Abstract
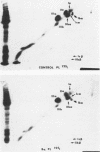
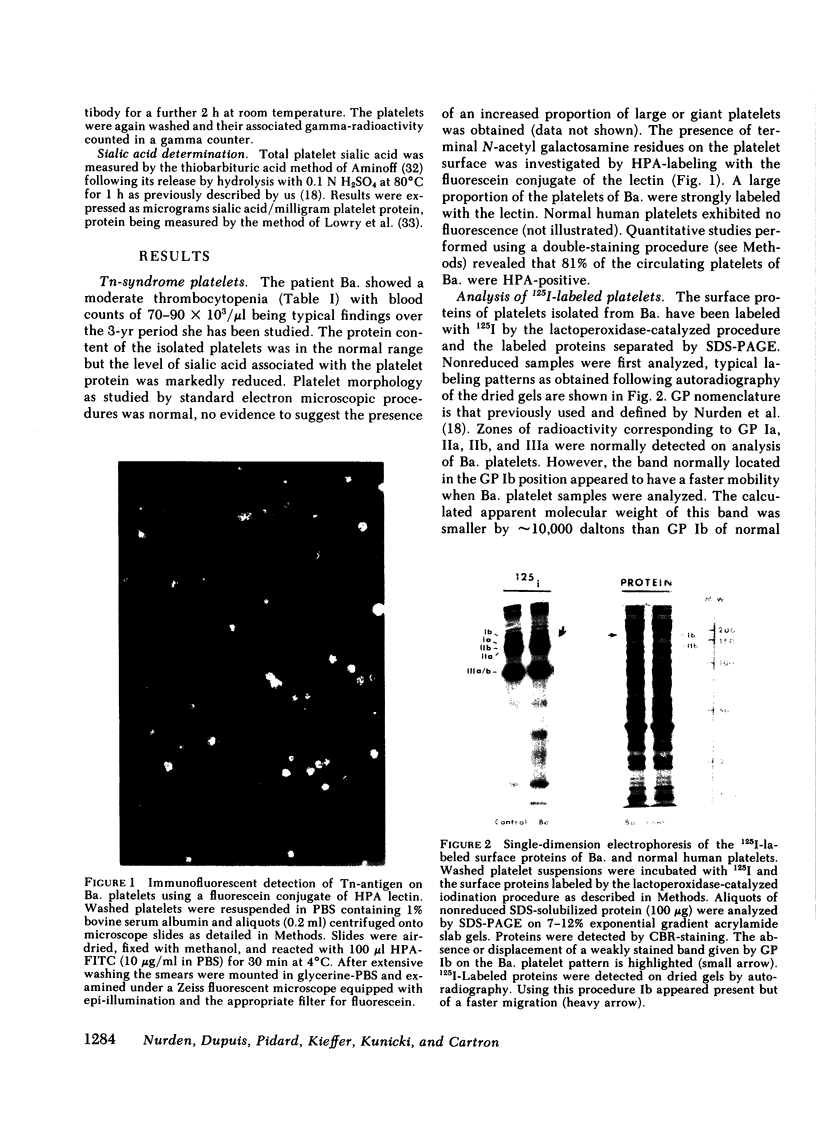
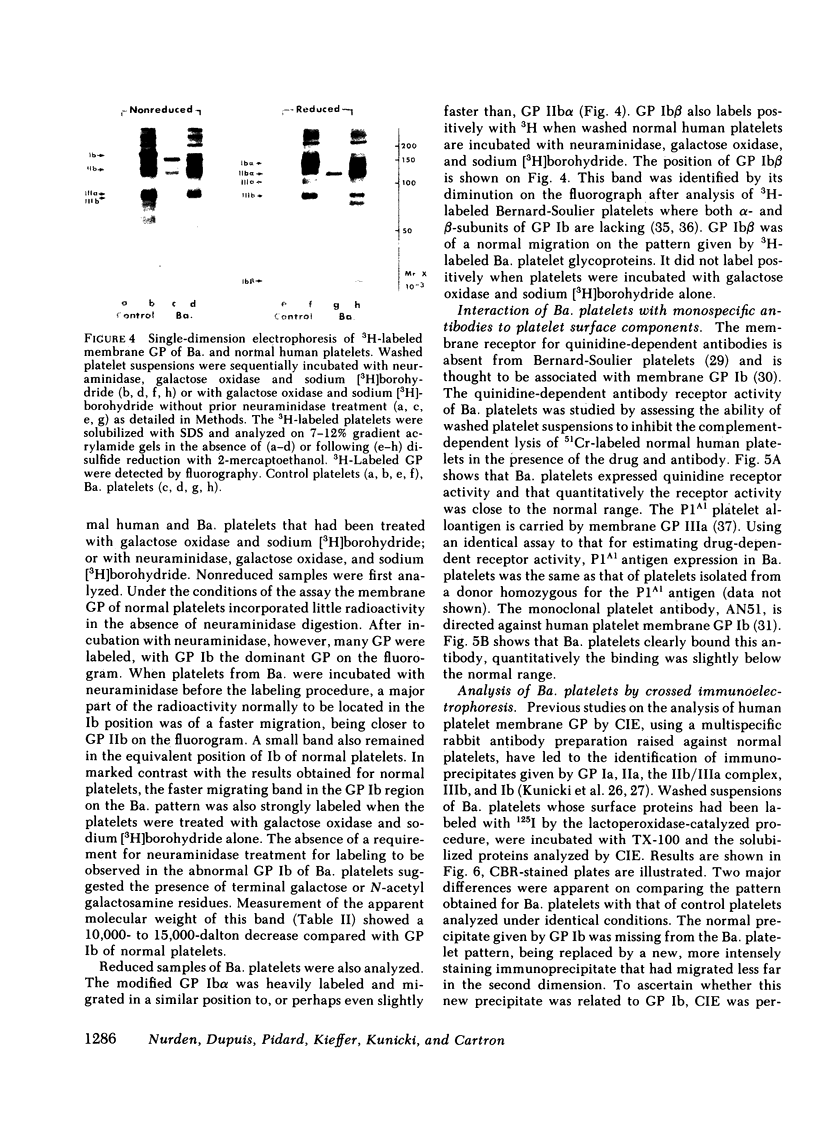
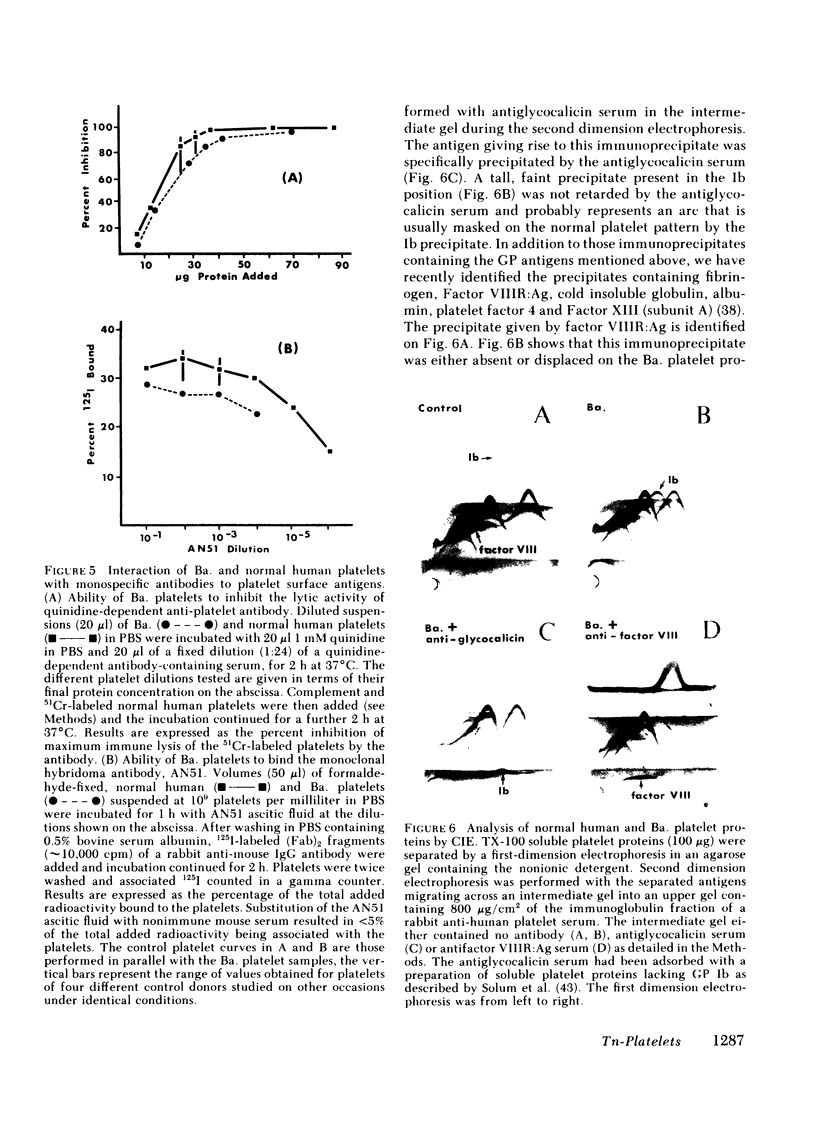
The Tn-syndrome is an acquired disorder characterized by the polyagglutination of blood cells and the pathological exposure of alpha-N-acetyl-D-galactosamine residues (Tn-antigen) at the cell surface. We now report studies on the platelet of a patient (Ba.) of which 81% reacted positively with a fluorescein conjugate of Helix pomatia agglutinin (HPA). The surface proteins of Ba. platelets were labeled with 125I by the lactoperoxidase-catalyzed procedure; single and two-dimensional electrophoresis on sodium dodecyl sulfate (SDS)-polyacrylamide gels was followed by autoradiography that revealed normal 125I-labeling of the major membrane glycoproteins (GP) but that GP Ib had a faster than normal migration. the abnormal GP Ib of Ba. platelets was strongly labeled when platelet suspensions were treated sequentially with neuraminidase, galactose oxidase, and sodium [3H]borohydride. Unlike the GP Ib of normal human platelets, it was also strongly labeled when Ba. platelets were treated with galactose oxidase and sodium [3H]borohydride alone. Both the alloantigen, PlA1, and quinidine-dependent antibody receptor activity were normally expressed by Ba. platelets, which also bound a monoclonal antibody (AN51) to GP Ib. Analysis of Ba. platelets by crossed immunoelectrophoresis using a rabbit anti-human platelet antibody preparation revealed the presence of an immunoprecipitate in the GP Ib position that had an abnormal appearance and migration in the second dimension. An altered position of the precipitate given by Factor VIIIR:Ag was also noted. Incorporation of HPA into the agarose gel during the first dimension electrophoresis resulted in the specific precipitation of the abnormal GP Ib of Ba. platelets. Our studies show that circulating Tn-platelets contain GP Ib with a modified oligosaccharide chain structure responsible for the platelet expression of Tn-antigen activity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee D. J. The blood group MNSs-active sialoglycoproteins. Semin Hematol. 1981 Jan;18(1):13–31. [PubMed] [Google Scholar]
- Baldwin M. L., Barrasso C., Ridolfi R. L. Tn-polyagglutinability associated with acute myelomonocytic leukemia. Am J Clin Pathol. 1979 Dec;72(6):1024–1027. doi: 10.1093/ajcp/72.6.1024. [DOI] [PubMed] [Google Scholar]
- Beck M. L., Hicklin B. L., Pierce S. R., Edwards R. L. Observations on leucocytes and platelets in six cases of Tn-polyagglutination. Med Lab Sci. 1977 Oct;34(4):325–329. [PubMed] [Google Scholar]
- Berman H. J., Smarto J., Issitt C. H., Issitt P. D., Marsh W. L., Jensen L. Tn-activation with acquired A-like antigen. Transfusion. 1972 Jan-Feb;12(1):35–45. doi: 10.1111/j.1537-2995.1972.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Shinton N. K., Wingham J. Persistent mixed-field polyagglutination. Br J Haematol. 1971 Oct;21(4):443–453. doi: 10.1111/j.1365-2141.1971.tb02705.x. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Wingham J., Pippard M. J., Hoult J. G., Melikian V. Erythrocyte membrane modification in malignant diseases of myeloid and lymphoreticular tissues. I. Tn-polyagglutination in acute myelocytic leukaemia. Br J Haematol. 1976 Jun;33(2):289–294. doi: 10.1111/j.1365-2141.1976.tb03540.x. [DOI] [PubMed] [Google Scholar]
- Caen J. P., Nurden A. T., Jeanneau C., Michel H., Tobelem G., Levy-Toledano S., Sultan Y., Valensi F., Bernard J. Bernard-Soulier syndrome: a new platelet glycoprotein abnormality. Its relationship with platelet adhesion to subendothelium and with the factor VIII von Willebrand protein. J Lab Clin Med. 1976 Apr;87(4):586–596. [PubMed] [Google Scholar]
- Cartron J. P., Andreu G., Cartron J., Bird G. W., Salmon C., Gerbal A. Demonstration of T-transferase deficiency in Tn-polyagglutinable blood samples. Eur J Biochem. 1978 Dec 1;92(1):111–119. doi: 10.1111/j.1432-1033.1978.tb12728.x. [DOI] [PubMed] [Google Scholar]
- Cartron J. P., Nurden A. T. Galactosyltransferase and membrane glycoprotein abnormality in human platelets from Tn-syndrome donors. Nature. 1979 Dec 6;282(5739):621–623. doi: 10.1038/282621a0. [DOI] [PubMed] [Google Scholar]
- Clemetson K. J., Naim H. Y., Lüscher E. F. Relationship between glycocalicin and glycoprotein Ib of human platelets. Proc Natl Acad Sci U S A. 1981 May;78(5):2712–2716. doi: 10.1073/pnas.78.5.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. A., Bennett W. P., Kreger A., Lyerly D., Wagner R. H. The effect of extracellular proteases from gran-negative bacteria on the interaction of von Willebrand factor with human platelets. J Lab Clin Med. 1981 Mar;97(3):379–389. [PubMed] [Google Scholar]
- DAUSSET J., MOULLEC J., BERNARD J. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (Anti-Tn). Blood. 1959 Oct;14:1079–1093. [PubMed] [Google Scholar]
- Dahr W., Uhlenbruck G., Bird G. W. Cryptic A-like receptor sites in human erythrocyte glycoproteins: proposed nature of Tn-antigen. Vox Sang. 1974;27(1):29–42. doi: 10.1111/j.1423-0410.1974.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Dahr W., Uhlenbruck G., Gunson H. H., Van Der Hart M. Molecular basis of Tn-polyagglutinability. Vox Sang. 1975;29(1):36–50. doi: 10.1111/j.1423-0410.1975.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Dahr W., Uhlenbruck G., Gunson H. H., van der Hart M. Studies on glycoproteins and glycopeptides from Tn-polyagglutinable erythrocytes. Vox Sang. 1975;28(3):249–252. doi: 10.1111/j.1423-0410.1975.tb02764.x. [DOI] [PubMed] [Google Scholar]
- Glöckner W. M., Kaulen H. D., Uhlenbruck G. Immunochemical detection of the Thomsen-Friedenreich antigen (T-antigen) on platelet plasma membranes. Thromb Haemost. 1978 Feb 28;39(1):186–192. [PubMed] [Google Scholar]
- Hagen I., Bjerrum O. J., Solum N. O. Characterization of human platelet proteins solubilized with Triton X-100 and examined by crossed immunoelectrophoresis. Reference patterns of extracts from whole platelets and isolated membranes. Eur J Biochem. 1979 Aug 15;99(1):9–22. doi: 10.1111/j.1432-1033.1979.tb13225.x. [DOI] [PubMed] [Google Scholar]
- Hagen I., Nurden A., Bjerrum O. J., Solum N. O., Caen J. Immunochemical evidence for protein abnormalities in platelets from patients with Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Clin Invest. 1980 Mar;65(3):722–731. doi: 10.1172/JCI109719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Murphy L. A., Goldstein I. J., Etzler M. E. Carbohydrate binding specificity of four N-acetyl-D-galactosamine- "specific" lectins: Helix pomatia A hemagglutinin, soy bean agglutinin, lima bean lectin, and Dolichos biflorus lectin. Biochemistry. 1977 Jun 14;16(12):2750–2755. doi: 10.1021/bi00631a025. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Aster R. H. Deletion of the platelet-specific alloantigen PlA1 from platelets in Glanzmann's thrombasthenia. J Clin Invest. 1978 May;61(5):1225–1231. doi: 10.1172/JCI109038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Aster R. H. Isolation and immunologic characterization of the human platelet alloantigen, P1A1. Mol Immunol. 1979 Jun;16(6):353–360. doi: 10.1016/0161-5890(79)90100-7. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Johnson M. M., Aster R. H. Absence of the platelet receptor for drug-dependent antibodies in the Bernard-Soulier syndrome. J Clin Invest. 1978 Sep;62(3):716–719. doi: 10.1172/JCI109181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Nurden A. T., Pidard D., Russell N. R., Caen J. P. Characterization of human platelet glycoprotein antigens giving rise to individual immunoprecipitates in crossed-immunoelectrophoresis. Blood. 1981 Dec;58(6):1190–1197. [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Cazenave J. P., Nurden A. T., Caen J. P. Inheritance of the human platelet alloantigen, PlA1, in type I Glanzmann's thrombasthenia. J Clin Invest. 1981 Mar;67(3):717–724. doi: 10.1172/JCI110088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Kunicki T. J., Russell N., Nurden A. T., Aster R. H., Caen J. P. Further studies of the human platelet receptor for quinine- and quinine-dependent antibodies. J Immunol. 1981 Feb;126(2):398–402. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGregor J. L., Clemetson K. J., James E., Dechavanne M. A comparison of techniques used to study externally oriented proteins and glycoproteins of human blood platelets. Thromb Res. 1979;16(3-4):437–452. doi: 10.1016/0049-3848(79)90091-4. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Rust N. A., Pilch J. R., Sochynsky R., Morton J., Mason D. Y., Ruan C., Tobelem G., Caen J. Monoclonal antibody to human platelet glycoprotein I. I. Immunological studies. Br J Haematol. 1981 Dec;49(4):501–509. doi: 10.1111/j.1365-2141.1981.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Ness P. M., Garratty G., Morel P. A., Perkins H. A. Tn polyagglutination preceding acute leukemia. Blood. 1979 Jul;54(1):30–34. [PubMed] [Google Scholar]
- Nurden A. T., Dupuis D., Kunicki T. J., Caen J. P. Analysis of the glycoprotein and protein composition of Bernard-Soulier platelets by single and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Clin Invest. 1981 May;67(5):1431–1440. doi: 10.1172/JCI110172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Dupuis D., Pidard D., Kunicki T., Caen J. P. Biochemistry and immunology of platelet membranes with reference to glycoprotein composition. Ann N Y Acad Sci. 1981;370:72–86. doi: 10.1111/j.1749-6632.1981.tb29723.x. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Kunicki T. J., Dupuis D., Soria C., Caen J. P. Specific protein and glycoprotein deficiencies in platelets isolated from two patients with the gray platelet syndrome. Blood. 1982 Apr;59(4):709–718. [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Phillips D. R. Surface labelling as a tool to determine structure-function relationships of platelet plasma membrane glycoproteins. Thromb Haemost. 1980 Feb 29;42(5):1638–1651. [PubMed] [Google Scholar]
- Ruan C., Tobelem G., McMichael A. J., Drouet L., Legrand Y., Degos L., Kieffer N., Lee H., Caen J. P. Monoclonal antibody to human platelet glycoprotein I. II. Effects on human platelet function. Br J Haematol. 1981 Dec;49(4):511–519. doi: 10.1111/j.1365-2141.1981.tb07259.x. [DOI] [PubMed] [Google Scholar]
- Shulman S., Karpatkin S. Crossed immunoelectrophoresis of human platelet membranes. Diminished major antigen in Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Biol Chem. 1980 May 10;255(9):4320–4327. [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Filion-Myklebust C., Stabaek T. Platelet glycocalicin. Its membrane association and solubilization in aqueous media. Biochim Biophys Acta. 1980 Apr 10;597(2):235–246. doi: 10.1016/0005-2736(80)90102-9. [DOI] [PubMed] [Google Scholar]
- Sturgeon P., McQuiston D. T., Taswell H. F., Allan C. J. Permanent mixed-field polyagglutinability (PMFP). I. Serological observations. Vox Sang. 1973 Dec;25(6):481–497. doi: 10.1111/j.1423-0410.1973.tb03543.x. [DOI] [PubMed] [Google Scholar]
- Vainchenker W., Testa U., Deschamps J. F., Henri A., Titeux M., Breton-Gorius J., Rochant H., Lee D., Cartron J. P. Clonal expression of the Tn antigen in erythroid and granulocyte colonies and its application to determination of the clonality of the human megakaryocyte colony assay. J Clin Invest. 1982 May;69(5):1081–1091. doi: 10.1172/JCI110543. [DOI] [PMC free article] [PubMed] [Google Scholar]