Abstract
Programmed death-1 (PD-1) is a co-inhibitory inducible receptor present on T-cells and macrophages. Tumor cells with increased programmed death ligand-1 (PD-L1) are believed to escape immunity through activation of PD-1/PD-L1 pathway and suppression of effector immune responses. Recent strategies targeting the PD-1/PD-L1 axis have shown promising results in patients with several tumors types, including lung carcinomas. Preliminary data suggests that PD-L1 protein expression might predictive response to such therapies. Sarcomatoid carcinomas (SCs) of the lung include rare subtypes of poorly differentiated non-small cell lung carcinomas (NSCLC) of high grade and aggressive behavior. The biology of these neoplasms is poorly understood and they frequently show increased local inflammatory and lymphocytic infiltration. Here, we report the expression of PD-L1 in 13 SCs from two large retrospective lung cancer cohorts. Using automated quantitative immunofloresence (aqIF/AQUA®) and a mouse monoclonal antibody directed against the extra-cellular domain of PD-L1, we show that 9 of 13 (69.2%) patients with SCs are positive for PD-L1 and their levels are higher than in conventional NSCLC. These results provide rationale for the potential use of targeted immunetherapy in lung SCs.
Keywords: Sarcomatoid carcinoma, programmed death receptor 1, Lung cancer, Immunotherapy
Background
Programmed death-1 (PD-1) is a negative regulator of T-lymphocytes and act as a co-inhibitory receptor to prevent off target immune activation.[1] Tumor cells from diverse locations overexpress PD-1 receptor ligand called PD-L1 (also B7-h1 or CD274). PD-L1 upregulation by neoplastic cells allows tumors to escape the effector immune responses. Recently, clinical trials using monoclonal antibodies targeting the PD-1/PD-L1 axis show promising anti-tumor activity in several malignancies including lung carcinomas.[2, 3] Preliminary data from these trials suggests that tumor PD-L1 expression predicts response to these treatments.
Sarcomatoid carcinoma (SC) is a rare form (0.3–1.3% of all lung carcinomas) of poorly differentiated non-small cell lung carcinoma (NSCLC) containing a component of malignant mesenchymal differentiation. According to the last WHO classification, SCs comprise 5 histopathologic subtypes: pleomorphic carcinoma (PC), spindle cell carcinoma (SCC), giant cell carcinoma (GCC), carcinosarcoma and pulmonary blastoma.[4] Combined lesions (e.g. pleomorphic carcinoma) include areas of conventional NSCLC (e.g. squamous, adenocarcinoma or large cell carcinoma) and the more common sarcomatous elements are SCC and GCC. SCCs are characterized by the presence of fascicles and nests of highly atypical elongated cells. GCCs are characterized by large pleomorphic mono- or multinucleated giant tumor cells and mixed inflammatory infiltrates composed predominantly of neutrophils and lymphocytes. In addition, engulfment of intact inflammatory cells by giant tumor cells (a phenomenon called, emperipolesis) is a distinctive finding. These tumors are aggressive and resistant to chemotherapy and radiotherapy.[5] Little is known about the biology of these neoplasms and the mechanism of chemo/radio-resistance and progression. Here, we measured the levels of PD-L1 protein using the AQUA® method of quantitative immunofluorescence (QIF) in 13 SCs samples from two retrospective lung cancer cohorts. Our results show that SCs show higher PD-L1 levels than other NSCLC, supporting a potential for the use of anti PD-1/PD-L1 targeted therapies.
Methods
Formalin fixed paraffin embedded tissue samples (FFPE) from 13 SCs were selected from two retrospective lung cancer cohorts including patients with NSCLC from the Yale University and Sotiria General Hospital in Greece (December 1988 and October 2003). The cohort descriptions were previously communicated.[6, 7] The 13 SCs examined were part of a broader study where 458 patients were evaluable for PD-L1 measurement. All the specimens were evaluated in a tissue microarray (TMA) format. Human placenta and tonsil were included as positive controls for endogenous PD-L1 along with cores from FFPE parental and PD-L1 overexpressing Mel624 cells. Culture conditions and cell-line TMA construction were performed as reported elsewhere[8]. TMA sections were deparaffinized in oven for 30 min at 60 °C followed by two serial xylenes incubations. Sections were then rehydrated in graded alcohols, and subjected to antigen retrieval using tris-EDTA buffer with 0.05% Tween (pH=9.0) and boilied for 20 min at 102 °C in a PT module (Lab Vision, Fermont, CA). Slides were incubated with ACE block at room temperature for 10 min and exposed overnight at 4 °C to a mouse monoclonal primary PD-L1 antibody (clone 5H1; Dr. Lieping Chen, Yale University) at 1:500 dilution and a rabbit monoclonal anti-human pancytokeratin antibody at 1:50 (AE1/AE3, M3515; DAKO). Sections were incubated for 1 hour at room temperature with Alexa 546-conjugated goat anti-rabbit secondary antibody (A11003; Molecular Probes) diluted 1:100 in mouse EnVision amplification reagent (K4003, Dako). Cyanine 5 (Cy5) directly conjugated to tyramide (FP1117; Perkin-Elmer) at a 1:50 dilution was used for target antibody detection. Prolong mounting medium (ProLong Gold, P36931; Molecular Probes) containing 4′,6-diamidino-2-phenylindole (DAPI) was used to highlight nuclei. Automated quantitative fluorescence analysis (QIF/AQUA®) was used for PD-L1 measurements allowing objective measurement of protein levels within user defined cellular compartments, as described in detail elsewhere.[9] AQUA® scores of PD-L1 protein in each histospot were measured in the area of the tumor mask (defined by the cytokeratin positive compartment). Comparisons of mean AQUA® scores of PD-L1 were performed using Mann-Whitney test and GraphPad Prism software.
Results
The levels of PD-L1 were measured using QIF in the lung cases and in control preparations (Figure 1). Specificity of the assay was tested in TMA based FFPE preparations containing term human placenta and Mel624 cell lines with or without PD-L1 transfection. As expected, PD-L1 signal was detected only in the placental trophoblastic cells (CK positive compartment) and in Mel624 transfectants, but not in the mesenchymal areas of the chorionic villi (Vimentin positive compartment) or in parental Mel624 cells (Figure 1B). In the control preparations, PD-L1 staining was relatively homogenous and with a predominant perinuclear staining pattern.
Figure 1. Sarcomatoid lung carcinomas show increased PD-L1 protein levels.
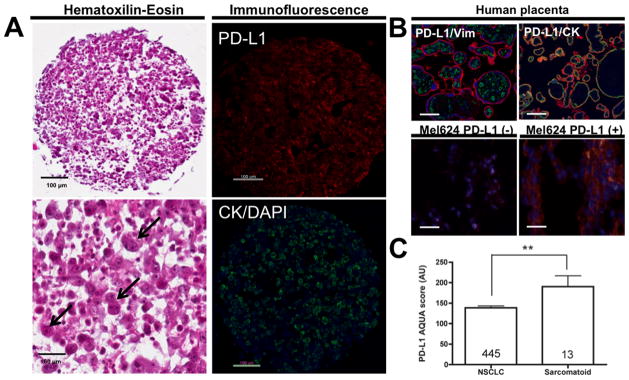
A) Representative microphotographs showing PD-L1 and cytokeratin positivity in Giant Cell lung carcinoma. Left panels include Hematoxilin & Eosin stained preparations at 200x (upper) and 400x magnification (lower panel). Giant cell carcinomas show characteristic lack of typical epithelial cohesiveness, a prominent mixed inflammatory infiltrate and bizarre giant tumor cells with single/multiple nuclei and focal leukocyte engulfment (emperipolesis, arrows). The right panels show fluorescence captions of PD-L1 (upper, red channel) and cytokeratin (lower, green channel) positivity of the same sample. Nuclei were stained with DAPI (blue channel).
B) Representative fluorescence microphotographs showing PD-L1 staining in FFPE term human placenta (upper panels) and Mel624 transfectants (lower panels). PD-L1 positivity in placental samples was restricted to the trophoblastic cells (cytokeratin positive compartment, CK, right panel) and absent in the stromal areas (vimentin positive compartment, Vim, left panel). Bar=100 μm. In the cell lines, PD-L1 signal was evident with a perinuclear/membranous pattern only in PD-L1 transfected Mel624 cells (bottom right panel) but not in parental cells (bottom left panel). Bar=50 μm.
C) Graph showing mean ± SEM PD-L1 AQUA scores in conventional NSCLC (left bar) and in Sarcomatous carcinomas (right bar). AQUA scores are expressed as arbitrary units (AU). Cases were measured in the same preparations. The number of cases in each group is indicated within each bar. **=p<0.01
In agreement with previous reports5, the studied SCs included a relatively large proportion of GCCs showing characteristic discohesive and highly atypical giant tumor cells, some of them multinucleated and with prominent neutrophilic infiltration (Figure 1A , H&E, left panels). The positivity of pancytokeratin staining in atypical cells confirmed the epithelial nature of the lesion and PD-L1 positivity was identified in both tumor and stromal cells (Figure 1A, right panels).
Of 13 SCs cases, 10 were GCCs, 1 SCC, 1 carcinosarcoma and 1 pleomorphic carcinoma (Table 1). Subjects affected by SC were predominantly male (10 of 13) with average 60.2 years, diverse clinical stages at presentation and history of tobacco use (Table 1). Of 13 SCs, 9 (69.2%) were positive for PD-L1 and as depicted in Figure 1C, PD-L1 levels were ~40% higher in SCs than in conventional NSCLC (AQUA score 138 and 190, respectively p=0.01). Only 122 of 445 (27.4%) conventional NSCLC in the studied cohorts were positive for PD-L1.
Table 1.
Main clinicopathological characteristics of subjects with sarcomatous carcinomas
| Age | Gender | Stage | Smoking | Histology | PD-L1 Status |
|---|---|---|---|---|---|
| 48 | Female | IB | Yes | Giant cell carcinoma | Negative |
| 66 | Male | IIB | NA | Giant cell carcinoma | Negative |
| 61 | Male | IIB | Yes | Giant cell carcinoma | Negative |
| 64 | Male | IIIA | Yes | Giant cell carcinoma | Negative |
| 61 | Male | IIIB | Yes | Giant cell carcinoma | Positive |
| 47 | Male | IV | Yes | Giant cell carcinoma | Positive |
| 54 | Male | IIB | Yes | Giant cell carcinoma | Positive |
| 55 | Male | IB | Yes | Giant cell carcinoma | Positive |
| 65 | Male | IB | Yes | Giant cell carcinoma | Positive |
| 42 | Female | IIB | Yes | Giant cell carcinoma | Positive |
| 83 | Male | IB | NA | Spindle cell carcinoma | Positive |
| 66 | Male | IA | NA | Carcinosarcoma (NSCLC and spindle/chondroid) | Positive |
| 71 | Female | IB | NA | Pleomorphic carcinoma (adenoca and giant/spindle cell) | Positive |
Discussion
SC is a rare subtype of NSCLC with a poorly understood biology and ominous outcome. The identification of actionable molecular targets for such infrequent and aggressive diseases is critical for design of new clinical trials.[10] In agreement with previously reported studies[11–13], the present data show that SC patients comprise predominantly adult male smokers with non-metastatic disease at diagnosis. Among SCs, GCC represents a frequent histologic tumor component/subtype and show characteristic morphology with highly atypical discohesive large tumor cells and increased inflammatory and lymphocytic response.
Increased tumor infiltration by neutrophils and lymphocytes in SCs suggest the presence of strong local anti-tumor immune responses. Despite the presence of rich inflammatory infiltrates patients with SCs have worse outcomes compared to other histologic subtypes of NSCLC. Elevated expression of PD-L1 by SC cells might account for this apparent contradiction, since local inactivation of effector immune cells through PD-1 receptor signaling could ultimately enhance the disease progression. Additional local immune inhibitory mechanisms such as infiltration by specific immune inhibitory cells (e.g. T regulatory cells and myeloid-derived suppressor cells), production of inhibitory cytokines (e.g. IL-6, IL-10, TGF-β) and activation of other T cell co-inhibitory pathways (e.g. CTLA-4, PD-L2, IDO-1) might also participate. Future studies will be required to clarify this.
Compared to conventional NSCLC, SCs are more aggressive and cytotoxic chemotherapy is generally ineffective. Recent advances in our understanding of the mechanisms of immune escape of tumors paved way for new therapeutic strategies. Although lung carcinomas have traditionally been considered to be poorly immunogenic, PD-1/PD-L1 interaction has been recognized as a key mechanism of immune evasion in NSCLC.[1, 14, 15] To our knowledge, this is the first report measuring PD-L1 protein levels in SCs and the results suggest the possibility of using novel immunotherapy approaches such as PD-1/PD-L1 blockers in this otherwise difficult to treat disease.
References
- 1.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nature reviews Immunology. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Fishback NF, Travis WD, Moran CA, Guinee DG, Jr, McCarthy WF, Koss MN. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73:2936–2945. doi: 10.1002/1097-0142(19940615)73:12<2936::aid-cncr2820731210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Anagnostou VK, Syrigos KN, Bepler G, Homer RJ, Rimm DL. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:271–278. doi: 10.1200/JCO.2008.17.0043. [DOI] [PubMed] [Google Scholar]
- 7.Anagnostou VK, Dimou AT, Botsis T, Killiam EJ, Gustavson MD, Homer RJ, Boffa D, Zolota V, Dougenis D, Tanoue L, Gettinger SN, Detterbeck FC, Syrigos KN, Bepler G, Rimm DL. Molecular classification of nonsmall cell lung cancer using a 4-protein quantitative assay. Cancer. 2012;118:1607–1618. doi: 10.1002/cncr.26450. [DOI] [PubMed] [Google Scholar]
- 8.McCabe A, Dolled-Filhart M, Camp RL, Rimm DL. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. Journal of the National Cancer Institute. 2005;97:1808–1815. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 9.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nature medicine. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 10.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Seminars in roentgenology. 2005;40:90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Venissac N, Pop D, Lassalle S, Berthier F, Hofman P, Mouroux J. Sarcomatoid lung cancer (spindle/giant cells): an aggressive disease? The Journal of thoracic and cardiovascular surgery. 2007;134:619–623. doi: 10.1016/j.jtcvs.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Ishida T, Tateishi M, Kaneko S, Yano T, Mitsudomi T, Sugimachi K, Hara N, Ohta M. Carcinosarcoma and spindle cell carcinoma of the lung. Clinicopathologic and immunohistochemical studies. The Journal of thoracic and cardiovascular surgery. 1990;100:844–852. [PubMed] [Google Scholar]
- 13.Vieira T, Cazes A, Pierre B, Zemoura L, Monnet I, De Cremoux H, Chouaid C, Duruisseaux M, Giroux Leprieur E, Belmont L, Lavole A, Cadranel J, Wislez M. Is conventional chemotherapy effective in advanced sarcomatoid lung cancers? ASCO Meeting Abstracts. 2012;30:e18102. [Google Scholar]
- 14.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 15.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]


