Abstract
Purpose
The objective of this study was to investigate the roles of the constitutive androstane receptor (CAR) in cyclophosphamide (CPA)- and ifosfamide (IFO)-mediated induction of hepatic drug-metabolizing enzymes (DME).
Methods
Induction of DMEs was evaluated using real-time RT-PCR and Western blotting analysis in human primary hepatocyte (HPH) cultures. Activation of CAR, pregnane X receptor (PXR), and aryl hydrocarbon receptor by CPA and IFO was assessed in cell-based reporter assays in HepG2 cells and/or nuclear translocation assays in HPHs.
Results
CYP2B6 reporter activity was significantly enhanced by CPA and IFO in HepG2 cells co-transfected with CYP2B6 reporter plasmid and a chemical-responsive human CAR variant (CAR1+A) construct. Real-time RT-PCR and Western blotting analysis in HPHs showed that both CPA and IFO induced the expressions of CYP2B6 and CYP3A4. Notably, treatment of HPHs with CPA but not IFO resulted in significant nuclear accumulation of CAR, which represents the initial step of CAR activation. Further studies in HPHs demonstrated that selective inhibition of PXR by sulforaphane preferentially repressed IFO- over CPA-mediated induction of CYP2B6.
Conclusion
These results provide novel insights into the differential roles of CAR in the regulation of CPA- and IFO-induced DME expression and potential drug-drug interactions.
Keywords: Cyclophosphamide, Ifosfamide, CYP2B6, CAR, Induction
INTRODUCTION
Cyclophosphamide (CPA), along with its close derivative ifosfamide (IFO), represent the most widely used alkylating agents over the last 40 years in the treatment of hematological malignancies and solid tumors (1, 2). CPA has also been used at higher doses in the treatment of aplastic anemia and leukemia prior to bone marrow transplantation and as a therapeutic immmnunosuppressor for several autoimmune disorders (3, 4). IFO was reported to exhibit higher efficacy against a number of malignant diseases in comparison with the CPA treatment (5). In clinical application, these oxazaphosphorines are frequently used in combination with other drugs and supportive therapies, aiming to produce synergistic or additive therapeutic benefits. Conversely, such combined regimens may also increase the potential for unintentional drug-drug interactions.
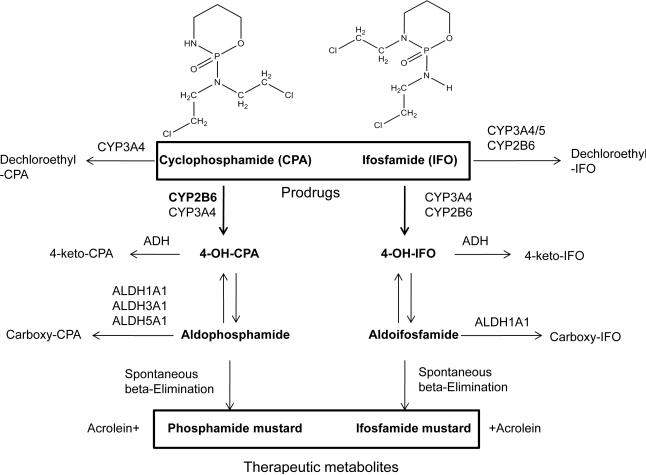
CPA and IFO are alkylating prodrugs requiring hepatic biotransformation mediated by cytochrome P450 (CYP) pathways through which 4-hydroxylation of both oxazaphosphorines leads to the formation of active alkylating moieties, while N-dechloroethylation yields neurotoxic metabolites (6, 7). To date, accumulating evidence suggests that many drug-metabolizing enzymes (DME) are involved in the metabolism of CPA and IFO in the liver (8, 9). Among these DMEs, CYP2B6 has been demonstrated to play a major role in the bioactivation of CPA and accounts for approximately 45% of 4-hydroxycyclophosphamide (4OH-CPA) generation, which ultimately produces the DNA-alkylating phosphoramide mustard leading to therapeutic cytotoxicity (Fig. 1) (10). On the other hand, CYP3A4 only moderately contributes to 4-hydroxylation of CPA but exclusively mediates N-dechloroethylation of CPA yielding neurotoxic byproduct (6, 10). In the case of IFO, CYP2B6 and CYP3A4 are believed to be the predominant isozymes responsible for both its 4-hydroxyal activation and N-dechloroethylated inactivation (11).
Figure 1.
Schematic pathways of cyclophosphamide (CPA) and ifosfamide (IFO) metabolism.
Given that hepatic CYP2B6 and CYP3A4 are highly inducible by a broad array of clinically used drugs and both CPA and IFO are often used in combination with other therapeutic agents, a dramatic interpatient variability has been observed in the pharmacokinetics of CPA and IFO in clinical investigations (12-14). Interestingly, an auto-inductive effect that increases their own metabolism and clearance has also been reported for CPA and IFO in human primary hepatocytes (HPH) and in rat liver in vivo, by which the mRNA and protein levels of relevant CYP2B and CYP3A were up-regulated (14-17). However, the molecular mechanisms underlying the autoinduction were not fully elucidated. Initial investigations suggested that activation of xenobiotic receptor pregnane X receptor (PXR) plays a critical role in the inductive effects of these oxazaphosphorines on CYP2B6 and CYP3A4 (18, 19). However, previous study demonstrated that CPA induces the expression of CYP2B6 to a significantly greater extent than that of CYP3A4 in HPHs (19). Since PXR was regarded as the primary regulator of CYP3A4 induction, the preferential induction of CYP2B6 by CPA may not be explained entirely by the PXR-mediated pathway. Notably, the sister xenobiotic sensor of PXR, constitutive androstane receptor (CAR), has been established as a compensatory transcription factor of PXR and shares a number of overlapping and distinct target genes as well as many chemical activators (20, 21). Given the close association of PXR/CAR and CYP3A4/CYP2B6 that dictates the biotransformation of CPA and IFO, it is important to explore the role of CAR in the inductive activity of these oxazaphosphorines.
In the current study, we investigated the hypothesis that human (h) CAR plays a role in CPA and IFO induced expression of several DMEs and contributes significantly to the CPA-mediated induction of CYP2B6. Cell-based transfection reporter assays in HepG2 cells were employed to assess the role of CPA and IFO in the activation of CAR, PXR and aryl hydrocarbon receptor (AhR). The expression of CYP2B6, CYP3A4, CYP1A2 and UGT1A1 were evaluated in HPHs. Additionally, activation-associated hCAR nuclear translocation was characterized in HPHs infected with adenovirus expressing enhanced yellow fluorescent protein-tagged hCAR (Ad/EYFP-hCAR). Our results indicate that both CPA and IFO significantly induced their major metabolizing enzymes CYP2B6 and CYP3A4. Importantly, although PXR may contribute equally to the metabolic inducibility of CPA and IFO, CAR appears to play an important and preferential role in CPA- over IFO-mediated CYP induction.
MATERIALS AND METHODS
Chemicals and biological reagents
1-(2-chlorophenyl-Nmethylpropyl)-3-isoquinoline-carboxamide (PK11195), sulforaphane (SFN), CPA, IFO, phenobarbital (PB), rifampicin (RIF), and 3-methylcholanthrene (3MC) were purchased from Sigma-Aldrich (St. Louis, MO). 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl) oxime (CITCO) was from BIOMOL Research Laboratories (Plymouth Meeting, PA). Oligonucleotide primers were synthesized by Integrated DNA technologies (Coralville, IA). The Dual-Luciferase Reporter Assay System was purchased from Promega (Madison, WI). FuGENE® 6 transfection reagent was obtained from Roche (Basel, Switzerland). Matrigel, insulin and ITS+ culture supplements were from BD Biosciences (Bedford, MA). Other cell culture and molecular reagents were purchased from Invitrogen (Calsbad, CA) or Sigma-Aldrich.
Plasmid constructs
The pCR3-hCAR expression vector and the UGT1A1-gtPBREM containing a 290 base pairs (bp) sequence from UGT1A1 promoter (-3483-3194) were kindly provided by Dr. Masahiko Negishi (National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC). The pSG5-hPXR expression vector was obtained from Dr. Steve Kliewer (University of Texas Southwestern Medical Center, Dallas, TX). The pCR3-hCAR1+A expression vector, and the CYP2B6 reporter construct containing both the PBREM and the distal XREM (CYP2B6–2.2kb), were generated as described previously (22, 23). The CYP3A4-PXRE/XREM reporter vector was obtained from Dr. Bryan Goodwin (GlaxoSmithKline, Research Triangle Park, NC). The pRL-TK Renilla luciferase plasmid used to normalize firefly luciferase activities was from Promega.
Culture and treatment of human primary hepatocytes
Human liver tissues were obtained following surgical resection by qualified pathology staff after diagnostic criteria were met and prior approval from the Institutional Review Board at the University of Maryland at Baltimore. Hepatocytes were isolated from human liver specimens by a modification of the two-step collagenase digestion method as described previously (24), or obtained from Life Technologies Corporation (Durham, NC). Cultures of hepatocytes were maintained in 6-well or 12-well BioCoat plates as described previously (25). Thirty-six hours (hr) after seeding, hepatocytes were treated with vehicle control (0.1% DMSO), RIF (10 μM), CITCO (1 μM), 3MC (5 μM), or CPA and IFO (500 and 1000 μM) for another 24 or 72 hr for the detection of mRNA and protein, respectively. Cell culture medium was replaced on a daily base.
Real-time PCR analysis
Total RNA was isolated from treated hepatocytes using the RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed using High Capacity cDNA Archive kit (Applied Biosystems, Foster, CA) following the manufacturers’ instructions. CYP2B6 and CYP3A4 mRNA expression was normalized against that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). RT-PCR assays were performed in 96-well optical plates on an ABI Prism 7000 Sequence Detection System with SYBR Green PCR Master Mix (Applied Biosystems). Primers for CYP2B6, CYP3A4, UGT1A1, CYP1A2, and GAPDH mRNA detection are as follows: CYP2B6: 5’-AGACGCCTTCAATCCTGACC-3’ (forward), 5’-CCTTCACCAAGACAAATCCGC-3’ (reverse); CYP3A4: 5’-GTGGGGCTTTTATGATGGTCA-3’ (forward), 5’-GCCTCAGATTTCTCACCAACACA-3’ (reverse); UGT1A1: 5’-CCTTCACCAAAATCCACTATCCC-3’ (forward), 5’-CGTGTTGTTCGCAAGATTCGAT-3’(reverse); CYP1A2: 5’-TCGACCCTTACAATCAGGTGG-3’ (forward), 5’-GCAGGTAGCGAAGGATGGG-3’(reverse); and GAPDH: 5’-CCCATCACCATCTTCCAGGAG-3’ (forward), 5’- GTTGTCATGGATGACCTTGGC-3’ (reverse). Fold induction values were calculated according to the equation: fold = 2ΔΔCt, where ΔCt represents the differences in cycle threshold numbers between the target gene and GAPDH, and ΔΔCt represents the relative change in these differences between control and treatment groups.
Western Blot Analyses
Homogenate proteins (20 μg each) from treated HPHs were resolved on SDS -polyacrylamide gels, and electrophoretically transferred onto Immobilon-P polyvinylidene difluoride membranes. Subsequently, membranes were incubated with specific antibodies against CYP2B6 or CYP3A4 (Millipore-Chemicon, Temecula, CA) diluted 1:4000 and 1:5000, respectively. β-actin (Sigma-Aldrich) was used for normalization of protein loading. After incubating with horseradish peroxidase goat anti-rabbit IgG antibody diluted 1:4000, membranes were developed using ECL Western blotting detection reagent (GE Healthcare, Piscataway, NJ). The relative quantity of CYP2B6 or CYP3A4 protein was estimated by densitometry analysis using Adobe Photoshop (CD3 Extended, V.10.0.1)
Cellular distribution of CAR in HPHs
The Ad/EYFP-hCAR was generated as described previously (25). Human hepatocyte cultures in 24-well BioCoat plates were infected with 5 μl of Ad/EYFP-hCAR for 12 h before treatment with the vehicle control (0.1% DMSO), PB (1 mM), CPA (500 μM) or IFO (500 μM) for another 24 hr. After fixed for 30 min in 4% buffered paraformaldehyde, cells were stained with 4,6-diamidine-2-phenylindole dihydrochloride (DAPI) for 30 min. Stained hepatocytes were subjected to microscopic detection as described previously (25). The subcellular localization of hCAR was visualized and quantitatively characterized as nuclear, cytosolic, or mixed (nuclear + cytosolic) expressions by counting 100 Ad/EYFP-hCAR-expressing hepatocytes per treatment group.
Luciferase reporter assays in HepG2 cells
HepG2 cells in 24-well plates were transfected with hPXR, hCAR1 or hCAR1+A expression vector, in the presence of CYP2B6-2.2kb or CYP3A4-PXRE/XREM reporter construct; or transfected with UGT1A1-gtPBREM reporter in the absence of exogenous nuclear receptors using Fugene 6 Transfection Kit following the manufacturer's instruction. Twenty-four hr after transfection, cells were treated for another 24 hr with vehicle control (0.1% DMSO), RIF (10 μm), CITCO (1 μM), PB (1 mM), PK11195 (10 μM), 3MC (5 μM), or CPA and IFO (500 and 1000 μM); or cotreated with PK11195 plus CPA or IFO at concentrations as indicated in figures. Cell lysates were assayed for firefly activities normalized against the activities of cotransfected Renilla luciferase using Dual-Luciferase Kit (Promega). Data were represented as mean ± S.D. of three individual transfections.
Statistical analysis
All data represent at least three independent experiments and are expressed as the mean ± S.D. Statistical comparisons were made using one-way analysis of variance followed by a post hoc Dunnett's test and Student's t test where appropriate. The statistical significance was set at p values < 0.05 (*), or < 0.01 (**).
RESULTS
Effects of CPA and IFO on the expression of DMEs in HPHs
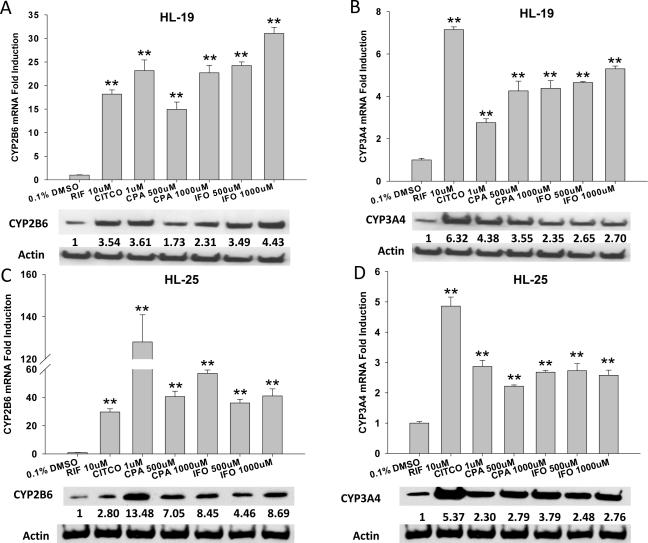
HPHs are well accepted as an appropriate in vitro model mimicking human liver for drug metabolism and pre-clinical induction studies (26). In the current investigation, we evaluated the effects of CPA and IFO on the expression of several DMEs in HPH cultures using RT-PCR and Western Blotting assays. As demonstrated in Figure 2, the mRNA and protein expressions of CYP2B6 and CYP3A4 were significantly induced by CPA and IFO at concentrations bracketing their pharmacological relevant levels (19, 27); and there was no identifiable cytotoxicity observed throughout the experiments. Notably, CYP2B6 expression was induced to a greater extent by both CPA and IFO treatment than that of CYP3A4, in particular, at the mRNA level. In HPH preparation from donor HL-19, the maximal induction of CYP2B6 mRNA by CPA and IFO challenges the induction by positive controls (RIF and CITCO). In parallel experiments, we further investigated the role of CPA and IFO in the induction of UGT1A1 and CYP1A2, which are also involved in the metabolism of a broad spectrum of drugs and environmental compounds. Results showed that CPA and IFO treatment of HPHs resulted in a clear induction of UGT1A1 mRNA expression, while exhibiting no effects on the regulation of CYP1A2 expression (Fig. 3A, 3B). These results indicate that CPA and IFO may enhance the expression of CYP2B6, CYP3A4, and UGT1A1 through shared transcriptional regulatory machineries that are distinct from the induction of CYP1A2.
Figure 2.
Induction of CYP2B6 and CYP3A4 by CPA and IFO in human primary hepatocytes. Human hepatocytes from liver donors (HL-19 and HL-25) were treated with CITCO (1μM), RIF (10μM) or CPA and IFO at indicated concentrations for 24 hr (mRNA) and 72 hr (protein), respectively. The expression of mRNA for CYP2B6 (A, C) and CYP3A4 (B, D) was analyzed using RT-PCR; and related protein expression was detected using immunoblotting analysis as outlined in “Materials and Methods”. RT-PCR data are expressed as mean ± SD (n=3). **: p < 0.01. Densitometry of CYP2B6 and CYP3A4 proteins was normalized against β-actin expression.
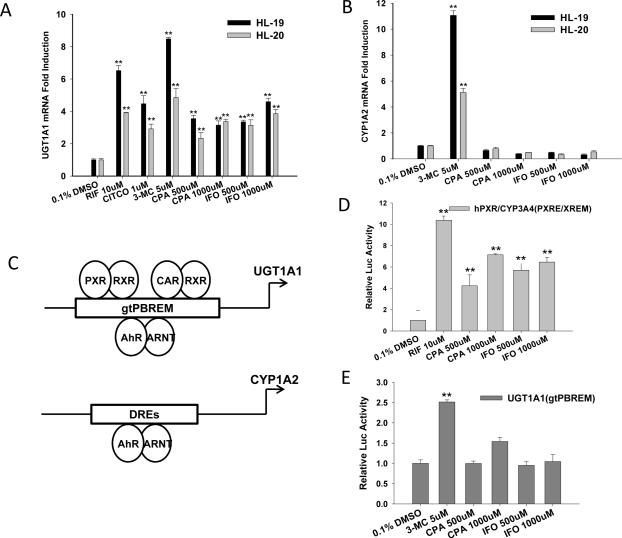
Figure 3.
CPA and IFO induce the expression of UGT1A1 but not CYP1A2. Human primary hepatocytes from liver donors (HL-19, and HL-20) were treated with CITCO (1 μM) , RIF (10 μM) , 3MC (5 μM) or CPA and IFO at indicated concentrations for 24 hr. The mRNA expression of UGT1A1 (A) and CYP1A2 (B) was analyzed using real-time RT-PCR. In parallel experiments, CPA- and IFO-mediated activation of PXR (D) and AhR (E) was assessed in HepG2 cell-based reporter assays as described in “Materials and Methods”. All data are presented as mean ± SD (n = 3), **: p < 0.01. Panel (C) represents a schematic diagram depicting the CAR, PXR, and AhR response elements in the UGT1A1 and CYP1A2 promoters.
CPA and IFO activate human PXR but not AhR
It has been well established that drug induced expression of UGT1A1 is primarily regulated at the transcriptional level by three xenobiotic receptors PXR, CAR and AhR, while CYP1A2 is predominantly transactivated by AhR (Fig 3C) (28-30). To illustrate mechanisms pertaining to the differential induction of UGT1A1 and CYP1A2 by CPA and IFO, we tested the role of these oxazaphosphorines in the activation of PXR and AhR in HepG2 cell-based reporter assays. In agreement with previous reports, CPA and IFO significantly enhanced PXR-mediated transactivation of CYP3A4 luciferase activity (Fig. 3D) (18, 19). In determining the contribution of AhR in CPA- and IFO-mediated UGT1A1 induction, HepG2 cells, which express endogenous AhR abundantly (31), were transfected with UGT1A1-gtPBREM reporter construct alone. As expected 3MC, a prototypical AhR activator, remarkably transactivated UGT1A1 luciferase activity, while neither CPA nor IFO activated AhR (Fig. 3E), indicating the CPA- and IFO-mediated induction of UGT1A1 is AhR-independent.
Differential activation of human CAR by CPA and IFO
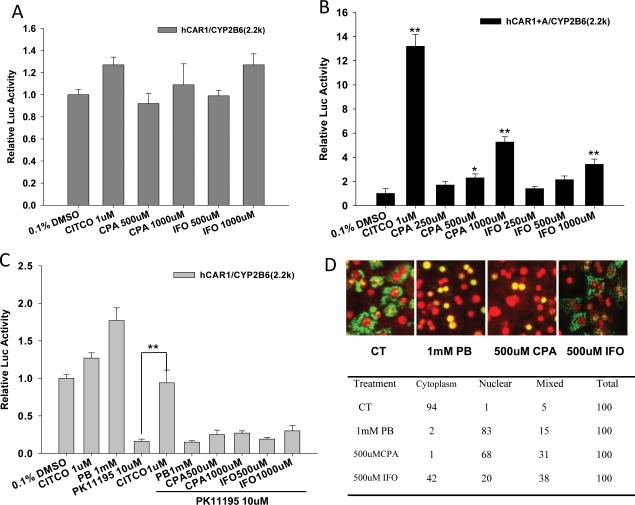
Since CYP2B6 and CYP3A4 are typical target genes for both CAR and PXR, and CPA- and IFO-mediated induction of UGT1A1 is AhR-independent, we speculated that PXR and CAR play pivotal roles in the overall metabolic elevation by these oxazaphosphorines. In particular, the role of CAR in the metabolic inducibility of CPA and IFO remains unexplored, due partly to its high basal activity and lack of response to chemical stimulation in immortalized cell lines (Fig. 4A) (20). Utilizing a recently established chemical-responsive hCAR variant with an alanine insertion at 271 (hCAR1+A) (23), our reporter assays revealed that CPA and IFO can enhance CYP2B6 luciferase activity through activation of CAR in a concentration-related manner, and CPA appears to be a stronger CAR activator than IFO (Fig. 4B). Additional luciferase reporter experiments demonstrated that PK11195, a potent hCAR antagonist, deactivated CAR activity (80% of the constitutive control) could be efficiently reactivated by the direct hCAR activator CITCO but not by the indirect activator PB (Fig. 4C). CPA and IFO failed to reactivate PK11195 repressed hCAR activity in this assay, suggesting these oxazaphosphorines may activate hCAR through PB-type indirect mechanism.
Figure 4.
Differential activation of hCAR by CPA and IFO. HepG2 cells were transfected with hCAR1 (A, C) or hCAR1+A (B) expression vector in the presence of CYP2B6-2.2kb reporter construct and the pRL-TK control vector. Transfected cells were treated for 24 hr with CITCO (1 μM), PB (1 mM), PK11195 (10 μM) or CPA and IFO at indicated concentrations alone; or cotreated with PK11195 as depicted in 4C. Luciferase activities were determined and expressed as fold relative to vehicle control. All data are expressed as mean ± SD (n=3). **: p < 0.01. In separate experiment, human primary hepatocytes (HL-20) were infected with Ad/EYFP-hCAR and treated with negative control (0.1% DMSO), positive control (PB, 1 mM), or CPA and IFO (500 μM) for 24 hr. After fixation and DAPI staining, hepatocytes were subjected to confocal microscopy analysis as described in “Materials and Methods”. Representative Ad/EYFP-hCAR localization was demonstrated in (D). The table shows the percentage of hepatocytes exhibiting the different types of hCAR localization from each treatment group.
Different from the constitutive activation in immortalized cell lines, CAR is primarily localized in the cytoplasm of HPHs prior to activation; and nuclear accumulation occurs only after treatment with CAR modulators (32). To further examine the capability of CPA and IFO in hCAR activation, a hCAR nuclear translocation experiment was carried out in cultured HPHs infected with the Ad/EYFP-hCAR, which has demonstrated exceptional efficiency in infecting HPH (25). As expected, in the virus infected HPHs, the EYFP-hCAR was primarily expressed in the cytoplasm (94%) without activation (vehicle control) and accumulated in nucleus (83%) upon PB treatment (Fig. 4D). Intriguingly, treatment with CPA at 500 μM resulted in remarkable EYFP-hCAR nuclear translocation (63%), while IFO at the same concentration only led a moderate nuclear accumulation of CAR (20%) (Fig. 4D). In agreement with aforementioned reporter assays, these results suggest that CPA is a stronger hCAR activator in comparison to IFO, and IFO may exert its inductive activity primarily through the activation of PXR.
Effects of selective inhibition of PXR on the induction of CYP2B6 by CPA and IFO
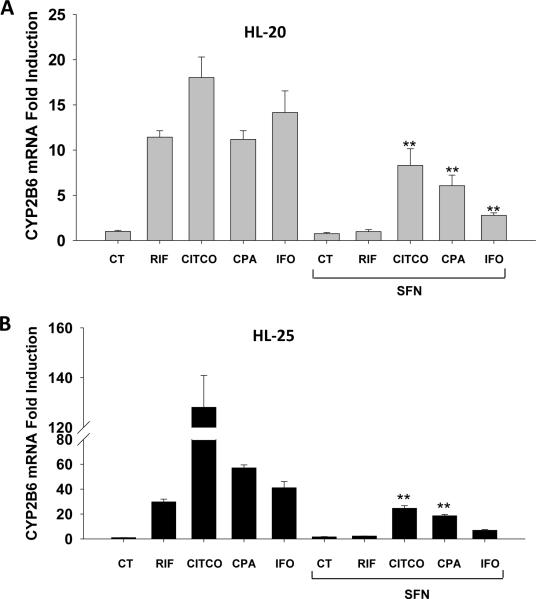
Through mechanistic cross-talking, PXR and CAR share a large number of target genes as well as many xenobiotic activators (20). To further explore the contribution of CAR in CPA and IFO induction of DME, a cotreatment experiment was carried out in HPHs using sulforaphane (SFN) as a selective inhibitor of hPXR (33). As expected, concomitant treatment of SFN and the selective hPXR activator RIF completely abolished RIF-mediated induction of CYP2B6, while CITCO-mediated CYP2B6 induction was only moderately repressed by SFN (Fig. 5) (33, 34). Notably, although CPA-mediated induction of CYP2B6 in HPHs was suppressed moderately by the cotreatment of SFN, the induction by IFO was dramatically decreased (Fig. 5). Taken together, these findings corroborate the differential contribution of CAR in CPA- and IFO-mediated induction of CYP2B6.
Figure 5.
Effects of PXR inhibition on the induction of CYP2B6 by CPA and IFO. Human primary hepatocytes from donors (HL-20 and HL-25) were treated for 24 hr with CITCO (1 μM), RIF (10 μM), SFN (25 μM), CPA (1 mM), and IFO (1 mM), and cotreatment of SFN (25 μM) with CITCO, RIF, CPA, and IFO, respectively. The expression of CYP2B6 mRNA was assessed in real-time RT-PCR assays (Materials and Methods). All data are expressed as mean ± SD (n=3). **: p < 0.01.
Discussion
Although CPA and IFO have long been used as anti-cancer alkylating agents in clinical practice with their pharmacology, metabolism and pharmacokinetic/pharmacodynamic profiles well elucidated, there have been rising concerns regarding drug-drug interactions, and autoinduction along with the increase of combinatory therapeutic strategies involving these drugs (35). Accumulating evidence thus far, revealed that hepatic metabolism of CPA and IFO is auto-inducible and repeated administration of CPA and IFO was associated with elevated 4-hydroxylation of the oxazaphosphorines and expression of several CYP enzymes (15, 27). However, the molecular mechanisms behind the induction are not fully understood. In this report, we demonstrated that in addition to the promiscuous xenobiotic receptor PXR, CAR plays an important role in the enzymatic autoinduction of these oxazaphosphorines. More importantly, although PXR appears to be equally involved in the inductive activity of CPA and IFO, CAR functions as a preferential mediator of CPA- rather than IFO-mediated induction of hepatic DMEs. This evidence suggests that co-administration of drugs that selectively disturbing the expression and function of CAR may differentially affect the autoinduction and drug-drug interactions of CPA rather than IFO.
In order to accommodate their own metabolism and clearance, xenobiotics including drugs and environmental chemicals can alter the expression of DMEs and transporters through the transactivation of a group of xenobiotic receptors. Previously, PXR was reported as the mediator of CPA and IFO autoinduction by transcriptional up-regulation of CYP2B6 and CYP3A4 (18, 19). Both CPA and IFO increased PXR activity in luciferase reporter assays at the concentrations that significantly induced CYP2B6 and CYP3A4 expression in HPH cultures (18, 19). Interestingly, we showed that CPA and IFO treatment resulted in greater increase in CYP2B6 mRNA than that of CYP3A4, which is in agreement with data from a previous report (19). Given that CYP2B6 is a favorable target gene of CAR over PXR and potent induction of CYP2B6 by CPA and IFO was observed, activation of PXR alone by these oxazaphosphorines may not be able to sufficiently accommodate the extent of DME induction in hepatocytes. Accordingly, it is conceivable that CAR, the closest relative of PXR, may mediate a compensatory role in CPA and IFO associated induction. However, unlike that of PXR, investigation of CAR activation in vitro has been hindered by several specific features of this receptor including that: 1) CAR is constitutively activated and spontaneously nuclear localized in immortalized cell lines; and 2) it can be activated by both direct ligand binding and indirect ligand-independent mechanisms (32, 36). Recently, we have generated a chimerical construct namely hCAR1+A with an alanine insertion at 271, which converts the constitutive wide-type hCAR into a chemically responsive xenobiotic sensor (23). In cell-based reporter assays utilizing this chimerical vector, our results revealed that CPA and IFO dose-dependently enhanced CYP2B6 luciferase expression through the activation of hCAR1+A. Given that over 90% of known hCAR activators positively response to this chimerical constructs (23), this initial experiment supports the involvement of CAR in the inductive activity of CPA and IFO.
Alternatively, CAR exhibits significant nuclear translocation from cytoplasm of primary cultured hepatocytes in the presence of CAR activators such as PB and CITCO (25). This initial step of CAR activation in primary hepatocytes has been successfully established as an efficient approach to identify hCAR activators in vitro (25). Remarkably, both direct activators (e. g. CITCO, and artemicinin) and indirect activators (e. g. PB, and phenytoin) are effective in the nuclear accumulation of CAR in hepatocyte cultures (25, 32, 36). Intriguingly, our results showed that CPA but not IFO displayed marked effects on nuclear accumulation of CAR in HPHs. Although activation of CAR is a multistep process, the lack of initial nuclear translocation of CAR by IFO suggests that IFO is less likely to be an effective activator of hCAR.
In contrast to other nuclear receptors, activation of CAR doesn't require ligand binding (indirect activation), and as a matter of fact the majority of known hCAR activators identified thus far activate CAR through PB-like indirect machinery rather than CITCO-like ligand binding (25, 32). Recent reports from this laboratory demonstrated that in HepG2 cells, the PK11195, a prototypical peripheral benzodiazepine receptor and potent hCAR deactivator, repressed hCAR activity can be efficiently reactivated by direct activator CITCO but not the prototypical indirect activator PB (34). Akin to PB, CPA was unable to reactive PK11195 inhibited CAR activity, suggesting CPA may function as a PB-type indirect CAR activator.
To date, mounting evidence suggests that CAR and PXR share their target genes through recognizing of, and binding to, xenobiotic responsive elements located in the upstream of these genes (37, 38). An asymmetrical cross-regulation of CYP2B6 and CYP3A4 by hCAR but not hPXR has been established in that hCAR exhibits preferential induction of CYP2B6 over CYP3A4, while hPXR exerts less-selective induction of both genes (39). In an effort to determine the relative involvement of CAR in CPA- and IFO-mediated CYP2B6 induction, experiments in HPHs revealed that SFN, a selective inhibitor of hPXR, dramatically repressed IFO induced expression of CYP2B6 mRNA, while to a lesser extent in response to the CPA-mediated induction. In concert with aforementioned luciferase reporter and nuclear translocation data, these results strongly suggest that CPA is a stronger activator of hCAR compared to IFO.
Conclusion
In summary, our results from the current study indicate that although PXR indiscriminately mediates the inductive activity of both CPA and IFO, CAR exhibits marked differential roles in CPA and IFO associated induction of DMEs. CPA can activate both PXR and CAR, IFO appears primarily to transactivate PXR. Furthermore, CPA functions most likely through PB-type indirect mechanisms, by which it predominantly influences the nuclear translocation of CAR with no obvious effects on the nuclear localized CAR activity. It is noteworthy that due to the insufficient understanding of the mechanism(s) underlying indirect activation of CAR, particularly the lack of knowledge regarding which molecule these activators initially interact with, it is difficult to explain the differential roles on CAR activation between the structurally similar CPA and IFO. Moreover, extra caution is required in the interpretation of these in vitro discoveries due to the range of drug concentrations where a response was observed. Nevertheless, these findings may be potentially of clinical importance where drug-mediated manipulation of hCAR activity could selectively alter the autoinduction and pharmacokinetic profile of oxazaphosphorines.
Acknowledgement
The authors thank Dr. Masahiko Negishi (National Institute of Environmental and Health Sciences, National Institutes of Health, Research Triangle Park, NC) for providing hCAR expression and UGT1A1 reporter constructs; Dr. Steve Kliewer (University of Texas, Southwestern Medical Center, Dallas, TX) for the pSG5-hPXR expression vector; and Dr. Bryan Goodwin (GlaxoSmithKline, Research Triangle Park, NC) for the CYP3A4 reporter plasmid. This research was supported in part by the National Institute of Health Grant (R01, DK061652) and the Seed Grant from University of Maryland Greenebaum Cancer Center.
Abbreviations
- AhR
aryl hydrocarbon receptor
- CAR
constitutive androstane receptor
- Ad/EYFP-hCAR
adenovirus expressing enhanced yellow fluorescent protein-tagged human CAR
- CITCO
6-(4-chlorophenyl) imidazo[2,1-b][1,3]- thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl) oxime
- CPA
cyclophosphamide
- CYP
cytochrome P450
- DMSO
dimethyl sulfoxide
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HPH
human primary hepatocytes
- IFO
ifosfamide
- 3MC
3-methylcholanthrene
- PB
phenobarbital
- PK11195
1-(2-chlorophenyl-Nmethylpropyl)-3-isoquinoline-carboxamide
- PXR
pregnane X receptor
- RT-PCR
reverse transcription-polymerase chain reaction
- RIF
rifampicin
- SFN
sulforaphane
References
- 1.Rao R, Shammo JM, Enschede SH, Porter C, Adler SS, Venugopal P, Gregory SA. The combination of fludarabine, cyclophosphamide, and granulocyte-macrophage colony-stimulating factor in the treatment of patients with relapsed chronic lymphocytic leukemia and low-grade Non-Hodgkin's lymphoma. Clin Lymphoma. 2005;6:26–30. doi: 10.3816/clm.2005.n.023. [DOI] [PubMed] [Google Scholar]
- 2.Chrystal K, Cheong K, Harper P. Chemotherapy of small cell lung cancer: state of the art. Curr Opin Oncol. 2004;16:136–140. doi: 10.1097/00001622-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, Macro M, Pertuiset E, Dreyfus F, Mariette X, Boccacio C, Brouet JC. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 4.Demirer T, Buckner CD, Appelbaum FR, Clift R, Storb R, Myerson D, Lilleby K, Rowley S, Bensinger WI. High-dose busulfan and cyclophosphamide followed by autologous transplantation in patients with advanced breast cancer. Bone Marrow Transplant. 1996;17:769–774. [PubMed] [Google Scholar]
- 5.Bramwell VH, Mouridsen HT, Santoro A, Blackledge G, Somers R, Verweij J, Dombernowsky P, Onsrud M, Thomas D, Sylvester R, et al. Cyclophosphamide versus ifosfamide: a randomized phase II trial in adult soft-tissue sarcomas. The European Organization for Research and Treatment of Cancer [EORTC], Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol. 1993;31(Suppl 2):S180–184. [PubMed] [Google Scholar]
- 6.Huang Z, Roy P, Waxman DJ. Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochem Pharmacol. 2000;59:961–972. doi: 10.1016/s0006-2952(99)00410-4. [DOI] [PubMed] [Google Scholar]
- 7.Boddy AV, English M, Pearson AD, Idle JR, Skinner R. Ifosfamide nephrotoxicity: limited influence of metabolism and mode of administration during repeated therapy in paediatrics. Eur J Cancer. 1996;32A:1179–1184. doi: 10.1016/0959-8049(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Tian Q, Yung Chan S, Chuen Li S, Zhou S, Duan W, Zhu YZ. Metabolism and transport of oxazaphosphorines and the clinical implications. Drug Metab Rev. 2005;37:611–703. doi: 10.1080/03602530500364023. [DOI] [PubMed] [Google Scholar]
- 9.Chenand L, Waxman DJ. Cytochrome P450 gene-directed enzyme prodrug therapy (GDEPT) for cancer. Current pharmaceutical design. 2002;8:1405–1416. doi: 10.2174/1381612023394566. [DOI] [PubMed] [Google Scholar]
- 10.Chen CS, Lin JT, Goss KA, He YA, Halpert JR, Waxman DJ. Activation of the anticancer prodrugs cyclophosphamide and ifosfamide: identification of cytochrome P450 2B enzymes and site-specific mutants with improved enzyme kinetics. Mol Pharmacol. 2004;65:1278–1285. doi: 10.1124/mol.65.5.1278. [DOI] [PubMed] [Google Scholar]
- 11.Chang TK, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res. 1993;53:5629–5637. [PubMed] [Google Scholar]
- 12.Moore MJ, Erlichman C, Thiessen JJ, Bunting PS, Hardy R, Kerr I, Soldin S. Variability in the pharmacokinetics of cyclophosphamide, methotrexate and 5-fluorouracil in women receiving adjuvant treatment for breast cancer. Cancer Chemother Pharmacol. 1994;33:472–476. doi: 10.1007/BF00686503. [DOI] [PubMed] [Google Scholar]
- 13.Ren S, Kalhorn TF, McDonald GB, Anasetti C, Appelbaum FR, Slattery JT. Pharmacokinetics of cyclophosphamide and its metabolites in bone marrow transplantation patients. Clinical pharmacology and therapeutics. 1998;64:289–301. doi: 10.1016/S0009-9236(98)90178-3. [DOI] [PubMed] [Google Scholar]
- 14.Kerbusch T, de Kraker J, Keizer HJ, van Putten JW, Groen HJ, Jansen RL, Schellens JH, Beijnen JH. Clinical pharmacokinetics and pharmacodynamics of ifosfamide and its metabolites. Clin Pharmacokinet. 2001;40:41–62. doi: 10.2165/00003088-200140010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Chang TK, Yu L, Maurel P, Waxman DJ. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997;57:1946–1954. [PubMed] [Google Scholar]
- 16.Afsharian P, Terelius Y, Hassan Z, Nilsson C, Lundgren S, Hassan M. The effect of repeated administration of cyclophosphamide on cytochrome P450 2B in rats. Clin Cancer Res. 2007;13:4218–4224. doi: 10.1158/1078-0432.CCR-07-0320. [DOI] [PubMed] [Google Scholar]
- 17.Xie H, Afsharian P, Terelius Y, Mirghani RA, Yasar U, Hagbjork AL, Lundgren S, Hu Y, Rane A, Hassan M. Cyclophosphamide induces mRNA, protein and enzyme activity of cytochrome P450 in rat. Xenobiotica. 2005;35:239–251. doi: 10.1080/00498250500057369. [DOI] [PubMed] [Google Scholar]
- 18.Harmsen S, Meijerman I, Beijnen JH, Schellens JH. Nuclear receptor mediated induction of cytochrome P450 3A4 by anticancer drugs: a key role for the pregnane X receptor. Cancer Chemother Pharmacol. 2009;64:35–43. doi: 10.1007/s00280-008-0842-3. [DOI] [PubMed] [Google Scholar]
- 19.Lindley C, Hamilton G, McCune JS, Faucette S, Shord SS, Hawke RL, Wang H, Gilbert D, Jolley S, Yan B, LeCluyse EL. The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:814–822. doi: 10.1124/dmd.30.7.814. [DOI] [PubMed] [Google Scholar]
- 20.Wangand H, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet. 2003;42:1331–1357. doi: 10.2165/00003088-200342150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem. 2003;278:14146–14152. doi: 10.1074/jbc.M212482200. [DOI] [PubMed] [Google Scholar]
- 23.Chen T, Tompkins LM, Li L, Li H, Kim G, Zheng Y, Wang H. A single amino acid controls the functional switch of human constitutive androstane receptor (CAR) 1 to the xenobiotic-sensitive splicing variant CAR3. J Pharmacol Exp Ther. 2010;332:106–115. doi: 10.1124/jpet.109.159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, Richert L. Isolation and culture of primary human hepatocytes. Methods Mol Biol. 2005;290:207–229. doi: 10.1385/1-59259-838-2:207. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Chen T, Cottrell J, Wang H. Nuclear translocation of adenoviral-enhanced yellow fluorescent protein-tagged-human constitutive androstane receptor (hCAR): a novel tool for screening hCAR activators in human primary hepatocytes. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:1098–1106. doi: 10.1124/dmd.108.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–368. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- 27.Kerbusch T, Mathot RA, Keizer HJ, Kaijser GP, Schellens JH, Beijnen JH. Influence of dose and infusion duration on pharmacokinetics of ifosfamide and metabolites. Drug metabolism and disposition: the biological fate of chemicals. 2001;29:967–975. [PubMed] [Google Scholar]
- 28.Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, Owens IS, Negishi M, Sueyoshi T. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology (Baltimore, Md. 2001;33:1232–1238. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- 29.Gardner-Stephen D, Heydel JM, Goyal A, Lu Y, Xie W, Lindblom T, Mackenzie P, Radominska-Pandya A. Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug metabolism and disposition: the biological fate of chemicals. 2004;32:340–347. doi: 10.1124/dmd.32.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L, Li AP, Kaminski DL, Ruh MF. 2,3,7,8 Tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in cultured rat and human hepatocytes. Chem Biol Interact. 2000;124:173–189. doi: 10.1016/s0009-2797(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 31.Davarinosand NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J Biol Chem. 1999;274:28708–28715. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 32.Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou C, Poulton EJ, Grun F, Bammler TK, Blumberg B, Thummel KE, Eaton DL. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71:220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Chen T, Stanton JD, Sueyoshi T, Negishi M, Wang H. The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol. 2008;74:443–453. doi: 10.1124/mol.108.046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giraud B, Hebert G, Deroussent A, Veal GJ, Vassal G, Paci A. Oxazaphosphorines: new therapeutic strategies for an old class of drugs. Expert Opin Drug Metab Toxicol. 2010;6:919–938. doi: 10.1517/17425255.2010.487861. [DOI] [PubMed] [Google Scholar]
- 36.Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, Moore JT. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- 37.Smirlis D, Muangmoonchai R, Edwards M, Phillips IR, Shephard EA. Orphan receptor promiscuity in the induction of cytochromes p450 by xenobiotics. J Biol Chem. 2001;276:12822–12826. doi: 10.1074/jbc.M005930200. [DOI] [PubMed] [Google Scholar]
- 38.Xie W, Barwick JL, Simon CM, Pierce AM, Safe S, Blumberg B, Guzelian PS, Evans RM. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14:3014–3023. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J Pharmacol Exp Ther. 2006;317:1200–1209. doi: 10.1124/jpet.105.098160. [DOI] [PubMed] [Google Scholar]