Abstract
Recent advances in assisted reproduction treatment have enabled some couples with severe infertility issues to conceive, but the methods are not successful in all cases. Notwithstanding the significant financial burden of assisted reproduction treatment, the emotional scars from an inability to conceive a child enacts a greater toll on affected couples. While methods have circumvented some root causes for male and female infertility, often the underlying causes cannot be treated, thus true cures for restoring a patient’s fertility are limited. Furthermore, the procedures are only available if the affected patients are able to produce gametes. Patients rendered sterile by medical interventions, exposure to toxicants or genetic causes are unable to utilize assisted reproduction to conceive a child – and often resort to donors, where permitted. Stem cells represent a future potential avenue for allowing these sterile patients to produce offspring. Advances in stem cell biology indicate that stem cell replacement therapies or in-vitro differentiation may be on the horizon to treat and could cure male and female infertility, although significant challenges need to be met before this technology can reach clinical practice. This article discusses these advances and describes the impact that these advances may have on treating infertility.
Keywords: assisted reproduction treatment, female infertility, differentiation, in-vitro gametogenesis, male infertility, stem cells
Current treatment options for infertility
In recent years, several advancements have been made in assisted reproduction treatment such that now more than 80% of couples experiencing infertility issues can conceive a child (Schlegel, 2009). Infertility is a global phenomenon that affects up to 15% of couples, with male infertility as the sole cause, representing up to 30% of these cases (Schlegel, 2009). Approximately 40% of couples are affected by combined male and female factors (http://publications.nice.org.uk/fertility-cg156). In cases of male infertility contributing to a couple’s inability to conceive, there are limited and some invasive procedures that can be implemented. For patients able to provide a sperm sample, IVF can be achieved by either incubation of the sperm sample with a partner’s oocyte or by intracytoplasmic sperm injection (ICSI) of the oocyte with a single spermatozoon (Palermo et al., 2009). In cases where functional spermatozoa or elongated spermatids for ICSI are rare, three invasive procedures can be used to obtain samples suitable for ICSI: testicular biopsy, testicular sperm extraction or percutaneous sperm aspiration (Esteves et al., 2011). Testicular sperm extraction, which involves a needle biopsy, has been widely successful for isolating testicular spermatozoa capable of fertilizing a partner’s oocyte by ICSI for even highly azoospermic cases, including men with Klinefelter syndrome (Schiff et al., 2005, Silber, 2010).
For couples diagnosed with infertility, all assisted reproduction options are highly invasive and often involve hormone therapies for stimulating ovulation, which can lead to ovarian hyperstimulation syndrome due to increased vascular permeability mediated by vasoactive substances from ovary stimulation, often leading to additional health concerns (Gomez et al., 2010; Prakash and Mathur, 2013). These concerns underscore that current infertility treatment options for women are invasive and potentially present health risks, notwithstanding the financial burden of these treatments. Current infertility treatment options include intrauterine insemination, ovarian drilling and ovulation induction for IVF/ICSI, which carries the ethical and financial dilemma of the implantation of too many blastocysts post IVF/ICSI, resulting in potentially undesired multiple pregnancies and births (Van Voorhis and Ryan, 2010).
For both male and female patients experiencing infertility, current treatment options rely solely on the premise that both partners produce functional haploid gametes. For those couples where one partner is unable to produce a functional gamete (oocyte or spermatozoon), no treatment options are available other than use of donor gametes. Several factors contribute to a patients’ infertility that may result in a loss of gamete formation. These include genetic factors resulting in primary ovarian insufficiency/premature ovarian failure in females and sterility/failure to produce spermatozoa through spermatogenesis in males, exposure to environmental and industrial toxicants and medical interventions such as chemotherapies and immune suppressant treatments (Bahadur, 2000, Deutsch et al., 2007, Schlegel, 2009, Skrzypek and Krause, 2007, Wallace, 2011). For these patients, there are no cures for their infertility/sterility and they are unable to conceive a child with their partner.
Preserving fertility following medical therapies
Previously, medical professionals were focused on treating cancers in prepubescent boys and girls to extend their lives with little concern over preserving patients’ fertility. As cancer survival rates in adolescents have risen (Bahadur, 2000, Wallace, 2011, Woodruff, 2010, Wyns et al., 2011, Wyns et al., 2010, Jahnukainen et al., 2011, Keros et al., 2007, Levine et al., 2010, Orwig and Schlatt, 2005), the focus has shifted to preserving fertility. One of the benevolent goals of the Oncofertility Consortium is to preserve fertility in patients undergoing rigorous cancer treatments such as high dose chemotherapies (Woodruff, 2010). However, fertility preservation can be extended to any medical treatment that impacts fertility, such as immune suppressant treatment which has been shown to cause permanent sterility in some male patients (Deutsch et al., 2007, Skrzypek and Krause, 2007). For female patients, cryopreservation of oocytes (Cobo et al., 2011) or ovarian tissue (Amorim et al., 2011; Andersen et al., 2012) prior to medical treatments is an option. Oocytes can be later utilized for IVF. For adult male patients, cryopreservation of a sperm sample is the least invasive procedure, but for prepubescent patients or those patients unable to provide a sperm sample, cryopreservation of testicular tissue has become the latest innovation (Wallace, 2011) to preserve a patient’s fertility. Several studies in mouse models have shown the ability to reintroduce spermatogonial stem cells (SSC) obtained from testis biopsies to restore fertility in sterilized mice (Brinster, 2007, Brinster and Avarbock, 1994, Brinster and Zimmermann, 1994, Nagano et al., 1998, Oatley and Brinster, 2006, Ryu et al., 2003). Recently, this work has been extended to non-human primates (Hermann et al., 2012), which showed that isolated rhesus macaque SSC obtained from biopsies taken prior to busulphan-mediated chemosterilization can be retransplanted back into the testis to recolonize the testes, resulting in the production of functional spermatozoa. This outstanding breakthrough now paves the way for reintroducing isolated human SSC from tissue biopsies taken from adolescent male patients prior to high dose chemotherapy.
This method of fertility preservation carries the concern of reintroducing cancer cells after chemotherapy. While improvements have been made to separate SSC from cancer cells (Hermann et al., 2011), the risk must still be considered. Furthermore, SSC recolonization requires that the somatic environment of the testes must remain intact after the medical intervention. For patients with damaged somatic environments (Zhang et al., 2007) or genetic defects in the somatic environment that prevent SSC expansion and differentiation, this type of strategy is ineffective at curing a patient’s infertility.
Pluripotent stem cell treatment options
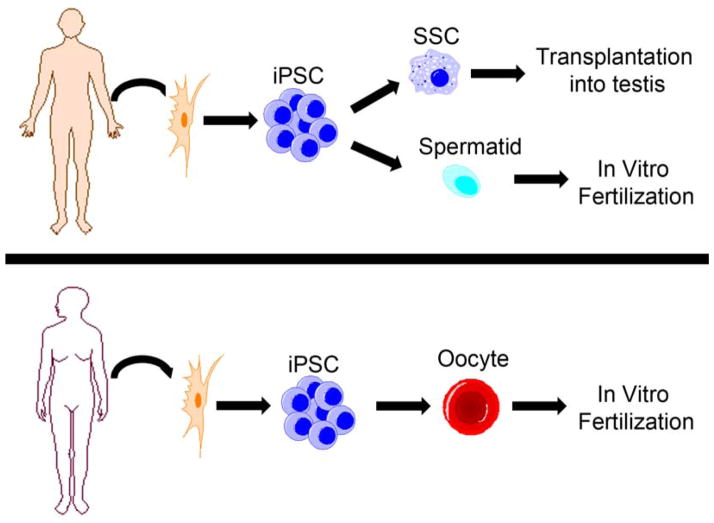
Recent evidence reported by several independent groups has shown the ability of human, non-human primate and mouse pluripotent stem cells to differentiate into primordial germ cells (Buaas et al., 2004, Eguizabal et al., 2011, Geijsen et al., 2004, Kee et al., 2009, Kee et al., 2006, Ko et al., 2010, Nayernia et al., 2006, Panula et al., 2011, Park et al., 2009, Teramura et al., 2007, Tilgner et al., 2008, Toyooka et al., 2003, Yamauchi et al., 2009, Hayashi et al., 2011), precursor cells that contribute to gametogenesis in both males and females. Studies with mouse embryonic stem cells have even shown the ability to make functional spermatozoa (Nayernia et al., 2006, Zhao et al., 2010). The recent work by Hayashi et al. (2011) demonstrates that stem cells can be differentiated into a primordial germ cell-like state and then transplanted into a sterile mouse testis for recolonization and the generation of functional haploid sperm cells. While primordial germ cells have shown a limited capacity to recolonize sterile testes in rodents, this technique has not been fully examined in other mammalian models, including non-human primates. Thus, the possibility exists that pluripotent stem cells can be differentiated into a germ cell lineage more suitable for recolonization and restoration of spermatogenesis. In fact, the current study group recently published that human embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC) can be differentiated into SSC-like cells (Easley et al., 2012), and this germ cell stage has been shown in several animal models to be capable of recolonizing the testis (Brinster, 2007, Hermann et al., 2012). With the recently shown ability to generate GMP (good manufacturing practice) human iPSC for potential clinical uses (Karumbayaram et al., 2012, Okita et al., 2012), it may become possible to generate iPSC from patients sterilized by medical interventions, environmental or industrial toxicants or injury. Advances in the current study group’s protocol to GMP/animal-component-free conditions, combined with the work of Hermann and colleagues, could lead to the first stem cell replacement therapy for male infertility, whereby iPSC differentiation into SSC-like cells followed by transplantation of these SSC-like cells into the patient’s testes restores fertility (Figure 1).
Figure 1.
Using human pluripotent stem cells to treat infertility. Male (top) and female (bottom) infertility could be treated by deriving patient-specific induced pluripotent stem cells (iPSC) from patient somatic cell samples. For males, these iPSC can be differentiated into spermatogonial stem cells (SSC) for transplantation into the testis to recolonize and generate spermatozoa in vivo. iPSC can also be differentiated in vitro into haploid cell products capable of fertilizing an oocyte by IVF. Similarly, female iPSC can be differentiated in vitro into oocytes capable of being fertilized by a partner’s spermatozoa to produce offspring by IVF.
As mentioned above, one limiting step for stem cell replacement therapy is the testis somatic environment. If the somatic environment is damaged and not receptive to SSC transplant, then SSC from iPSC would not be able to restore a patient’s fertility. However, complete in-vitro differentiation into functional spermatids could be a possible workaround solution (Figure 1). This study group recently demonstrated that human ESC and human iPSC can be differentiated in vitro into advanced spermatogenic stages, including round spermatids (Easley et al., 2012). While round spermatids have not been successful in fertilizing oocytes in higher-order mammals, the results indicate that it is at least feasible to differentiate pluripotent stem cells into haploid spermatids. Improvement in the differentiation strategy could lead to the maturation of round spermatids into elongated spermatids, which are capable of fertilizing an oocyte in IVF clinics. Future potential cures for infertility/sterility could target in-vitro differentiation into functional spermatids and thus not necessitate testis cell transplantation.
In-vitro germ cell models for deriving novel cures for infertility
Previously mentioned uses for stem cells and the treatment of male infertility have focused on those patients rendered sterile by exposure and not due to a genetic component. In-vitro differentiation may alleviate some patients with genetic root causes for their infertility, such as those patients that experience defects in the somatic environment or possibly Sertoli cell only syndrome patients, but may not be useful for those with spermatogenic defects such as DAZ family deletions and Klinefelter syndrome. However, development of in-vitro spermatogenic models is critical for understanding and identifying root causes for known and idiopathic infertility cases. These models could then be adapted for high-throughput screening to identify novel compounds capable of restoring spermatogenesis in vitro to generate functional haploid spermatids or spermatozoa for fertilizing a partner’s oocyte in IVF clinics. These types of models are critical for uncovering novel underlying problems that contribute to infertility. This study group’s recent work highlighted the ability to differentiate human ESC and iPSC into various cell lineages found in spermatogenesis, including SSC, premeiotic spermatocytes, post-meiotic spermatocytes and round spermatids, although it has not yet shown whether individual cells track through all stages of spermatogenesis (Easley et al., 2012).
Stem cell therapies for infertile women
Until recently, most of the major advances involving germ cell differentiation into haploid cells have been in male stem cells. To date, work in human pluripotent stem cells has generated haploid female cells but nothing that resembles an oocyte nor is predicated to possess a functional ooplasm capable of being fertilized (Eguizabal et al., 2011). However, the recent work by Hayashi et al. (2012) showed that mouse stem cells could be differentiated in an in-vitro/in-vivo system into oocyte-like cells that are capable of being fertilized by spermatozoa and generating normal progeny. This outstanding advancement further shows the ability of pluripotent stem cells to differentiate into all cells of the adult organism. Whether the work by Hayashi and colleagues can be adapted for human stem cells remains to be seen, but this advancement is a critical step forward in generating functional de-novo oocytes from human iPSC from female patients rendered sterile by medical interventions, exposure to toxicants or by premature ovarian failure (Figure 1).
Mutations in mitochondrial DNA (mtDNA), inherited maternally, have been linked to severe human disorders including myopathies, neurodegenerative diseases, diabetes, cancer and even infertility (Solano et al., 2001, Tachibana et al., 2009). If severe enough, these mtDNA mutations are capable of preventing a woman from producing an offspring with her partner. Recently, Tachibana et al. (2009, 2012) showed, using a non-human primate model, that mtDNA defects can be circumvented by spindle–chromosomal complex transfer from a mature metaphase-II oocyte into an enucleated mature donor oocyte. These oocytes are capable of being fertilized and giving rise to offspring that lack the deleterious mtDNA mutation, but maintain the maternal genomic DNA signature (Tachibana et al., 2009, 2012). This novel approach, while currently not confirmed in human oocytes, has the potential to remove deleterious mtDNA mutations that contribute to infertility and would enable affected women to produce healthy offspring with their partner.
Recently, the work by White et al. (2012) has identified a rare population of mitotically active germ cells in human ovaries that can be purified and cultured in vitro to spontaneously form oocytes. This work highlights a unique potential to generate oocytes in vitro from isolated cells in reproductive-aged women who may have a depleted follicle pool from such genetic defects as Fragile X-associated primary ovarian insufficiency. This recent advance, along with those described above, highlight the unique methodologies being developed to combat female-factor infertility.
Conclusions
The novel innovation by Yamanaka and others of reprogramming adult somatic cells into embryonic stem-like cells has revolutionized patient-specific stem cell therapies in medicine, especially as GMP protocols for deriving iPSC are being established. Recent advances have shown the ‘promiscuity’ of stem cells to differentiate not only into somatic lineages but also into gametic lineages (Schatten, 2012). The ability to differentiate a patient’s iPSC into functional haploid products is an important step not only for providing material suitable for IVF, but also for developing a model system for chemical screens to identify novel compounds capable of curing a patient’s infertility. The generation of functional haploid products from patient-specific stem cells is a noble quest, but one that needs to be rigorously examined in non-human primate models before being utilized in a clinical setting. Long-term studies will need to be conducted to examine whether healthy offspring can be generated from pluripotent stem cell-derived gametes. The best short-term uses for human research will be to develop in-vitro models for spermatogenesis and oogenesis for use with drug screens evaluating whether new medical treatments impact gametogenesis or to design novel contraceptives.
Infertility is a condition that affects an estimated 15% of couples worldwide. Recent advances in assisted reproduction treatment have enabled some couples with severe infertility issues to conceive, but the methods are not successful for all cases. Notwithstanding the significant financial burden of assisted reproduction treatment, the emotional scars from an inability to conceive a child enacts a greater toll on affected couples. Stem cells represent a future potential avenue for allowing sterile patients to produce offspring. Advances in stem cell biology indicate that stem cell replacement therapies may be on the horizon to treat and possibly cure male and female infertility, although significant challenges need to be met before this technology can reach clinical practice. Here, we discuss these recent advances and describe the impact that these advances may have on treating infertility.
Biography

Charles A Easley, PhD is a research professor in the department of cell biology at Emory University School of Medicine in the newly formed Laboratory of Translational Cell Biology. He completed his graduate work at Virginia Commonwealth University under Robert Tombes and completed his postdoctoral fellowship under Gerald Schatten, where he developed a novel protocol for differentiating human pluripotent stem cells into advanced spermatogenic lineages. His current work focuses on using pluripotent stem cells to model genetic forms of male infertility in order to uncover novel root causes and develop potential therapies.
Footnotes
Declaration: The authors report no financial or commercial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23 (2):160–186. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Silber SJ, Berghold SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports Reprod. BioMed Online. 2012;25 (2):128–132. doi: 10.1016/j.rbmo.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Cobo A, Remohi J, Chang CC, Nagy ZP. Oocyte cryopreservation for donor egg banking. Reprod Biomed Online. 2011;23 (3):341–346. doi: 10.1016/j.rbmo.2011.05.014. [DOI] [PubMed] [Google Scholar]
- BAHADUR G. Fertility issues for cancer patients. Mol Cell Endocrinol. 2000;169:117–22. doi: 10.1016/s0303-7207(00)00364-6. [DOI] [PubMed] [Google Scholar]
- BRINSTER RL. Male germline stem cells: from mice to men. Science. 2007;316:404–5. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINSTER RL, AVARBOCK MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–7. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINSTER RL, ZIMMERMANN JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUAAS FW, KIRSH AL, SHARMA M, MCLEAN DJ, MORRIS JL, GRISWOLD MD, DE ROOIJ DG, BRAUN RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–52. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- DEUTSCH MA, KACZMAREK I, HUBER S, SCHMAUSS D, BEIRAS-FERNANDEZ A, SCHMOECKEL M, OCHSENKUEHN R, MEISER B, MUELLER-HOECKER J, REICHART B. Sirolimus-associated infertility: case report and literature review of possible mechanisms. Am J Transplant. 2007;7:2414–21. doi: 10.1111/j.1600-6143.2007.01929.x. [DOI] [PubMed] [Google Scholar]
- EASLEY CAT, PHILLIPS BT, MCGUIRE MM, BARRINGER JM, VALLI H, HERMANN BP, SIMERLY CR, RAJKOVIC A, MIKI T, ORWIG KE, SCHATTEN GP. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2:440–6. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGUIZABAL C, MONTSERRAT N, VASSENA R, BARRAGAN M, GARRETA E, GARCIA-QUEVEDO L, VIDAL F, GIORGETTI A, VEIGA A, IZPISUA BELMONTE JC. Complete meiosis from human induced pluripotent stem cells. Stem Cells. 2011;29:1186–95. doi: 10.1002/stem.672. [DOI] [PubMed] [Google Scholar]
- ESTEVES SC, MIYAOKA R, AGARWAL A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- GEIJSEN N, HOROSCHAK M, KIM K, GRIBNAU J, EGGAN K, DALEY GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–54. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- GOMEZ R, SOARES SR, BUSSO C, GARCIA-VELASCO JA, SIMON C, PELLICER A. Physiology and pathology of ovarian hyperstimulation syndrome. Semin Reprod Med. 2010;28:448–57. doi: 10.1055/s-0030-1265670. [DOI] [PubMed] [Google Scholar]
- HAYASHI K, OGUSHI S, KURIMOTO K, SHIMAMOTO S, OHTA H, SAITOU M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338:971–5. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- HAYASHI K, OHTA H, KURIMOTO K, ARAMAKI S, SAITOU M. Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells. Cell. 2011;146:1–14. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- HERMANN BP, SUKHWANI M, SALATI J, SHENG Y, CHU T, ORWIG KE. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Hum Reprod. 2011;26:3222–31. doi: 10.1093/humrep/der343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMANN BP, SUKHWANI M, WINKLER F, PASCARELLA JN, PETERS KA, SHENG Y, VALLI H, RODRIGUEZ M, EZZELARAB M, DARGO G, PETERSON K, MASTERSON K, RAMSEY C, WARD T, LIENESCH M, VOLK A, COOPER DK, THOMSON AW, KISS JE, PENEDO MC, SCHATTEN GP, MITALIPOV S, ORWIG KE. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–26. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHNUKAINEN K, EHMCKE J, HOU M, SCHLATT S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab. 2011;25:287–302. doi: 10.1016/j.beem.2010.09.007. [DOI] [PubMed] [Google Scholar]
- KARUMBAYARAM S, LEE P, AZGHADI SF, COOPER AR, PATTERSON M, KOHN DB, PYLE A, CLARK A, BYRNE J, ZACK JA, PLATH K, LOWRY WE. From skin biopsy to neurons through a pluripotent intermediate under Good Manufacturing Practice protocols. Stem Cells Transl Med. 2012;1:36–43. doi: 10.5966/sctm.2011-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEE K, ANGELES VT, FLORES M, NGUYEN HN, REIJO PERA RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–5. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEE K, GONSALVES JM, CLARK AT, PERA RA. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–7. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- KEROS V, HULTENBY K, BORGSTROM B, FRIDSTROM M, JAHNUKAINEN K, HOVATTA O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22:1384–95. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- KO K, HUEBNER K, MUELLER-KEUKER J, SCHOELER HR. In vitro derivation of germ cells from embryonic stem cells. Front Biosci. 2010;15:46–56. doi: 10.2741/3605. [DOI] [PubMed] [Google Scholar]
- LEVINE J, CANADA A, STERN CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4831–41. doi: 10.1200/JCO.2009.22.8312. [DOI] [PubMed] [Google Scholar]
- NAGANO M, AVARBOCK MR, LEONIDA EB, BRINSTER CJ, BRINSTER RL. Culture of mouse spermatogonial stem cells. Tissue Cell. 1998;30:389–97. doi: 10.1016/s0040-8166(98)80053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAYERNIA K, NOLTE J, MICHELMANN HW, LEE JH, RATHSACK K, DRUSENHEIMER N, DEV A, WULF G, EHRMANN IE, ELLIOTT DJ, OKPANYI V, ZECHNER U, HAAF T, MEINHARDT A, ENGEL W. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–32. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- OATLEY JM, BRINSTER RL. Spermatogonial stem cells. Methods Enzymol. 2006;419:259–82. doi: 10.1016/S0076-6879(06)19011-4. [DOI] [PubMed] [Google Scholar]
- OKITA K, YAMAKAWA T, MATSUMURA Y, SATO Y, AMANO N, WATANABE A, GOSHIMA N, YAMANAKA S. An Efficient Non-viral Method to Generate Integration-Free Human iPS Cells from Cord Blood and Peripheral Blood Cells. Stem Cells. 2012 doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- ORWIG KE, SCHLATT S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005:51–6. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- PALERMO GD, NERI QV, TAKEUCHI T, ROSENWAKS Z. ICSI: where we have been and where we are going. Semin Reprod Med. 2009;27:191–201. doi: 10.1055/s-0029-1202309. [DOI] [PubMed] [Google Scholar]
- PANULA S, MEDRANO JV, KEE K, BERGSTROM R, NGUYEN HN, BYERS B, WILSON KD, WU JC, SIMON C, HOVATTA O, REIJO PERA RA. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20:752–62. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK TS, GALIC Z, CONWAY AE, LINDGREN A, VAN HANDEL BJ, MAGNUSSON M, RICHTER L, TEITELL MA, MIKKOLA HK, LOWRY WE, PLATH K, CLARK AT. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27:783–95. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash A, Mathur R. Ovarian Hyperstimulation Syndrome. The Obstetrician and Gynaecologist. 2013;15:31–35. [Google Scholar]
- RYU BY, ORWIG KE, AVARBOCK MR, BRINSTER RL. Stem cell and niche development in the postnatal rat testis. Dev Biol. 2003;263:253–63. doi: 10.1016/j.ydbio.2003.07.010. [DOI] [PubMed] [Google Scholar]
- SCHATTEN G. Cellular promiscuity: explaining cellular fidelity in vivo against unrestrained pluripotency in vitro. EMBO Rep. 2012 doi: 10.1038/embor.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIFF JD, PALERMO GD, VEECK LL, GOLDSTEIN M, ROSENWAKS Z, SCHLEGEL PN. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90:6263–7. doi: 10.1210/jc.2004-2322. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL PN. Evaluation of male infertility. Minerva Ginecol. 2009;61:261–83. [PubMed] [Google Scholar]
- SILBER SJ. Sperm retrieval for azoospermia and intracytoplasmic sperm injection success rates--a personal overview. Hum Fertil (Camb) 2010;13:247–56. doi: 10.3109/14647273.2010.534529. [DOI] [PubMed] [Google Scholar]
- SKRZYPEK J, KRAUSE W. Azoospermia in a renal transplant recipient during sirolimus (rapamycin) treatment. Andrologia. 2007;39:198–9. doi: 10.1111/j.1439-0272.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- SOLANO A, PLAYAN A, LOPEZ-PEREZ MJ, MONTOYA J. Genetic diseases of the mitochondrial DNA in humans. Salud Publica Mex. 2001;43:151–61. [PubMed] [Google Scholar]
- TACHIBANA M, AMATO P, SPARMAN M, WOODWARD J, SANCHIS DM, MA H, GUTIERREZ NM, TIPPNER-HEDGES R, KANG E, LEE HS, RAMSEY C, MASTERSON K, BATTAGLIA D, LEE D, WU D, JENSEN J, PATTON P, GOKHALE S, STOUFFER R, MITALIPOV S. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2012 doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACHIBANA M, SPARMAN M, SRITANAUDOMCHAI H, MA H, CLEPPER L, WOODWARD J, LI Y, RAMSEY C, KOLOTUSHKINA O, MITALIPOV S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–72. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERAMURA T, TAKEHARA T, KAWATA N, FUJINAMI N, MITANI T, TAKENOSHITA M, MATSUMOTO K, SAEKI K, IRITANI A, SAGAWA N, HOSOI Y. Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning Stem Cells. 2007;9:144–56. doi: 10.1089/clo.2006.0070. [DOI] [PubMed] [Google Scholar]
- TILGNER K, ATKINSON SP, GOLEBIEWSKA A, STOJKOVIC M, LAKO M, ARMSTRONG L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26:3075–85. doi: 10.1634/stemcells.2008-0289. [DOI] [PubMed] [Google Scholar]
- TOYOOKA Y, TSUNEKAWA N, AKASU R, NOCE T. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci U S A. 2003;100:11457–62. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN VOORHIS BJ, RYAN GL. Ethical obligation for restricting the number of embryos transferred to women: combating the multiple-birth epidemic from in vitro fertilization. Semin Reprod Med. 2010;28:287–94. doi: 10.1055/s-0030-1255176. [DOI] [PubMed] [Google Scholar]
- WALLACE WH. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer. 2011;117:2301–10. doi: 10.1002/cncr.26045. [DOI] [PubMed] [Google Scholar]
- WHITE YA, WOODS DC, TAKAI Y, ISHIHARA O, SEKI H, TILLY JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–21. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODRUFF TK. The Oncofertility Consortium--addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7:466–75. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYNS C, CURABA M, PETIT S, VANABELLE B, LAURENT P, WESE JF, DONNEZ J. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod. 2011;26:737–47. doi: 10.1093/humrep/deq387. [DOI] [PubMed] [Google Scholar]
- WYNS C, CURABA M, VANABELLE B, VAN LANGENDONCKT A, DONNEZ J. Options for fertility preservation in prepubertal boys. Hum Reprod Update. 2010;16:312–28. doi: 10.1093/humupd/dmp054. [DOI] [PubMed] [Google Scholar]
- YAMAUCHI K, HASEGAWA K, CHUMA S, NAKATSUJI N, SUEMORI H. In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PLoS One. 2009;4:e5338. doi: 10.1371/journal.pone.0005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Z, SHAO S, MEISTRICH ML. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol. 2007;211:149–58. doi: 10.1002/jcp.20910. [DOI] [PubMed] [Google Scholar]
- ZHAO XY, LI W, LV Z, LIU L, TONG M, HAI T, HAO J, WANG X, WANG L, ZENG F, ZHOU Q. Viable fertile mice generated from fully pluripotent iPS cells derived from adult somatic cells. Stem Cell Rev. 2010;6:390–7. doi: 10.1007/s12015-010-9160-3. [DOI] [PubMed] [Google Scholar]