Abstract
The prototypic head and neck squamous cell carcinoma (HNSCC) arises from the mucosal lining of the upper aerodigestive tract, demonstrates squamous differentiation microscopically, involves older men with a long history of cigarette smoking and alcohol consumption, and is treated by multimodality therapy. HNSCC has long been regarded as a uniform disease process requiring a methodical and unwavering therapeutic approach. Divergence in epidemiologic trends among HNSCCs arising from different anatomic sites has introduced a view that, morphologic repetition aside, head and neck cancers form a heterogeneous group. This view has been supported at the molecular genetic level. A more complete understanding of the molecular genetics of head and neck cancer is providing new insights into long-held but poorly comprehended concepts such as field cancerization and is introducing various biomarkers with potential application for diagnosing, staging, monitoring, and prognosticating HNSCC.
Keywords: genetics, human papillomavirus, pathology, vaccines, tyrosine kinase inhibitors
INTRODUCTION
Head and neck cancer is not a specific entity, but rather a broad category of diverse tumor types arising from various anatomic structures including the craniofacial bones, soft tissues, salivary glands, skin, and mucosal membranes. The vast majority (more than 90%) are squamous cell carcinomas, such that the term head and neck cancer is often used to describe all carcinomas arising from the epithelium lining the sinonasal tract, oral cavity, pharynx, and larynx and showing microscopic evidence of squamous differentiation. Long regarded as a uniform group of tumors that differed only as a function of anatomic site, ongoing studies indicate that these HNSCCs may not be as homogenous as previously supposed. Recognition of distinct molecular genetic profiles now permits finer resolution of HNSCC into distinct subtypes that differ with respect to risk factors, pathogenesis, and clinical behavior. This review covers recent advances toward defining the molecular genetic makeup of HNSCC, including the unique molecular genetic profile of human papillomavirus (HPV)-related carcinoma that warrants its separation as a distinct subtype. These advances, in turn, occasion novel opportunities for the application of molecular genetic characterization of HNSCC in the clinical arena.
CHANGING TRENDS IN THE EPIDEMIOLOGY OF HEAD AND NECK CANCER
Head and neck cancer is the eighth most common cancer worldwide with approximately 650,000 new cases reported annually (1). In the United States, 45,660 new cases of HNSCC were diagnosed in 2007, accounting for 3.2% of all incident malignancies (2). Tracking the epidemiology of head and neck cancer over extended periods of time has shown that these tumors are in flux with respect to both incidence and associated mortality. The direction and degree of these shifting trends are site specific. Between 1973 and 1999, 5-year survival rates significantly improved for carcinomas of the nasopharynx, oropharynx, and hypopharynx, and they declined for regional-stage oral cavity cancer and early-stage cancer of the larynx (3). With regard to trends in tumor incidence, the declining overall incidence of head and neck cancer in the United States and elsewhere has been partially offset by a disturbing rise in the incidence of oropharynx carcinoma (Figure 1) (4–7). Although the age-adjusted incidence of laryngeal, oral cavity, and hypopharyngeal cancers has been in decline, the incidence of oropharyngeal cancer has been on the rise, particularly among individuals under 45 years of age. This change in the distribution of primary tumor site is reflected in the populations of patients enrolled in clinical studies, and this shifting distribution could partially confound meaningful comparison of treatment responses across different time periods. Compared to studies conducted in earlier decades, patients enrolled in current large multicenter clinical trials tend to be younger and have cancers of the oropharynx (8, 9).
Figure 1.
General incident trends of head and neck squamous cell carcinoma (HNSCC) as a function of yearly cigarette consumption. In the United States, the overall incidence of HNSCC (orange arrow) has paralleled the yearly annual cigarette consumption, with a peak incidence in the 1970s. A distinct departure from this trend has been noted among white males under 60, in whom the incidence of oropharyngeal carcinoma (red arrow) has been on the rise since the early 1970s.
This anomalous rise of oropharyngeal carcinoma bucks the prevailing notion that HNSCC incident rates can be controlled through smoking prevention and cessation (Figure 1) (4). Indeed, the escalating incidence of oropharyngeal carcinoma in the absence of a parallel rise in smoking and alcohol consumption in the United States and Western Europe points to the participation of nontraditional behavioral and environmental factors driving these epidemiologic trends.
RISK FACTORS
Carcinogen exposure, diet, oral hygiene, infectious agents, family history, and preexisting medical conditions all play a role, individually or in combination, in the development of HNSCC. Of these, tobacco smoking is well established as a dominant risk factor for HNSCC, and this risk is correlated with the intensity and duration of smoking. Smoking cessation reduces but does not eliminate the risk of cancer development (10). Environmental exposure to tobacco smoke (i.e., passive smoking) also appears to increase the risk of developing HNSCC, even for individuals who have never actively smoked (11). This increased risk is largely attributable to the genotoxic effects of carcinogens in tobacco smoke, including nitrosamines and polycyclic hydrocarbons. There is an ongoing effort to identify the molecular targets of cigarette smoke and to discern the unique profile of tobacco-induced mutations. TP53 mutations in HNSCCs, for example, occur more frequently in patients who smoke than in patients who do not smoke (12). Clearly not everyone who smokes acquires TP53 mutations and develops HNSCC, indicating that individual differences in carcinogen-metabolizing enzymes may modify cancer risk. Researchers have focused much attention on genetic polymorphisms in those enzymes that activate pro-carcinogens and detoxify carcinogens, but a straightforward casual association has been difficult to establish (13). In reality, risk may be related to a complex interaction among exposure, activation, detoxification, and repair of DNA damage.
Heavy alcohol consumption is also recognized as an independent risk factor for HN-SCC, particularly for cancers of the hypopharynx (14). Alcohol consumption, however, is most relevant for its ability to magnify the effects of tobacco smoke in a synergistic manner: The risk of cancer development among heavy smokers and drinkers is much higher than expected based on the additive effects of the individual risks (15). Although alcohol itself is not a direct carcinogen, its metabolite—acetaldehyde—forms DNA adducts that interfere with DNA synthesis and repair (16). Polymorphisms in the enzyme that metabolize alcohol to acetaldehyde (i.e., alcohol dehydrogenase) have not been conclusively associated with modification of cancer risk. The ability of alcohol to potentiate the effects of smoking more likely resides in its nature as a chemical solvent, enhancing and prolonging mucosal exposure to the carcinogens present in tobacco smoke.
Although tobacco exposure and alcohol consumption are responsible for the vast majority of HNSCCs that occur in the oral cavity, larynx, and hypopharynx, their role in tumorigenesis of the oropharynx is much less consequential. Instead, oncogenic HPV, particularly type 16, has been established as a causative agent in up to 70% of oropharyngeal cancers (17–19). With declining cigarette smoking in many areas of the world, HPV-16 infection is becoming a preeminent risk factor and is shifting the demographics of HNSCC toward younger patients who do not smoke or drink. Indeed, traditional risk factors, namely tobacco and alcohol exposure, do not appear to play a contributive role in HPV-mediated carcinogenesis of the oropharynx (20). Instead, HPV-associated HNSCC is strongly associated with oral HPV infection and certain sexual practices that facilitate repeated viral exposure; these include early age of sexual debut, a high number of lifetime vaginal and oral sexual partners, frequent oral-genital and oral-anal contact, and infrequent use of barriers during sexual activity (20, 21).
Although the risk factors for viral transmission are well recognized, those associated with subsequent HPV-induced tumorigenesis are now only coming into focus. Certain conditions and behaviors that alter antitumor immunity have been implicated as potential contributors to viral persistence and tumor progression. For example, oral HPV is more frequently detected in individuals who are HIV positive than in those who are HIV negative, and the virus is much more likely to persist in the oral cavity of these HIV-positive individuals over extended periods of time (22, 23). Marijuana use has recently been identified as an independent risk factor for HPV-positive HNSCC, and the strength of this association increases with intensity, duration, and cumulative years of marijuana smoking (24). Long scrutinized as a potential source of DNA-damaging carcinogens, marijuana smoke may be more relevant in the progression of HPV-positive HNSCCs for its immunomodulatory effects. Cannabinoids bind to the CB2 receptor expressed on B cells, T cells, NK cells, macrophages, and dendritic cells in human tonsillar tissue. Binding, in turn, can suppress immune responses, diminish host responses to viral pathogens, and attenuate an-titumor activity (25–27). In effect, marijuana use could affect all stages of HPV-induced tumorigenesis from onset of infection to viral persistence, tumor initiation, and growth and metastasis (24).
MICROANATOMY OF THE ORAL CAVITY AND OROPHARYNX: IMPLICATIONS FOR TUMORIGENESIS
The oral cavity is lined by a stratified squamous epithelium that varies slightly with respect to thickness and keratinization as a function of exposure to forces of mastication. Its interface with the underlying lamina propria is abrupt and delineated by a contiguous basement membrane—a structure that regulates differentiation and migration of the epithelial cells and serves as a barrier to stromal invasion during tumorigenesis. The mucosa lining the lingual and palatine tonsils of the oropharynx is notable for its intimate relationship with the lymphoid tissue of Waldheyer’s ring—a circular collection of submucosal lymphoid tissue that guards the opening of the aerodigestive tract and serves as the first line of defense against airborne and ingested antigens. The surface area of the tonsillar epithelium is maximized by the presence of numerous blind-ending crypts that extend through the full thickness of the palatine tonsil (Figure 2a) (28). Patches of the tonsillar crypt are lined by a reticulated (netlike) squamous epithelium that is uniquely structured to facilitate transport of foreign antigens from the external environment of the oropharynx to the tonsillar lymphoid tissue (Figure 2b) (29). The basal cell layer is incomplete and its supporting basement membrane is disrupted and porous, thus allowing for the direct passage of lymphocytes and antigen-presenting cells. The intermediate layer is permeated by lymphocytes and antigen-presenting cells. The superficial layer is thin and fragile. Complete desquamation of the superficial cells exposes the internal environment of the tonsil to external pathogens.
Figure 2.
(a) Topography of the human palatine tonsil. The surface epithelium of the palatine tonsil deeply invaginates into a lymphoid stroma as blind-ending and ramifying crypts (boxed area) that increase the surface area of the tonsil by nearly 700%. Drawing by Max Brödel. Used with permission from Art as Applied to Medicine, the Johns Hopkins University School of Medicine. (b) The specialized reticulated epithelium lining the tonsillar crypts. The zones of squamous epithelium—the basal, intermediate, and superficial layers—are interrupted by migrating nonepithelial cells including lymphocytes and antigen-presenting cells. Loss of structural integrity leaves the basement membrane exposed to deposition of viral particles. Drawing by T. Phelps. Abbreviations: APG, antigen presenting group; HPV, human papillomavirus.
The vast majority of HPV-positive HNSCCs localize to the tonsillar crypts of the lingual and palatine tonsils (17, 18, 30, 31). The reticular epithelium may be well suited for its role in mucosal immune protection, but those same structural features render it vulnerable to attack by HPV. In the cervix, HPV infection requires disruption (e.g., mechanical abrasion) of the epithelium with subsequent deposition of the virus onto an exposed basement membrane (32). In the reticulated epithelium of the tonsil, epithelial disruption and unrest leave the basement membrane exposed to viral deposition without the need for mucosal trauma (Figure 2b). The subsequent steps culminating in malignant transformation of the HPV-infected basal cells are not well understood, but the microanatomy of the crypt epithelium may contribute to certain clinical characteristics of HPV-related tonsillar cancer such as the propensity of even small carcinomas to present as advanced regional metastases (33). Tumor origin from the deeply penetrant crypts conceals the presence of premalignant lesions, and discontinuity of the basement membrane may facilitate early invasion and metastasis of occult cancers.
HISTOPATHOLOGY OF HEAD AND NECK SQUAMOUS CELL CARCINOMA
Squamous dysplasia refers to neoplastic alterations of the surface epithelium prior to invasion of the subepithelial connective tissues. These changes include abnormal cellular organization, increased mitotic activity, and nuclear enlargement with pleomorphism. These alterations are typically graded on a scale of 1 to 3 based on the severity of the atypia. Although terminology varies, atypia limited to the lower one-third of the epithelium is generally referred to as mild dysplasia, atypia limited to the lower two-thirds as moderate dysplasia, and atypia involving the full thickness of the epithelium as severe dysplasia/carcinoma in situ. With progression, the carcinoma in situ breaks through the basement membrane and infiltrates the subepithelial connective tissue as cohesive nests and cords. With advanced tumor growth, nests of invasive tumor invade skeletal muscle, craniofacial bones, and facial skin. Invasion may be associated with tumor extension along nerves (i.e., perineural invasion) and involvement of lymphatic spaces.
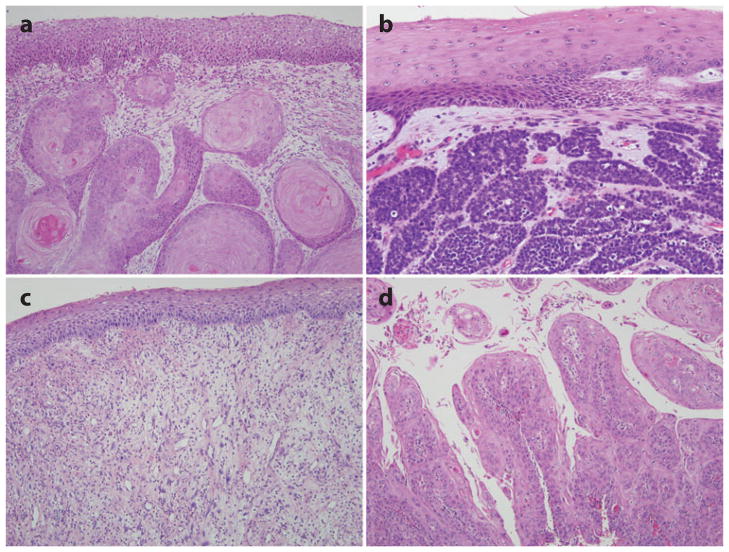
The microscopic appearance may vary as a function of tumor differentiation, but the prototypic HNSCC is moderately differentiated (Figure 3a). Figure 3 illustrates the subtypes of HNSCC. The spindle-cell variant is characterized by the proliferation of noncohesive spindle cells. Its microscopic appearance more closely resembles a sarcoma than a carcinoma. Verrucous carcinoma is seen clinically as an exophytic mass with a warty or papillary surface and is observed histologically as a markedly thickened squamous epithelium with “church spires” of parakerototic squamous cells. In contrast to conventional squamous cell carcinoma, verrucous carcinoma invades as a burrowing mass with broad pushing borders, lacks any significant atypia, and has no potential to metastasize. The papillary variant is characterized by a prominent exophytic component of papillary growth. In contrast to the benign squamous papilloma, the papillary fronds are lined by overtly malignant squamous cells.
Figure 3.
Histologic features of head and neck squamous cell carcinoma (HNSCC). The prototypic HNSCC is characterized by nests of squamous cells with pink cytoplasm, intercellular bridges and keratin pearl formation set in a background of stromal fibrosis (a). Subtypes of HNSCC include the basaloid variant (b), the spindle-cell variant (c), and the papillary variant (d ).
The basaloid squamous variant is identified as a distinct subtype of HNSCC based on its striking basaloid morphology (e.g., solid lobules of cells with peripheral pallisading, scant cytoplasm, and dark nuclei) and its highly aggressive behavior (Figure 3b) (34). It is generally regarded as an aggressive rapidly growing tumor associated with poor patient outcomes. The identity of basaloid squamous cell carcinoma as a distinct subtype of HNSCC has recently been blurred by descriptions of those HNSCCs caused by HPV that share this basaloid appearance (17, 30). Indeed, a recent study has shown that the basaloid squamous cell carcinoma is a mixed group of HPV-16-positive and HPV-16-negative carcinomas (35). The distinction is important, as HPV-16 status was found to have a profound influence on patient survival. The absence of HPV-16 was significantly associated with decreased overall survival even though patients with HPV-16-positive carcinomas were more likely to present with lymph node metastases. These HPV-positive cancers underscore the concept that distinct molecular genetic alterations drive morphologic divergence among HNSCCs and underlie differences in biologic behavior (Table 1).
Table 1.
Major differences between head and neck squamous cell carcinomas (HNSCCs) by human papillomavirus (HPV) status
| HPV-positive HNSCC | HPV-negative HNSCC | |
|---|---|---|
| Risk factors | High-risk sexual practices | Cigarette and alcohol use |
| Tumor site | Lingual and palatine tonsils | Nonoropharyngeal sites |
| Histopathology | Basaloid, nonkeratinizing, poorly differentiated | Keratinizing, moderately differentiated |
| Molecular genetic alterations | ||
| P53 pathway disturbances | Degradation of wt p53 by E6 | TP53 mutations, 17p LOH |
| Rb pathway disturbances | Degradation of wt Rb by E7 | p16INK4A-promoter hypermethylation, 9p LOH |
| Relative responsiveness to chemoradiation | Better | Worse |
| Relative prognosis | Improved | Worse |
Abbreviations: HPV, human papillomavirus; LOH, loss of heterozygosity; Rb, retinoblastoma.
GENETIC MODEL OF HEAD AND NECK SQUAMOUS CELL CARCINOMA TUMORIGENESIS
True to the current model of tumorigenesis (36), the initiation and progression of head and neck cancer is a complex multistep process that entails a progressive acquisition of genetic and epigenetic alterations. An early model of head and neck tumorigenesis based on microsatellite analysis of allelic imbalances showed that (a) the number of acquired genetic alterations progressively increases from squamous hyperplasia through graded dysplasia to invasive carcinoma; (b) this progressive accumulation of genetic alterations loosely follows a sequential order; (c) chromosomal loss and gain preferentially target critical components of key genetic pathways regulating cell growth, motility, and stromal interactions; and (d ) genetic damage often precedes microscopic changes (37) (Figure 4).
Figure 4.
Genetic progression model of head and neck tumorigenesis. Clinical and histologic progression from simple squamous hyperplasia through the advancing stages of squamous dysplasia to invasive squamous cell carcinoma is driven by the progressive accumulation of genetic alterations. Some alterations, such as loss of heterozygosity (LOH) at chromosomal loci 3p and 9p, occur earlier in this sequence than do other alterations. Figure illustrated by Robert Morreale, CMI and contributed by Joseph Califano, MD. Reproduced with permission from New England Journal of Medicine.
The TP53 and retinoblastoma (Rb) pathways are almost universally disrupted in HNSCCs, indicating the importance of these pathways in head and neck tumorigenesis. The TP53 pathway controls cell growth by regulating cell-cycle progression and responses to stress via apoptosis. Over 50% of HNSCCs harbor inactivating TP53 gene mutations (38–40), and over 50% demonstrate chromosomal loss at 17p—the site where the TP53 gene resides. The most-targeted component of the Rb pathway is the p16INK4A tumor-suppressor gene. Its gene product prohibits cells from entering the cell cycle by inhibiting the cyclin-dependent kinases 4 and 6. Inactivation of p16INK4A can occur by any combination of promoter hypermethylation, gene mutation, and loss of heterozygosity (LOH). Indeed, LOH at chromosomal region 9p21 (where p16INK4A resides) occurs in up 80% of HNSCCs.
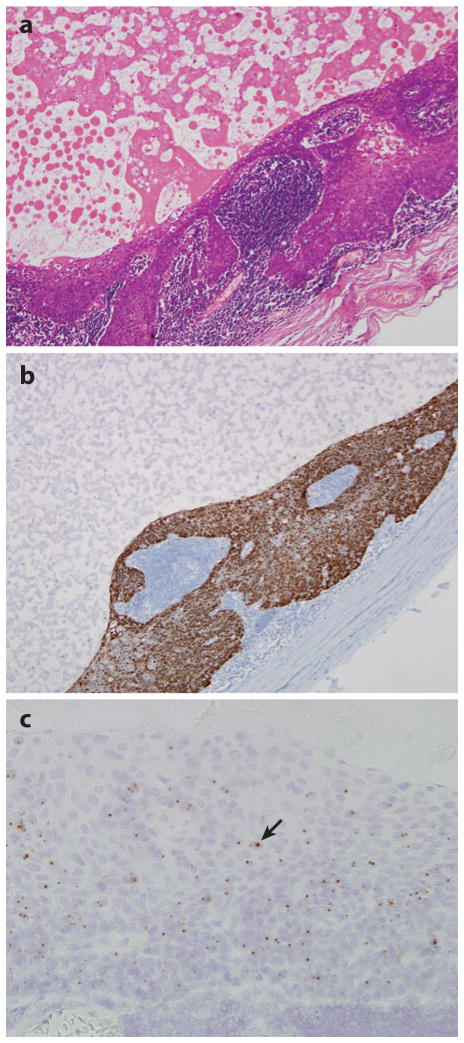
The absence of TP53 and p16INK4A silencing in a subset of HNSCCs points to the presence of alternative mechanisms of TP53 and Rb pathway disruption. Most notably, abrogation of p53 function can be mediated by degradation of wild-type p53 protein by the HPV viral oncoprotein E6 (41, 42). Indeed, HPV-positive HNSCCs are much less likely to harbor a TP53 mutation than HPV-16-negative HNSCCs (17, 43, 44). Although HPV-16 infection and p53 mutations coexist in a small subset of HNSCCs, the observed TP53 mutations are not the type that severely disrupt DNA binding and are associated with poor clinical outcomes (38, 45). Similarly, expression of the viral oncoprotein E7 binds and degrades wild-type Rb protein, rendering upstream inactivation of p16INK4A unnecessary in HPV-positive HN-SCCs (43). In sharp contrast to HPV-negative cancers, HPV-positive cancers consistently express wild-type p16 protein, such that immunohistochemical detection of p16 protein expression can be used to discern the HPV status of HNSCCs (Figure 5) (31, 46). The recognition of divergent molecular genetic profiles compelled by distinct environmental exposures supports an emerging view that HNSCCs form a heterogeneous group of cancers that can be categorized by HPV status.
Figure 5.
Metastatic head and neck squamous cell carcinoma from an unknown primary involving a cervical lymph node. This cystic metastasis with hematoxylin and eosin stain (a) shows P16 overexpression by immunohistochemical staining (b), andHPV-16 in-situ hybridization (c) demonstrates the presence of punctuate hybridization signals within the nuclei of tumor cells (arrow). The presence ofHPV- 16 points to the lingual or palatine tonsil as a highly likely site of tumor origin.
FIELD CANCERIZATION
Second tumors of the aerodigestive tract have a sobering effect on the outlook for patients with HNSCC. Often fatal, these tumors develop in the head and neck, lungs, or esophagus of 10–40% of patients with HNSCC (47, 48). One explanation for this multifocal tumor origin was proposed more than 40 years ago by Slaughter (49). According to his field cancerization concept, multiple cell groups independently undergo neoplastic transformation under the stress of regional carcinogenic activity. Molecular genetic approaches have recently challenged the notion that independent transforming events are commonplace in the epithelium of patients with HNSCC. Indeed, when a primary HNSCC is compared with a second tumor elsewhere in the respiratory tract, the paired tumors often harbor identical patterns of genetic alterations (50–53). Presumably, a critical genetic alteration in a single cell provides a growth advantage over its neighboring cells. At some point after transformation, cells harboring these early genetic alterations migrate to populate contiguous tracts of mucosa, accumulate other alterations, acquire additional growth advantages, and ultimately transform into aggressive subclones separated by time and space (54).
Importantly, collective observations have supported the view that the epithelium of the upper respiratory tract may become populated by these clones of genetically damaged cells yet may lack histopathologic evidence of dysplasia (55, 56). The presence of morphologically intact but genetically damaged cells not only explains the phenomenon of field cancerization but accounts for certain distressing patterns of tumor behavior, such as local tumor recurrence following seemingly complete surgical resection. A growing lack of confidence in the pathologist’s ability to recognize the presence and extent of the neoplastic process in patients at risk for HNSCC has accelerated a search for novel biomarkers in the recognition and treatment of HNSCC.
BIOMARKERS AND THEIR APPLICATION IN THE CLINICAL ARENA
A biomarker is a biochemical, molecular, or genetic parameter that can be objectively measured and evaluated to discern the presence and progress of disease. With the onset of the molecular revolution, the armament of potential biomarkers has been greatly expanded as our understanding of the molecular pathways involved in tumor initiation and progression improves (Table 2). In the past, biomarkers have been used primarily as prognostic indicators for patients with HNSCC. More recently, the role of biomarkers has been greatly expanded to address all aspects of patient care, from early cancer detection, to more accurate tumor staging, to the selection of those patients most likely to benefit from specific therapies, to posttreatment tumor surveillance.
Table 2.
Potential biomarkers in head and neck cancers
| Major Classes | Member | Function |
|---|---|---|
| Cell-cycle regulation | p16INK4A | A tumor-suppressor gene regulating senescence and cell-cycle progression |
| TP53 | A tumor-suppressor gene regulating cell-cycle progression and cell survival | |
| PTEN | A tumor-suppressor gene regulating signaling pathways controlling cell proliferation and apoptosis | |
| Rb | A tumor-suppressor gene regulating cell-cycle progression and apoptosis | |
| Cyclin D1 | A proto-oncogene regulating cell-cycle progression | |
| Signal transduction | EGFR | A transmembrane TK that acts as a central transducer of multiple signaling pathways |
| VEGF | A transmembrane TK that promotes the proliferation, migration, and survival of endothelial cells during tumor growth | |
| Extracellular matrix degradation | MMPs | A family of zinc-dependent proteolytic enzymes that degrade the basement membrane and other components of the extracellular matrix |
| Prostaglandin metabolism | Cox-2 | A catalytic enzyme that decreases apoptosis, increases inflammation and immunosuppression, and enhances the potential for tumor progression |
| Oncoviruses | EBV | A causative agent for most nasopharyngeal carcinomas |
| HPV | A causative agent for most oropharyngeal cancers |
Abbreviations: EBV, Epstein-Barr virus; EGFR, epidermal growth factor receptor; HPV, human papillomavirus; MMPs, matrix metalloproteinases; Rb, retinoblastoma; TK, tyrosine kinase; TP53, tumor protein 53; VEGF, vascular endothelial growth factor.
Identification of High-Risk Premalignant Lesions
Although premalignant lesions (i.e., dysplasias) of the upper aerodigestive tract are at risk of progressing to overtly malignant HNSCCs, the measurement of histologic parameters to assess the likelihood of progression is fraught with difficulties: The histopathologic features of pre-malignant lesions can be subtle and can overlap with nonneoplastic reactive processes, and there is considerable variation among pathologists in the recognition and grading of premalignant lesions. Among the biomarkers that may help identify those precursor lesions most likely to develop into HNSCCs, LOH at defined chromosomal loci may be the most promising. Several studies on oral dysplasias have shown that dual LOH at 3p and 9p can reliably distinguish those lesions that are likely to progress to invasive carcinoma from those that will not (55, 57, 58).
Detection of Undiagnosed Head and Neck Squamous Cell Carcinoma
One of the more promising breakthroughs regarding early cancer diagnosis has been the ability to use saliva as a substrate for biomarker assessment. Saliva has been used as a noninvasive, inexpensive, and readily accessible diagnostic substrate to assess diverse biomarkers including HPV status (59, 60), promoter hypermethylation profiles (61, 62), TP53 gene mutations (63), telomerase activity (39), and differential gene expression profiles (64). For patients with oral HNSCCs, the methylation profiles and HPV status of their tumors can be discerned from molecular genetic analysis of their oral washes. Despite the documented feasibility of saliva-based strategies, early-detection saliva assays have thus far failed to yield a high enough sensitivity and specificity for broad population-based screening.
Tumor localization
A significant subset of patients with HNSCC present with metastatic spread to cervical lymph nodes in the absence of a primary tumor by clinical, radiographic, endoscopic, and even histopathologic evaluation. Biomarkers can be used to help pinpoint the primary site of tumor origin (65). One biomarker strategy takes advantage of the fact that some HNSCCs are caused by certain oncogenic viruses that target specific regions of the upper aerodigestive tract (66). In effect, detection of a specific virus in the metastasis implicates the site of tumor origin. Most notably, detection of Epstein-Barr virus in a neck metastasis reliably points to tumor origin from the nasopharynx. Although this approach is readily feasible for diagnostic laboratories with in situ hybridization capabilities, thus far application has been limited as only a few HNSCCs have been linked to a tumorigenic virus. The application of this approach has been expanded with the recognition that HPV-16 is an important causative factor for HNSCCs arising from the oropharynx but not for HNSCCs arising from nonoropharyngeal sites. In situ hybridization detection of HPV-16 in a metastatic implant reliably points to the oropharynx as the site of tumor origin (Figure 5) (46, 67). Significantly, HPV status can be determined from individual cells aspirated from metastatic implants (67).
Tumor extent, including its relationship to surgical margins
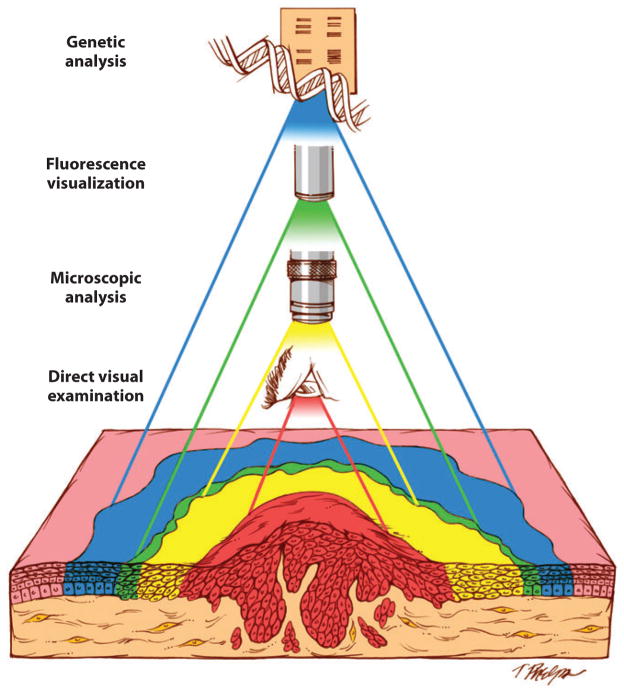
Time-honored methods that rely on clinical inspection and microscopic examination to detect the extent of the neoplastic process have been frustratingly ineffective when it comes to cancer of the head and neck. Inexplicably, excised tumors can recur in the face of microscopically free margins; patients effectively treated for oral cancer at one site can develop second tumors at other sites; and premalignant lesions that regress during chemoprevention can reappear and progress once therapy is halted. Clearly, novel methods are required to permit better mapping of this elusive cancer spread (Figure 6). Assisted visualization of oral neoplasia in the intraoperative setting is being developed along several lines. Some researchers have advocated staining the oral mucosa with the vital dye toluidine blue as a means of visualizing the subclinical disease. Abnormal staining of the oral mucosa has been shown to correlate with the presence of histologic dysplasia, the presence of LOH, and increased cancer risk (68, 69). Others have explored visual assessment of oral cavity luminescence under blue excitation light (400–460 nm) or following a chemical reaction (i.e., chemiluminescence) as a means of discriminating between normal and neoplastic mucosa (70, 71). Although the precise mechanisms are not well understood, changes in the metabolic activity of the surface epithelial cells induce spectral changes that impart tissue fluorescence signatures. At certain wavelengths, autofluorescence visualization of the oral mucosa provides a more complete topographical view of the neoplastic field, including its spatial relationship to surgical margins (Figure 6) (72).
Figure 6.
Invasive head and neck squamous cell carcinomas (HNSCCs) arise from genetically altered cells distributed throughout tracts of the mucosa. The aim of surgery is to remove the entire neoplastic field, but the ability to discern its boundaries in the operating room depends on the method of detection. Direct visualization examination (red zone) underestimates the full extent of histologic alterations as detected by intraoperative frozen section histology ( yellow zone). Fluorescence visualization ( green zone) is a simple, real-time method for visualizing optic changes associated with subclinical premalignant disease. The boundaries established by fluorescence visualization may even extend beyond the zone of histologic changes to more fully encompass the field of genetic alterations (blue zone). Figure reproduced with permission from Clinical Cancer Research.
The traditional definition of a negative surgical margin now demands redefinition in light of the propensity of genetically damaged cells to populate extended tracts of histologically normal oral mucosa. Indeed, the presence of genetically altered cells can be detected in histologically normal mucosal margins using a variety of strategies for detecting genetic alterations including TP53 mutations (73, 74), LOH (75), promoter hypermethylation (76, 77), and eIF4E proto-oncogene overexpression (78). Importantly, the presence of these genetically damaged cells has been shown to predict local tumor recurrence in patients who have undergone tumor resections with histologically clear margins. Unlike direct visualization techniques using vital dyes or autofluorescence, molecular genetic analysis does not provide instantaneous results. Nonetheless, automation and streamlining of methodologies is dramatically decreasing turnaround times, even to the point that genetic analysis of surgical margins may become feasible in the intraoperative setting (77, 79).
Prognostication
Despite a bewilderingly large body of studies evaluating the prognostic significance of cell proliferation (e.g., Ki67, PCNA), p53 immunohistochemical staining, apoptosis, aneuploidy, epidermal growth factor receptor (EGFR) overexpression, and many other biomarkers, none has consistently proved reliable across multiple studies, and none is currently used in routine surgical pathology practice.
The modest prognostic impact of currently used biomarkers is not altogether surprising, given the complexity of HNSCC tumorigenesis requiring the concerted actions of multiple genetic alterations and the differential impact of various gene-altering events. For example, about 50% of HNSCCs harbor TP53 gene mutations, but these mutations are highly divergent in their impact on p53 protein structure, stability, DNA binding properties, and clinical outcome, depending on where they occur (38). TP53 mutational analysis is apparently a much more powerful prognostic indicator when clinical outcomes are measured against the specific type of TP53 mutation. The TP53 mutations that occur within the core domain completely blocking DNA binding have been correlated with accelerated tumor progression, reduced therapeutic responsiveness, and decreased patient survival compared to HNSCCs that harbor less disruptive TP53 mutations (38, 80).
The presence of HPV-16 is now recognized as a highly favorable prognostic indicator for patients with HNSCC. Compared with patients with HPV-negative tumors, those with HPV-positive tumors have a lower risk of tumor progression and death (17, 81). In a recent prospective multi-institutional study of patients with HNSCCs uniformly treated with induction chemotherapy and radiation therapy, patients with HPV-positive tumors had a risk of progression that was 73% lower and a risk of death that was 64% lower than patients with HPV-negative tumors (82). The mechanisms underlying these clinical differences may involve the combined effects of immune surveillance to viral-specific tumor antigens, an intact apoptotic response to radiation, and the absence of widespread genetic alterations (i.e., field cancerization).
Patient selection for molecular-targeted therapy
Molecular-targeted therapies for HNSCC are rapidly becoming a reality. A number of phase III trials in patients with advanced HNSCC are already underway. The most promising of these drugs target EGFR (see below). Trial design now rests on the use of appropriate biomarkers to effectively identify those patients who will benefit from EGFR-targeted therapy. Confirmation of oncogenic HPV as an important causative agent in oropharyngeal cancer has opened the door for vaccine immunotherapy (see the following section). Determination of HPV status will soon be a routine part of the pathologic evaluation of HNSCCs, at least for those cancers arising from the oropharynx.
NOVEL STRATEGIES IN THE TREATMENT OF PATIENTS WITH HEAD AND NECK SQUAMOUS CELL CARCINOMA
Targeting the Epidermal Growth Factor Receptor Pathway
A growing understanding of the molecular genetic underpinning of HNSCC now permits the development of novel therapies that target specific components of the molecular genetic apparatus supporting tumor development and growth. One particularly intriguing target is EGFR—a transmembrane tyrosine kinase receptor that acts as a central transducer of multiple signaling pathways involved in tumor cell growth, invasion, angiogenesis, and invasion (83). In HNSCC, EGFR overexpression has been associated with a high local recurrence rate and poor survival (84–86). Although more than 90% of HNSCCs overexpress EGFR, only a small subset of HNSCCs demonstrate amplified copy numbers or mutational activation of the EGFR gene (83). Instead, EGFR activation in HNSCC is driven in part by high expression of its ligands, resulting in the formation of powerful autocrine and paracrine loops. Binding of ligands to EGFR induces dimerization of EGFR, autophosphorylation of its intracellular kinase domain, and activation of multiple oncogenic pathways.
Targeted therapy has taken aim at different points along this signal transduction sequence in an effort to blockade EGFR function. Monoclonal antibodies (e.g., cetuximab) directed against the extracellular receptor domain seek to block ligand binding, prevent receptor dimerization, induce receptor degradation, and activate antitumoral immune responses. Tyrosine kinase inhibitors (e.g., erlotinib, gefitnib) are small molecules that interact with the cellular domain of EGFR to inhibit its phosphorylation function. EGFR gene silencing can be accomplished by various posttranscriptional strategies including the use of sequence-specific anti-sense oligodeoxynucleotides and small interfering RNAs. Antisense oligodeoxynucleotides are antisense DNA strands that bind complementary EGFR mRNA and block protein synthesis. Small interfering RNAs are short double-stranded RNAs that bind a sequence-specific mRNA and trigger its destruction via the RNA interference pathway.
Despite the importance of overexpression in head and neck tumorigenesis, EGFR blockade as monotherapy has been only modestly successful in the treatment of patients with HNSCC (87–89). The limited success of monotherapy is not altogether surprising given the complexity and divergency of signaling pathways involved in tumor growth, invasion, and metastasis. Accordingly, current strategies seek to combine EGFR blockade with other traditional and nontraditional treatment modalities including the blockade of EGFR-independent signaling pathways. Cetuximab used in combination with radiation therapy has recently been established as an effective regimen for improving locoregional control and survival in patients with locoregionally advanced HNSCC (90, 91). Additional trials suggest that cetuximab and other EGFR inhibitors may similarly enhance the effects of platinum-based chemotherapy (92–94). Ongoing efforts to optimize EGFR blockade are combining agents with specific but nonoverlapping anti-EGFR activity, such as the combination of anti-EGFR monoclonal antibodies and tyro-sine kinase inhibitors (95). Another strategy involves the concomitant targeting of other signaling pathways that are independent of the EFGR pathway or that may intersect the EGFR pathway network. As one example, resistance to EGFR has been attributed, in part, to increased levels of vascular endothelial growth factor (VEGF). This finding, in turn, has generated interest in the use of dual inhibitors of both the EGFR and VEGF receptors (96).
Vaccine Strategies
The finding of HPV-16 in a distinct subset of HNSCCs provides a unique opportunity for the prevention and treatment of these cancers by vaccines designed to induce appropriate HPV virus–specific immune responses (97). The aim of a preventive vaccine is to prime the immune system so that it is able to induce high titers of neutralizing antibody upon exposure to a high-risk HPV to block cellular entry and prevent the development of an established infection. The ultimate prophylactic vaccine must: (a) prime an antibody response that is specific for neutralizing epitopes on HPV, (b) induce an immune response that is long lasting, and (c) impede infection at the point of HPV contact: the tonsillar crypts (98). A major breakthrough in preventive HPV vaccines came with the demonstration the HPV capsid proteins can self-assemble into virus-like particles (VLPs). These VLPs, in turn, can elicit high titers of neutralizing antibodies that inhibit the early stages of HPV cell binding and cell entry.
Therapeutic HPV vaccines aim to clear virally infected cells, inhibit progression of HPV-associated dysplasia, and eradicate established neoplasms. For established infections, utilization of T cell–mediated immunity against nonstructural viral antigens appears to be a more effective strategy in clearing infections and eradicating tumors. The HPV E6 and E7 proteins are ideal antigens for targeted immunotherapy: (a) The E6 and E7 proteins are uniquely expressed by tumor cells but not by normal tissues, (b) HPV-associated carcinoma represent 20–25% of all HNSCC and 60–70% of HNSCCs arising in the oropharynx, (c) constitutive expression of E6 and E7 is requisite for maintenance of the tumor phenotype such that evasion of immune responses is not likely to occur by E6/E7 downregulation, and (d ) these viral proteins are completely foreign and highly immunogenic.
A variety of HPV vaccines are under investigation including viral vector vaccines, bacterial vector vaccines, peptide/protein vaccines, DNA vaccines, and cell based vaccines. Preliminary data with an HPV DNA vaccine indicates that it is well tolerated in all patients, it is associated with minimal toxicity, and it generates significant levels of circulating HPV E7-specific immune cells postvacci-nation. Now that early phase clinical trials have demonstrated the safety and feasibility of DNA based HPV vaccines, efforts are now underway to enhance potency by optimizing delivery, frequency of immunizations, coadministration with adjuvant immune-enhancing agents, and two-staged delivery programs (i.e., prime-boost vaccines) (99–101).
In June 2006, the US Food and Drug Administration approved the HPV vaccine GardasilTM as an effective method of preventing cervical cancer and precancerous lesions due to HPV types 6, 11, 16, and 18. The Centers for Disease Control recommends routine vaccination of girls and young women as a means of reducing cases of cervical cancer. The growing recognition of HPV-related HNSCCs in women and men underscores the reality that HPV-induced neoplasia is not gender specific. HPV-related malignancies of all types, including HNSCCs, are expected to retreat in the face of effective HPV prophylaxis. Accordingly, HPV vaccination will likely be recommended for all individuals, boys and girls alike, prior to onset of sexual activity. Clinical trials are already under way using HPV vaccines in the treatment of HPV-associated HNSCCs. Based on animal models, HPV vaccines appear to be most effective for the eradication of low-volume disease. Accordingly, therapeutic vaccines may be most effective not as a monotherapy, but as adjunctive therapy to clear residual microscopic disease following conventional surgical excision and/or radiation.
CONCLUSIONS
Reflecting to some degree the limitations of conventional microscopy, HNSCCs have long been regarded as a tedious group with little variation from tumor to tumor. This notion, in turn, has fostered a one-size-fits-all attitude toward the management of patients with HNSCC. Today, molecular genetic analysis allows us to probe beneath the phenotypic surface to the underlying etiologic abnormalities. These new approaches are helping to elucidate long-recognized but poorly understood biologic concepts such as field cancerization and are helping to explain perplexing clinical patterns such as local tumor recurrence following seemingly complete resection. Analysis of the molecular genetic changes of HNSCCs discloses not just individual tumor differences, but also consistent large-scale differences that permit the recognition of important subtypes of HNSCCs (e.g., HPV-positive versus HPV-negative HNSCCs). An appreciation of these differences is critical to novel treatment strategies that aim to enhance immunologic responses to tumor-specific antigens and to target individual components of the molecular genetic apparatus supporting tumor development and growth.
SUMMARY POINTS.
HNSCCs are generally defined as malignant tumors that arise from the epithelium lining the sinonasal tract, oral cavity, pharynx, and larynx and show microscopic evidence of squamous differentiation.
Incident trends for HNSCC have generally paralleled smoking trends. The escalating incidence of oropharyngeal carcinoma in the absence of a parallel rise in smoking and alcohol consumption suggests that nontraditional behavioral and environmental factors are driving this aberration.
HPV, particularly type 16, has been established as a causative agent in up to 70% of oropharyngeal cancers. These HPV-positive HNSCCs differ in important respects from HPV-negative HNSCC.
Head and neck tumorigenesis is a multistep process that involves the accumulation of multiple genetic and epigenetic alterations. Clones of phenotypically intact but genetically damaged cells can populate extended tracts of the mucosa to give rise to second tumors.
The TP53 and Rb pathways regulating cell growth are frequently disrupted during head and neck tumorigenesis. The mechanisms of pathway disruption, however, may vary. In HPV-positive HNSCCs, the viral oncoproteins E6 and E7 bind and degrade wild-type p53 and Rb proteins, respectively. There is little need for the genetic and epigenetic silencing of the TP53 and p16INK4A genes, which occurs in most HPV-negative HNSCCs.
Unraveling the molecular genetic underpinning of HNSCC may help identify biomarkers to measure the presence, extent, and progress of disease. For example, dual LOH on 3p and 9p helps distinguish premalignant lesions likely to progress to invasive carcinoma from those lesions not likely to progress. The presence of HPV in lymph node metastases points to tumor origin from the oropharynx. The detection of TP53 mutations and other genetic alterations at the surgical margins is predictive of site-specific tumor recurrence. The presence of a highly disruptive TP53 mutation that blocks DNA binding is associated with poor patient outcome, whereas the presence of HPV is associated with improved patient outcome.
Over 90% of HNSCC overexpress EGFR, a central transducer of multiple signaling pathways involved in tumor cell growth, invasion, angiogenesis, and invasion. Targeted therapy takes aim at different points along this signal transduction sequence in an effort to blockade EGFR function. EGFR blockade may be most successful when used in combination with other treatment modalities, including inhibition of other signaling pathways.
FUTURE DIRECTIONS.
A more thorough understanding of the natural history of oropharyngeal HPV infection will provide a more rational approach for individual risk assessment and for the development of preventive and therapeutic vaccines.
Continuing advances in molecular genetic analysis will permit the resolution of specific molecular genetic profiles for each HNSCC. In turn, genetic profiling will permit individualized therapy targeting specific gene alterations and aberrant signaling pathways.
Characterization and standardization of biomarkers will be essential for assessing disease progression, particularly as novel therapeutic strategies utilizing tyrosine kinase inhibitors and other targeted therapies move into phase III clinical trials.
Glossary
- HPV
human papillomavirus
- Squamous dysplasia
morphologically and genetically altered squamous epithelium with an increased likelihood for progression to squamous cell carcinoma
- LOH
loss of heterozygosity
- Field cancerization
the observation that under the stress of prolonged exposure to carcinogens, the mucosa of the upper aerodigestive tract becomes preconditioned to undergo malignant transformation at multiple sites to give rise to multiple cancers
- Biomarker
a biochemical, molecular, or genetic parameter that can be objectively measured or evaluated to discern the presence and progress of disease
- EGFR
epidermal growth factor receptor
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Contributor Information
Sara I. Pai, Email: spai1@jhmi.edu.
William H. Westra, Email: wwestra@jhmi.edu.
LITERATURE CITED
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114:806–16. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 4.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus–associated cancers? Cancer. 2007;110:1429–35. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 5.Hammarstedt L, Dahlstrand H, Lindquist D, Onelov L, Ryott M, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127:988–92. doi: 10.1080/00016480601110170. [DOI] [PubMed] [Google Scholar]
- 6.Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, et al. Human papil-lomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–23. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 7.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–49. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 8.Garden AS, Harris J, Vokes EE, Forastiere AA, Ridge JA, et al. Preliminary results of Radiation Therapy Oncology Group 97–03: a randomized phase II trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol. 2004;22:2856–64. doi: 10.1200/JCO.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Harris J, Garden AS, Trotti A, Jones CU, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: radiation therapy oncology group phase II trial 99–14. J Clin Oncol. 2005;23:3008–15. doi: 10.1200/JCO.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 10.Schlecht NF, Franco EL, Pintos J, Kowalski LP. Effect of smoking cessation and tobacco type on the risk of cancers of the upper aero-digestive tract in Brazil. Epidemiology. 1999;10:412–18. doi: 10.1097/00001648-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Zhang ZF, Morgenstern H, Spitz MR, Tashkin DP, Yu GP, et al. Environmental tobacco smoking, mutagen sensitivity, and head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2000;9:1043–49. [PubMed] [Google Scholar]
- 12.Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–17. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 13.Ho T, Wei Q, Sturgis EM. Epidemiology of carcinogen metabolism genes and risk of squamous cell carcinoma of the head and neck. Head Neck. 2007;29:682–99. doi: 10.1002/hed.20570. [DOI] [PubMed] [Google Scholar]
- 14.Sturgis EM, Wei Q, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Semin Oncol. 2004;31:726–33. doi: 10.1053/j.seminoncol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Talamini R, Bosetti C, La Vecchia C, Dal Maso L, Levi F, et al. Combined effect of tobacco and alcohol on laryngeal cancer risk: a case-control study. Cancer Causes Control. 2002;13:957–64. doi: 10.1023/a:1021944123914. [DOI] [PubMed] [Google Scholar]
- 16.Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–93. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 18.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694–99. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 19.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 21.Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108:766–72. doi: 10.1002/ijc.11633. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza G, Fakhry C, Sugar EA, Seaberg EC, Weber K, et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007;121:143–50. doi: 10.1002/ijc.22667. [DOI] [PubMed] [Google Scholar]
- 23.Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189:686–98. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 24.Gillison ML, D’Souza G, Westra WH, Sugar E, Xiao W, et al. Distinct risk factor profiles for human papillomavirus–16 positive and negative head and neck cancer. J Natl Cancer Inst. 2008 doi: 10.1093/jnci/djn025. In press. [DOI] [PubMed] [Google Scholar]
- 25.Berglund BA, Boring DL, Howlett AC. Investigation of structural analogs of prostaglandin amides for binding to and activation of CB1 and CB2 cannabinoid receptors in rat brain and human tonsils. Adv Exp Med Biol. 1999;469:527–33. doi: 10.1007/978-1-4615-4793-8_77. [DOI] [PubMed] [Google Scholar]
- 26.Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, et al. δ-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor–mediated, cytokine-dependent pathway. J Immunol. 2000;165:373–80. doi: 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]
- 27.McKallip RJ, Nagarkatti M, Nagarkatti PS. δ-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol. 2005;174:3281–89. doi: 10.4049/jimmunol.174.6.3281. [DOI] [PubMed] [Google Scholar]
- 28.Abbey K, Kawabata I. Computerized three-dimensional reconstruction of the crypt system of the palatine tonsil. Acta Otolaryngol Suppl. 1988;454:39–42. doi: 10.3109/00016488809125002. [DOI] [PubMed] [Google Scholar]
- 29.Perry ME. The specialised structure of crypt epithelium in the human palatine tonsil and its functional significance. J Anat. 1994;185:111–27. [PMC free article] [PubMed] [Google Scholar]
- 30.El Mofty SK, Patil S. Human papillomavirus (HPV)–related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:339–45. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Klussmann JP, Gultekin E, Weissenborn SJ, Wieland U, Dries V, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–53. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–61. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 33.McQuone SJ, Eisele DW, Lee DJ, Westra WH, Koch WM. Occult tonsillar carcinoma in the unknown primary. Laryngoscope. 1998;108:1605–10. doi: 10.1097/00005537-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol. 1986;17:1158–66. doi: 10.1016/s0046-8177(86)80422-1. [DOI] [PubMed] [Google Scholar]
- 35.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Mod Pathol. 2008;21:231. doi: 10.1097/PAS.0b013e31816380ec. (Abstr.) [DOI] [PubMed] [Google Scholar]
- 36.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 37.Califano J, Van Der Riet P, Westra WH, Nawroz H, Clayman G, et al. Genetic progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996;56:2488–92. [PubMed] [Google Scholar]
- 38.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–61. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Califano J, Ahrendt SA, Meininger G, Westra WH, Koch WM, Sidransky D. Detection of telom-erase activity in oral rinses from head and neck squamous cell carcinoma patients. Cancer Res. 1996;56:5720–22. [PubMed] [Google Scholar]
- 40.Gonzalez MV, Pello MF, Lopez-Larrea C, Suarez C, Menendez MJ, Coto E. Loss of heterozygosity and mutation analysis of the p16 (9p21) and p53 (17p13) genes in squamous cell carcinoma of the head and neck. Clin Cancer Res. 1995;1:1043–49. [PubMed] [Google Scholar]
- 41.Olshan AF, Weissler MC, Pei H, Conway K. p53 mutations in head and neck cancer: new data and evaluation of mutational spectra. Cancer Epidemiol Biomarkers Prev. 1997;6:499–504. [PubMed] [Google Scholar]
- 42.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 43.Hafkamp HC, Speel EJ, Haesevoets A, Bot FJ, Dinjens WN, et al. A subset of head and neck squamous cell carcinomas exhibits integration of HPV 16/18 DNA and overexpression of p16INK4A and p53 in the absence of mutations in p53 exons 5–8. Int J Cancer. 2003;107:394–400. doi: 10.1002/ijc.11389. [DOI] [PubMed] [Google Scholar]
- 44.Scholes AG, Liloglou T, Snijders PJ, Hart CA, Jones AS, et al. p53 mutations in relation to human papillomavirus type 16 infection in squamous cell carcinomas of the head and neck. Int J Cancer. 1997;71:796–99. doi: 10.1002/(sici)1097-0215(19970529)71:5<796::aid-ijc17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus–16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:366–69. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 46.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003;9:6469–75. [PubMed] [Google Scholar]
- 47.Jones AS, Morar P, Phillips DE, Field JK, Husband D, Helliwell TR. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer. 1995;75:1343–53. doi: 10.1002/1097-0142(19950315)75:6<1343::aid-cncr2820750617>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz LH, Ozsahin M, Zhang GN, Touboul E, De Vataire F, et al. Synchronous and metachronous head and neck carcinomas. Cancer. 1994;74:1933–38. doi: 10.1002/1097-0142(19941001)74:7<1933::aid-cncr2820740718>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 49.Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer. 1953;6:953–68. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 50.Bedi GC, Westra WH, Gabrielson E, Koch W, Sidransky D. Multiple head and neck tumors: evidence for a common clonal origin. Cancer Res. 1996;56:2484–87. [PubMed] [Google Scholar]
- 51.Worsham MJ, Wolman SR, Carey TE, Zarbo RJ, Benninger MS, VanDyke DL. Common clonal origin of synchronous primary head and neck squamous cell carcinomas: analysis by tumor karyotypes and fluorescence in situ hybridization. Hum Pathol. 1995;26:251–61. doi: 10.1016/0046-8177(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 52.Scholes AGM, Woolgar JA, Boyle MA, Brown JS, Vaughan ED, et al. Synchronous oral carcinomas: independent or common clonal origin? Cancer Res. 1998;58:2003–6. [PubMed] [Google Scholar]
- 53.Leong PL, Banafsheh R, Koch WM, Reed A, Eisele D. Distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. J Natl Cancer Inst. 1998;90:972–77. doi: 10.1093/jnci/90.13.972. [DOI] [PubMed] [Google Scholar]
- 54.Califano J, Westra WH, Meininger G, Corio R, Koch WM, Sidransky D. Genetic progression and clonal relationship of recurrent premalignant head and neck lesions. Clin Cancer Res. 2000;6:347–52. [PubMed] [Google Scholar]
- 55.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2:682–85. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 56.Westra WH, Sidransky D. Phenotypic and genotypic disparity in premalignant lesions: of calm water and crocodiles. J Natl Cancer Inst. 1998;90:1500–1. doi: 10.1093/jnci/90.20.1500. [DOI] [PubMed] [Google Scholar]
- 57.Rosin MP, Cheng X, Poh C, Lam WL, Huang Y, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000;6:357–62. [PubMed] [Google Scholar]
- 58.Lee JJ, Hong WK, Hittelman WN, Mao L, Lotan R, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6:1702–10. [PubMed] [Google Scholar]
- 59.Zhao M, Rosenbaum E, Carvalho AL, Koch W, Jiang W, et al. Feasibility of quantitative PCR-based saliva rinse screening of HPV for head and neck cancer. Int J Cancer. 2005;117:605–10. doi: 10.1002/ijc.21216. [DOI] [PubMed] [Google Scholar]
- 60.Smith EM, Ritchie JM, Summersgill KF, Hoffman HT, Wang DH, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–55. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho AL, Jeronimo C, Kim MM, Henrique R, Zhang Z, et al. Evaluation of promoter hypermethylation detection in body fluids as a screening/diagnosis tool for head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:97–107. doi: 10.1158/1078-0432.CCR-07-0722. [DOI] [PubMed] [Google Scholar]
- 62.Rosas SL, Koch W, Costa Carvalho MG, Wu L, Califano J, et al. Promoter hypermethylation patterns of p16, O6-methylguanine-DNA-methyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res. 2001;61:939–42. [PubMed] [Google Scholar]
- 63.Boyle JO, Mao L, Brennan JA, Koch WM, Eisele DW, et al. Gene mutations in saliva as molecular markers for head and neck squamous cell carcinomas. Am J Surg. 1994;168:429–32. doi: 10.1016/s0002-9610(05)80092-3. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, St John MA, Zhou X, Kim Y, Sinha U, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442–50. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 65.Califano J, Westra WH, Koch W, Meininger G, Reed A, et al. Unknown primary head and neck squamous cell carcinoma: molecular identification of the site of origin. J Natl Cancer Inst. 1999;91:599–604. doi: 10.1093/jnci/91.7.599. [DOI] [PubMed] [Google Scholar]
- 66.Feinmesser R, Miyazaki I, Cheung R, Freeman JL, Noyek AM, Dosch HM. Diagnosis of nasopha-ryngeal carcinoma by DNA amplification of tissue obtained by fine-needle aspiration. N Engl J Med. 1992;326:17–21. doi: 10.1056/NEJM199201023260103. [DOI] [PubMed] [Google Scholar]
- 67.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus–16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:1186–91. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 68.Guo Z, Yamaguchi K, Sanchez-Cespedes M, Westra WH, Koch WM, Sidransky D. Allelic losses in OraTest-directed biopsies of patients with prior upper aerodigestive tract malignancy. Clin Cancer Res. 2001;7:1963–68. [PubMed] [Google Scholar]
- 69.Epstein JB, Zhang L, Poh C, Nakamura H, Berean K, Rosin M. Increased allelic loss in toluidine blue-positive oral premalignant lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:45–50. doi: 10.1067/moe.2003.97. [DOI] [PubMed] [Google Scholar]
- 70.Epstein JB, Gorsky M, Lonky S, Silverman S, Jr, Epstein JD, Bride M. The efficacy of oral lumenoscopy (ViziLite) in visualizing oral mucosal lesions. Spec Care Dentist. 2006;26:171–74. doi: 10.1111/j.1754-4505.2006.tb01720.x. [DOI] [PubMed] [Google Scholar]
- 71.Svistun E, Alizadeh-Naderi R, El Naggar A, Jacob R, Gillenwater A, Richards-Kortum R. Vision enhancement system for detection of oral cavity neoplasia based on autofluorescence. Head Neck. 2004;26:205–15. doi: 10.1002/hed.10381. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L, Williams M, Poh CF, Laronde D, Epstein JB, et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Res. 2005;65:8017–21. doi: 10.1158/0008-5472.CAN-04-3153. [DOI] [PubMed] [Google Scholar]
- 73.Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–35. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 74.van Houten VM, Leemans CR, Kummer JA, Dijkstra J, Kuik DJ, et al. Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients: a prospective study. Clin Cancer Res. 2004;10:3614–20. doi: 10.1158/1078-0432.CCR-03-0631. [DOI] [PubMed] [Google Scholar]
- 75.Sardi I, Franchi A, Ferriero G, Frittelli A, Bruschini L, et al. Prediction of recurrence by mi-crosatellite analysis in head and neck cancer. Genes Chromosom Cancer. 2000;29:201–6. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1031>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 76.Martone T, Gillio-Tos A, De Marco L, Fiano V, Maule M, et al. Association between hypermethylated tumor and paired surgical margins in head and neck squamous cell carcinomas. Clin Cancer Res. 2007;13:5089–94. doi: 10.1158/1078-0432.CCR-07-0119. [DOI] [PubMed] [Google Scholar]
- 77.Goldenberg D, Harden S, Masayesva BG, Ha P, Benoit N, et al. Intraoperative molecular margin analysis in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:39–44. doi: 10.1001/archotol.130.1.39. [DOI] [PubMed] [Google Scholar]
- 78.Nathan CO, Liu L, Li BD, Abreo FW, Nandy I, De Benedetti A. Detection of the proto-oncogene eIF4E in surgical margins may predict recurrence in head and neck cancer. Oncogene. 1997;15:579–84. doi: 10.1038/sj.onc.1201216. [DOI] [PubMed] [Google Scholar]
- 79.Harden SV, Thomas DC, Benoit N, Minhas K, Westra WH, et al. Real-time gap ligase chain reaction: a rapid semiquantitative assay for detecting p53 mutation at low levels in surgical margins and lymph nodes from resected lung and head and neck tumors. Clin Cancer Res. 2004;10:2379–85. doi: 10.1158/1078-0432.ccr-03-0405. [DOI] [PubMed] [Google Scholar]
- 80.Erber R, Conradt C, Homann N, Enders C, Finckh M, et al. TP53 DNA contact mutations are selectively associated with allelic loss and have a strong clinical impact in head and neck cancer. Oncogene. 1998;16:1671–79. doi: 10.1038/sj.onc.1201690. [DOI] [PubMed] [Google Scholar]
- 81.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–47. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 82.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, et al. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–69. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 83.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–72. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 84.Psyrri A, Yu Z, Weinberger PM, Sasaki C, Haffty B, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–62. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 85.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–56. [PubMed] [Google Scholar]
- 86.Rubin GJ, Melhem MF, Gooding WE, Day R, Holst VA, et al. Levels of TGF-α and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–32. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 87.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–77. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 88.Cohen EE, Kane MA, List MA, Brockstein BE, Mehrotra B. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11:8418–24. doi: 10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 89.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 90.Curran D, Giralt J, Harari PM, Ang KK, Cohen RB, et al. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol. 2007;25:2191–97. doi: 10.1200/JCO.2006.08.8005. [DOI] [PubMed] [Google Scholar]
- 91.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 92.Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–78. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- 93.Herbst RS, Arquette M, Shin DM, Dicke K, Vokes EE, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5578–87. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 94.Siu LL, Soulieres D, Chen EX, Pond GR, Chin SF, et al. Phase I/II trial of erlotinib and cisplatin in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a Princess Margaret Hospital phase II consortium and National Cancer Institute of Canada Clinical Trials Group Study. J Clin Oncol. 2007;25:2178–83. doi: 10.1200/JCO.2006.07.6547. [DOI] [PubMed] [Google Scholar]
- 95.Matar P, Rojo F, Cassia R, Moreno-Bueno G, Di Cosimo S, et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin Cancer Res. 2004;10:6487–501. doi: 10.1158/1078-0432.CCR-04-0870. [DOI] [PubMed] [Google Scholar]
- 96.Bozec A, Formento P, Lassalle S, Lippens C, Hofman P, Milano G. Dual inhibition of EGFR and VEGFR pathways in combination with irradiation: antitumour supra-additive effects on human head and neck cancer xenografts. Br J Cancer. 2007;97:65–72. doi: 10.1038/sj.bjc.6603791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Badaracco G, Venuti A. Human papillomavirus therapeutic vaccines in head and neck tumors. Expert Rev Anticancer Ther. 2007;7:753–66. doi: 10.1586/14737140.7.5.753. [DOI] [PubMed] [Google Scholar]
- 98.Devaraj K, Gillison ML, Wu TC. Development of HPV vaccines for HPV-associated head and neck squamous cell carcinoma. Crit Rev Oral Biol Med. 2003;14:345–62. doi: 10.1177/154411130301400505. [DOI] [PubMed] [Google Scholar]
- 99.Lin CT, Hung CF, Juang J, He L, Lin KY, et al. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies and antitumor effects of HPV-16 E7-expressing Sindbis virus replicon particles. Mol Ther. 2003;8:559–66. doi: 10.1016/s1525-0016(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 100.Mackova J, Stasikova J, Kutinova L, Masin J, Hainz P, et al. Prime/boost immunotherapy of HPV16-induced tumors with E7 protein delivered by Bordetella adenylate cyclase and modified vaccinia virus Ankara. Cancer Immunol Immunother. 2006;55:39–46. doi: 10.1007/s00262-005-0700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006;16:1075–81. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]