scholar]
- 31.Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. Early Intervention of Tyrosine Nitration Prevents Vaso-Obliteration and Neovascularization in Ischemic Retinopathy. J Pharmacol Exp Ther. 2010;332:125–134. doi: 10.1124/jpet.109.157941. [DOI] [PubMed] [Google Scholar]
- 32.Niesman MR, Johnson KA, Penn JS. Therapeutic effect of liposomal superoxide dismutase in an animal model of retinopathy of prematurity. Neurochemical Research. 1997;22:597–605. doi: 10.1023/a:1022474120512. [DOI] [PubMed] [Google Scholar]
- 33.Budd SJ, Hartnett ME. Increased Angiogenic Factors Associated With Peripheral Avascular Retina and Intravitreous Neovascularization: A Model of Retinopathy of Prematurity. Arch Ophthalmol. 2010;128:589–595. doi: 10.1001/archophthalmol.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H, Byfield G, Jiang Y, Smith GW, McCloskey M, Hartnett ME. VEGF-mediated STAT3 activation inhibits retinal vascularization by downregulating erythropoietin expression. Am J Path. 2012;180(3):1243–1253. doi: 10.1016/j.ajpath.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tawfik A, Sanders T, Kahook K, Akeel S, Elmarakby A, Al-Shabrawey M. Suppression of Retinal Peroxisome Proliferator-Activated Receptor γ in Experimental Diabetes and Oxygen-Induced Retinopathy: Role of NADPH Oxidase. Invest Ophthalmol Vis Sci. 2009;50:878–884. doi: 10.1167/iovs.08-2005. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Wang JJ, Yu Q, Chen K, Mahadev K, Zhang SX. Inhibition of Reactive Oxygen Species by Lovastatin Downregulates Vascular Endothelial Growth Factor Expression and Ameliorates Blood-Retinal Barrier Breakdown in db/db Mice. Diabetes. 2010;59:1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and Localization of NOX2 and NOX4 in Primary Human Endothelial Cells. Antioxidants & Redox Signaling. 2005:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 38.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cellular Signalling. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important Role of Nox4 Type NADPH Oxidase in Angiogenic Responses in Human Microvascular Endothelial Cells In Vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 41.Craige SM, Chen K, Pei Y, et al. NADPH Oxidase 4 Promotes Endothelial Angiogenesis Through Endothelial Nitric Oxide Synthase Activation / Clinical Perspective. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2009;296:C422–C432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schröder K, Zhang M, Benkhoff S, et al. Nox4 Is a Protective Reactive Oxygen Species Generating Vascular NADPH Oxidase / Novelty and Significance. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 44.Beauchamp MH, Sennlaub F, Speranza G, et al. Redox-dependent effects of nitric oxide on microvascular integrity in oxygen-induced retinopathy. Free Radic Biol Med. 2004;37:1885–1894. doi: 10.1016/j.freeradbiomed.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Geisen P, Peterson L, Martiniuk D, Uppal A, Saito Y, Hartnett M. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in an ROP model. Molecular Vision. 2008;14:345–357. [PMC free article] [PubMed] [Google Scholar]
- 46.Hartnett ME, Martiniuk DJ, Byfield GE, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in rat model of ROP: Relevance to plus disease. Invest Ophthalmol Vis Sci. 2008 Mar 31;:7–3107. doi: 10.1167/iovs.08-1780. epub[49] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kermorvant-Duchemin E, Sennlaub F, Sirinyan M, et al. Trans-arachidonic acids generated during nitrative stress induce a thrombospondin-1-dependent microvascular degeneration. Nat Med. 2005;11:1339–1345. doi: 10.1038/nm1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joyal JSb, Omri S, Sitaras N, Rivera JC, Sapieha P, Chemtob S. Neovascularization in retinopathy of prematurity: opposing actions of neuronal factors GPR91 and semaphorins 3A. Acta Paediatr. 2012;101(8):819–826. doi: 10.1111/j.1651-2227.2012.02692.x. [DOI] [PubMed] [Google Scholar]
- 49.Saugstad OD. Oxidative stress in the newborn--a 30-year perspective. Biol Neonate. 2005;88(3):228–236. doi: 10.1159/000087586. [DOI] [PubMed] [Google Scholar]
- 50.Kermorvant-Duchemin E, Sapieha P, Sirinyan M, et al. Understanding ischemic retinopathies: emerging concepts from oxygen-induced retinopathy. Doc Ophthalmol. 2010;120:51–60. doi: 10.1007/s10633-009-9201-x. [DOI] [PubMed] [Google Scholar]
- 51.Barnett JM, McCollum GW, Penn JS. Role of Cytosolic Phospholipase A2 in Retinal Neovascularization. Investigative Ophthalmology Visual Science. 2010;51:1136–1142. doi: 10.1167/iovs.09-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardy P, Beauchamp M, Sennlaub F, et al. New insights into the retinal circulation: Inflammatory lipid mediators in ischemic retinopathy. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;72:301–325. doi: 10.1016/j.plefa.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Lambert IH, Pedersen SF, Poulsen KA. Activation of PLA2 isoforms by cell swelling and ischaemia/hypoxia. Acta Physiol (Oxf) 2006;187(1–2):75–85. doi: 10.1111/j.1748-1716.2006.01557.x. [DOI] [PubMed] [Google Scholar]
- 54.Weidemann A, Krohne TU, Aguilar E, et al. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan-Ling T, Stone J. Degeneration of astrocytes in feline retinopathy of prematurity causes failure of the blood-retinal barrier. Invest Ophthalmol Vis Sci. 1992;33:2148–2159. [PubMed] [Google Scholar]
- 56.Brafman A, Mett I, Shafir M, et al. Inhibition of Oxygen-Induced Retinopathy in RTP801-Deficient Mice. Investigative Ophthalmology Visual Science. 2004;45:3796–3805. doi: 10.1167/iovs.04-0052. [DOI] [PubMed] [Google Scholar]
- 57.Cash TP, Pan Y, Simon MC. Reactive oxygen species and cellular oxygen sensing. Free Radic Biol Med. 2007;43:1219–1225. doi: 10.1016/j.freeradbiomed.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mettu P, Agron E, Samtani S, Chew EY, Wong WT. Genotype-Phenotype Correlation in Ocular von Hippel-Lindau (VHL) Disease: The Effect of Missense Mutation Position on Ocular VHL Phenotype. Invest Ophthalmol Vis Sci. 2010;51(9):4464–4470. doi: 10.1167/iovs.10-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sears JE, Hoppe G, Ebrahem Q, Anand-Apte B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy. PNAS. 2008;105:19898–19903. doi: 10.1073/pnas.0805817105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida T, Mett I, Bhunia AK, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med. 2010;16:767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoshani T, Faerman A, Mett I, et al. Identification of a Novel Hypoxia-Inducible Factor 1-Responsive Gene, RTP801, Involved in Apoptosis. Mol Cell Biol. 2002;22:2283–2293. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shoshani T, Faerman A, Mett I, et al. Identification of a Novel Hypoxia-Inducible Factor 1-Responsive Gene, RTP801, Involved in Apoptosis. Mol Cell Biol. 2002;22:2283–2293. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res. 2007;313:3285–3297. doi: 10.1016/j.yexcr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling. Role of NAD(P)H oxidase. Molecular and Cellular Biochemistry. 2004;V264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]