Abstract
Diabetic kidney disease causes significant morbidity and mortality among people with type 1 diabetes (T1D). Intensive glucose and blood pressure control have thus far failed to adequately curb this problem and therefore a major need for novel treatment approaches exists. Multiple observations link serum uric acid levels to kidney disease development and progression in diabetes and strongly argue that uric acid lowering should be tested as one such novel intervention. A pilot of such a trial, using allopurinol, is currently being conducted by the Preventing Early Renal Function Loss (PERL) Consortium. Although the PERL trial targets T1D individuals at highest risk of kidney function decline, the use of allopurinol as a renoprotective agent may also be relevant to a larger segment of the population with diabetes. As allopurinol is inexpensive and safe, it could be cost-effective even for relatively low-risk patients, pending the completion of appropriate trials at earlier stages.
Keywords: uric acid, kidney disease, diabetes, diabetic kidney disease, glomerular filtration rate, allopurinol, diabetic nephropathy, randomized clinical trial, type 1 diabetes, PERL trial
Introduction
The past 20 years have witnessed major improvement in the management of patients with type 1 diabetes (T1D) including better glucose and blood pressure (BP) control and the use of drugs to inhibit the renin-angiotensin aldosterone system (RAAS). While such improvements in clinical care have been effective at delaying the development of diabetic complications1, the overall incidence of end-stage renal disease (ESRD) due to diabetes has not declined2, 3 and diabetic nephropathy (DN) remains a major cause of morbidity and mortality among diabetic patients4–6. Thus, novel strategies are urgently needed to complement the existing ones in order to prevent kidney function loss in diabetes.
Multiple longitudinal cohort studies have shown that elevated serum uric acid levels are associated with higher risk of subsequent DN events such as the onset of albuminuria and its progression and the sustained decline of glomerular filtration rate (GFR) among people with T1D7–9. These observational data combined with animal10, 11 and laboratory12–15 studies support the hypothesis that decreasing serum uric acid levels could prevent or slow the rate of GFR decline among people with T1D who are at high risk for development and progression of DN. Proof of concept for this hypothesis has been provided by two small clinical trials showing that the uric acid-lowering agent allopurinol effectively slowed GFR decline in hyperuricemic and normouricemic persons with moderately reduced GFR16, 17.
Below we first discuss the limitations of the current strategies to prevent kidney function loss in diabetes. We then review the body of evidence that links uric acid to DN etiology and progression, and, finally, we describe a randomized clinical trial currently being planned by the PERL Consortium to test whether lowering serum uric acid with allopurinol can halt or slow kidney function loss in T1D patients.
Limitations of current approaches to prevent ESRD in diabetes
Among the chronic complications of diabetes, DN imposes the highest individual, social and economic burden. After 40 years of diabetes, about one in three patients with T1D develop kidney abnormalities, which frequently progress to ESRD18. As recently shown by the Finn-Diane19 and the Epidemiology of Diabetes Complications20 studies, in the absence of diabetic kidney disease the health outcomes of diabetic subjects are similar to those of non-diabetic individuals whereas the outcomes progressively worsen with increasing stages of baseline kidney disease. This highlights the critical need for preventing the onset of DN and stopping its progression to ESRD.
Two interventions are currently available to accomplish these goals. One is intensive glycemic control, which has been shown by the Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) to effectively prevent the development of albuminuria as well as the progressive loss of renal function21. The other is lowering systemic BP and intraglomerular pressure by means of RAAS blockers and other antihypertensive drugs22. Although effective, both approaches have significant limitations. Maintaining blood glucose near normal levels through intensive glycemic control is challenging and up to 50% of diabetic patients fail to lower their HbA1c values below the target level of 7%23. Data from the Type 1 Diabetes Exchange indicate that this is also the case early in the course of the disease and that the American Diabetes Association targets for A1c, BP, lipids, and BMI are met infrequently in children and adolescents24. RAAS blockers are effective in slowing GFR decline in T1D subjects, but this beneficial effect has been demonstrated only among patients with established proteinuria and serum creatinine values ≥1.5 mg/dl4, corresponding to a GFR <50–60 ml/min. Such findings are consistent with those of the Renin Angiotensin System Study, in which RAAS blockers did not prevent the progression of kidney lesions in normotensive/normoalbuminuric adults with T1D26, although effective in reducing progression from microalbuminuria to macroalbuminuria27, 28. Due to the relatively late stage at which these drugs are effective, and the relatively small magnitude of their effect, the use of these agents yields on average a less than two year delay in the progression to ESRD2. As a result, the annual incidence of ESRD due to diabetes in the US population has continued to steadily rise and is at historically high levels despite the introduction of these interventions in the mid-1990s6. Unfortunately, the ONTARGET and ALTITUDE studies have further highlighted the limitations of RAAS inhibition, since combination therapies with multiple RAAS blockers increase the risk of serious adverse events29, 30. Therefore, a large residual DN risk remains despite current evidence-based intervention strategies and novel therapies to safely complement glucose and RAAS inhibition are urgently needed3, 26.
Uric acid as a potential pathogenic factor for diabetic nephropathy
A large body of evidence has been generated recently indicating that serum uric acid levels are strong determinants of the development of DN and the loss of kidney function among people with diabetes (Table 1). Prospective data from the clinic-based Joslin Kidney Study (JKS) study identified clinical predictors of GFR loss among 355 non-proteinuric T1D patients with preserved renal function (estimated GFR (eGFR) ≥ 60 ml/min) who were not treated with diuretics or allopurinol at baseline. eGFR was determined from serum cystatin C (median of 5 measures) over a 4 to 6 year follow-up. Study subjects were divided into two groups according to the presence (n=79) or absence (n=276) of early GFR loss, defined as an eGFR decline >3.3%/year (the 95th percentile of the rate of GFR decline in the general population). Patients with early GFR loss were older and had longer duration of T1D, lower GFR, and higher urinary AER at baseline. Importantly, these patients had higher serum uric acid levels at baseline than patients with stable renal function (median 5.1 [IQR 3.7–5.3] vs. 4.5 mg/dl [IQR 4.4–5.7], p<0.0001). Moreover, a “dose-response relationship” was evident between baseline serum uric acid levels and future risk of early GFR loss. The unadjusted relative risk of developing increased GFR loss was 1.5 (95% CI 1.3–1.9, p=0.0002) for each mg/dl increase in serum uric acid. This translated into a 2.4-fold increase in the risk of early GFR loss for uric acid levels above the median (4.5 mg/dl) as compared to uric acid levels below this value. The magnitude of this effect did not significantly change after adjustment for urinary AER, gender, HbA1c, or, importantly, baseline GFR.
Table 1.
Summary of the epidemiological evidence linking serum uric acid to diabetic kidney disease.
| Study | Diabetes type | Length of follow-up | Outcome | Increased risk associated with baseline serum uric acid | Ref. |
|---|---|---|---|---|---|
| Joslin Kidney Study | Type 1 | 4–6 yrs | GFR loss (>3.3% per year) | OR=1.50 (1.3–1.9) per mg | 9 |
| Steno Diabetes Center | Type 1 | 18 yrs | Macroalbuminuria | HR=1.66 (1.02–2.7) per mg | 8 |
| CACTI | Type 1 | 6 yrs | Micro- or macroalbuminuria | OR=1.80 (1.2–2.8) per mg | 7 |
| Verona Diabetes Study | Type 2 | 5 yrs | Overt proteinuria or GFR< 60 ml/min | OR=1.20 (1.03–1.57) per 1-SD | 43 |
The Coronary Artery Calcification in Type 1 Diabetes (CACTI) study also found that serum uric acid predicted the transition from normo- to micro- or macro-albuminuria as well as the progression of subclinical atherosclerosis 31, 32. Among the 324 T1D study participants, every 1 mg/dl increase in baseline serum uric acid was associated with an 80% increase in the predicted odds of developing micro- or macroalbuminuria after 6 years of follow-up (OR=1.8, 95% CI: 1.2–2.8, p=0.005)31. The Steno group also reported an association between uric acid and development of persistent macroalbuminuria. In an inception cohort study of 263 individuals with newly diagnosed T1D, serum uric acid measured shortly after the onset of T1D was a significant independent predictor of macroalbuminuria 18 years later (HR=2.37, 95% CI: 1.04–5.37, p=0.04, for every 100umol/L [or 1.7 mg/dl] increase in uric acid)8. Also noteworthy, in the CACTI study baseline uric acid was associated with coronary artery calcification progression (a marker of subclinical atherosclerosis) with a 30% increased odds (95% CI: 1.07–1.58, p=0.007) for every 1 SD (0.2 mg/dl) increase in serum uric acid, independent of other known cardiovascular disease risk factors32.
These findings are especially relevant in the context of an increased prevalence of the metabolic syndrome among young individuals with T1D and may potentially explain the large residual risk for ESRD given the rise in prevalence of overweight/obesity33, and metabolic syndrome34, 35 in T1D. Increased serum uric acid levels are associated with metabolic syndrome36–38 and hyperuricemia can result from overproduction (due to high dietary intake of purines for example), under secretion (e.g. diuretic therapy), or both39, 40. The association of uric acid to cardiorenal disease has been reviewed previously5.
The role of uric acid as a predisposing factor for DN does not appear to be limited to T1D. In an Italian cohort of type 2 diabetic patients with normal kidney function and without overt proteinuria, the risk of chronic kidney disease (CKD) during a 5 year follow-up was significantly higher in participants with versus without hyperuricemia (29.5 vs. 11.4%, p<0.001). After adjusting for potential confounders, each 1-SD increment in serum uric acid was associated with a 21% increase in the risk of CKD42. Furthermore, a post-hoc analysis of the Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) Trial found that the lowering of serum uric acid induced by losartan (an effect that seems specific to this RAAS blocker) accounted for 20% of the renoprotective benefit of this medication43.
The consistency and strength of these prospective data and their independence from other DN risk factors and potential confounders strongly suggests that moderately elevated serum uric acid may have a role in the pathogenesis of DN and the deterioration of kidney function observed in T1D. This hypothesis is supported by population-based studies44–47 in which hyperuricemia predicted chronic renal failure. In animal models mild uric acid elevation has also been shown to cause renal disease10, 11. Hypothesized pathogenic mechanisms of elevated uric acid in kidney disease include alterations of nitric oxide (NO) pathways, activation of the RAAS48, induction of pro-inflammatory cytokines12, 13, and increased oxidative stress resulting from the generation of uric acid by xanthine oxidase14, 15. In vitro, uric acid leads to decreased NO production49, increased CRP50, and induction of cyclooxygenase-210. In addition to suppressing NO production, uric acid may directly deplete NO51. Consistent with the in vitro data, experimental hyperuricemia induced in the rat by a uricase inhibitor has led to endothelial dysfunction and similar associations have been made in humans52, 53. Increased uric acid has also been shown to activate the intrarenal RAAS, leading to tubulointerstitial disease in animals and humans13, 39, 54, 55. Other mechanisms by which uric acid may contribute to DN include stimulation of cytokines such as TNF-α, TGFβ-156, 57 and chemokines such as monocyte chemoattractant protein-110, 58 (Figure 1). While the extent of the pathogenic role of uric acid in endothelial dysfunction, inflammation, and kidney disease in humans is still debated, such studies lend plausibility to a contribution of hyperuricemia to the development and progression of diabetic kidney disease.
Figure 1.
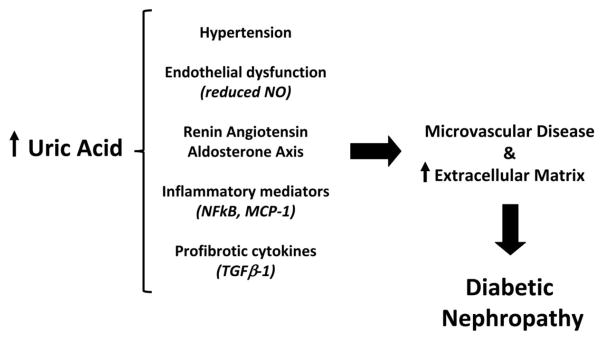
Mechanisms through which increased uric acid may contribute to the development of diabetic nephropathy.
Lowering serum uric acid to prevent renal function loss in diabetes
If increased serum uric acid itself predisposes to diabetic nephropathy and kidney function loss in diabetes, lowering its levels may help prevent this complication of diabetes and could be added to current therapies as a widespread intervention to curb the epidemic of ESRD in the diabetic population. This hypothesis is especially attractive because of the availability of a drug – allopurinol – characterized by a proven efficacy in lowering serum uric acid, a good safety profile, extensive post-marketing surveillance, and low cost owing to its generic drug status59 (Table 2). Allopurinol (1,5-dihydro-4H-pyrazolo [3,4-d]pyrimidin-4-one) is an inhibitor of xanthine oxidase, the enzyme responsible for the conversion of hypoxanthine to xanthine and of xanthine to uric acid. Allopurinol has been on the market since 1964 as the main drug for the therapy of symptomatic hyperuricemia (gout) and asymptomatic hyperuricemia (cancer treatment) and for uric acid kidney stones. At the average dosage (300 mg/day), allopurinol causes a 30–40% reduction in serum uric acid60–62, but up to a 60% reduction can be obtained using the maximum dosage of 600 mg/day63. Uric acid lowering with allopurinol reverses many of the adverse physiological effects of uric acid, leading to suppression of the RAAS, reduced oxidative stress, improved NO bioavailability and endothelial function, and a decline in urinary inflammatory biomarkers11, 57, 64–67.
Table 2.
Characteristics of allopurinol.
| Characteristic | Description |
|---|---|
| Class | Xanthine oxidase inhibitor |
| Efficacy | 20–60% reduction of serum uric acid |
| Dosage flexibility | 100 to 600 mg per day in 100 mg increments |
| Post-marketing surveillance | On the market since 1964 |
| Side effects | Skin rashes, rarely severe (Steven-Johnson syndrome). Liver damage, usually reversible. Bone marrow depression. |
| Use with impaired renal function | It can be used when GFR is <60 ml/min but dosage must be adjusted. |
| Cardiovascular safety | Excellent |
| Cost | Inexpensive (available as generic) |
While allopurinol is eliminated through the kidneys, it can be used in patients with impaired renal function after appropriate dose adjustments. Skin rashes are the most commonly reported adverse effects (up to 3% of patients). Rashes may be followed by more severe hypersensitivity reactions such as exfoliative lesions and the Stevens-Johnson syndrome (SJS), but such occurrences are very rare, in the order of 1 in 10,00068 and their likelihood can be further reduced by excluding subjects who are HLA-B*5801 positive69, 70. About 60% of allopurinol induced cases of SJS and toxic epidermal necrolysis (TEN) carry the HLA-B*5801 allele70 and HLA-B*5801 positive persons are estimated to be about 300 times more likely to develop SJS/TEN than those without this allele71. Other potential adverse effects include gout flares (if there is a history of gout), hepatotoxicity, and, rarely, bone marrow depression. Effective uric acid-lowering can also be achieved with newer agents such as the xanthine oxidase inhibitor febuxostat61, 62, which likely has similar neurohormonal and anti-inflammatory effects compared to allopurinol61, 72. While febuxostat has not been associated with serious skin reactions, this agent is still being examined in a large cardiovascular safety trial (NCT01101035) because of a numerically (though not statistically) higher risk of cardiovascular events associated with this medication in Phase III trials. Although it seems unlikely that febuxostat will be shown to increase cardiovascular risk, the use of this medication in high cardiovascular risk patients, such as those with diabetes, for indications other than gout and hyperuricemia should be carefully evaluated before further data become available.
Two small clinical trials have provided proof of concept for using allopurinol to lower the risk of kidney function loss. The first of these studies included 51 subjects, 25% with diabetes, who had proteinuria or serum creatinine of 1.35–4.5 mg/dl and a serum uric acid >7.6 mg/dl. Participants were treated for one year with allopurinol 100 to 300 mg/day. At the end of the treatment period, 4 of 25 subjects (16%) in the allopurinol arm had experienced significant worsening of their kidney function (defined as >40% increase in serum creatinine) as compared to 12 of 26 subjects (46%) in the placebo arm (p=0.015)16. The second study included 113 subjects, 37% of whom with diabetes, who had GFR <60 ml/min and relatively stable renal function (<50% serum creatinine increase in the previous 3 months). Participants were treated with allopurinol 100 mg/day for 24 months. At the end of the study, the GFR had increased, on average, by 1 ml/min in the treatment arm versus a 3 ml/min loss in the placebo arm17. Together, these two studies suggest that lowering uric acid by means of allopurinol may indeed halt or slow the progression of kidney disease. However, these trials have important limitations. First, sample size was small and as such could only yield modest statistical significance, leaving room for the possibility of false positive results. Second, the generalizability of their results is unclear. Due to the inclusion criterion of a serum uric acid >7.6 mg/dl, the participants in the first study had very high serum uric acid (about 10 mg/dl in both study arms). Whether the positive results observed in that study would also apply to subjects with lower uric acid levels, for instance in the 5–7 mg/dl range, remains unclear. The second study may not be generalizable because of the relatively old age of its participants (71 years on average), which was perhaps accounted for by the requirement for a relatively stable renal function. Whether allopurinol would have the same effect among younger subjects with more aggressive forms of kidney disease remains to be determined. Finally, and importantly for the topic of this paper, the nature of kidney disease was quite heterogeneous in both studies and only a minority of participants had diabetic nephropathy.
The PERL Consortium
In order to conduct an adequately powered clinical trial testing the hypothesis of a beneficial effect of allopurinol on kidney disease specifically among diabetic subjects, we have established a unique international consortium of investigators from 6 academic centers where large rosters of T1D patients are available along with long-standing expertise in the study of diabetic complications, especially DN, and in DN clinical trials. Three of these centers have been involved in clinical and basic research that is directly related to uric acid and renal disease development and progression8, 9, 31, 32. Included in this initiative are the Joslin Diabetes Center, the Universities of Minnesota, Colorado, Toronto, and Michigan, and the Steno Diabetes Center in Copenhagen, Denmark. The Consortium has been named PERL (Preventing Early Renal Function Loss in Diabetes) to emphasize the Consortium’s focus on intervening early in the course of kidney disease, when renal damage is most likely to be arrestable or reversible, and interventions are, thus, more likely to be effective in delaying late-stage clinical outcomes such as hemodialysis, renal transplantation, and death. A distinctive feature of the PERL Consortium is the choice of outcome on which to measure allopurinol effectiveness. Past clinical trials of interventions at the early stages of DN have used albuminuria as the primary outcome73. By contrast, the Consortium has selected GFR as the primary outcome variable. This choice is based on the recent results of prospective studies showing a dissociation between the natural history of GFR decline and that of albuminuria in T1D74–76. For example, the DCCT-EDIC study found that 24% of subjects who progressed to GFR <60 ml/min/1.73m2 did so without first developing microalbuminuria77. In the JKS, an increased rate of GFR decline (>3.3%/yr) was observed in 9% of normoalbuminuric and 31% of microalbuminuric T1D patients78. Since it is the loss of renal function that drives the increased morbidity and mortality associated with DN, it has been decided by the Consortium that this, rather than albuminuria, should be the outcome on which the efficacy of an intervention should be measured.
A clinical trial to test the efficacy of allopurinol in preventing kidney function loss in diabetes
Based on the above considerations, the PERL Consortium has designed a randomized clinical trial to evaluate the efficacy of the uric acid-lowering drug allopurinol, as compared to placebo, in reducing GFR loss among subjects with T1D. The study has the following characteristics:
a. Design
The study will be an international multi-center, stratified, double-blind, placebo-controlled, parallel-group randomized clinical trial.
b. Participating Centers
The trial will be carried out at the Joslin Diabetes Center, the Universities of Minnesota, Colorado, Toronto, and Michigan, and the Steno Diabetes Center in Copenhagen, Denmark. The participation of multiple centers will allow us to complete the recruitment of all study subjects within a reasonable period of time. While each of the centers has access to relatively large populations of T1D subjects, the inclusion and exclusion criteria of the study would make it challenging for a single center to recruit the entire study sample.
c. Study population
Four hundred T1D subjects at high risk for GFR loss because of the presence of micro- or macroalbuminuria and a relatively high serum uric acid (≥4.5 mg/dl), who still have only mildly or moderately decreased renal function (GFR=45–100 ml/min/1.73 m2) will be included in the study. The focus on subjects with these characteristics is based on the following considerations:
T1D. The association between uric acid and GFR has been thus far demonstrated only among T1D patients. While this effect may also be present in T2D, it seems to be wise to limit the first study to T1D, given that kidney damage and clinical progression may be more heterogeneous in T2D79.
GFR in the 45–100 ml/min/1.73 m2 range. The rationale for focusing on this GFR interval is two-fold. First, this stage of nephropathy is early enough to greatly increase the potential benefits of an intervention in terms of delay to ESRD. Second, this stage is sufficiently advanced as to enrich the study population for “GFR decliners” who have already started to lose renal function and on whom the efficacy of the intervention can be tested in a clinical trial of practical length. Although not so among non-diabetics, 100 ml/min/1.73 m2 is a relatively low value for people with T1D80.
Microalbuminuria or moderate macroalbuminuria. While albuminuria is not considered an adequate surrogate outcome for testing the efficacy of DN interventions73, it is still one of the strongest predictors of future renal function loss81. Therefore, targeting subjects with increased urinary albumin excretion will further enrich the study population with “GFR decliners,” increasing the power to detect a meaningful benefit in a trial of achievable size and practical duration.
Serum uric acid ≥4.5 mg/ml. This value of serum uric acid corresponds to the median value in the JKS. In that population, serum uric acid concentrations above this level were independently associated with a 2.4 fold increase in the odds of early renal function decline (95% CI 1.3–4.2)9. Thus, patients with uric acid above 4.5 mg/ml are highly prevalent, have an increased risk of GFR loss, and are, thus, an ideal target population for a uric acid-lowering intervention.
d. Treatment
After a 10 week run-in period, during which RAAS inhibition will be standardized and BP normalized, if needed, patients will be randomized 1 to 1 to receive either oral allopurinol or placebo. The plan is to titrate the allopurinol dose from 100 to 400 mg per day based on serum uric acid and GFR, with the goal of decreasing serum uric acid to 2.5–4.5 mg/dl, with a reduction of at least 30% from baseline levels. Since allopurinol is eliminated through the kidneys and the baseline GFR will range from 45 to 100 ml/min/1.73 m2, we expect inter-individual variability in allopurinol dose requirements. We also expect a fairly wide range of baseline serum uric acid levels (from 4.5 to ~9.0 mg/dl based on JKS data). Thus, using a variable allopurinol dose titrated on the basis of serum uric acid values and kidney function, as we propose, will allow us to maximize the reduction of serum uric acid, and consequently the power of the study, without decreasing the uric acid concentrations to non-physiological levels and/or triggering adverse reactions due to an excessive allopurinol dose relative to kidney function. Treatment will continue for 3 years, during which study participants will be followed by monitoring renal function, albuminuria, serum uric acid concentration, and the occurrence of adverse effects. The follow-up will continue for 12 weeks after allopurinol is discontinued and the GFR measured again in order to exclude the possibility of transient effects of this drug on GFR that are unrelated to permanent effects on the disease process.
e. Study outcomes
The GFR at the end of the intervention (as measured by the plasma clearance of non-radioactive iohexol and adjusted for the GFR at randomization) will be the primary outcome for the measure of the intervention’s efficacy. With this approach, it will be possible to detect beneficial effects on the rate of kidney function decline before hard end-points such as ESRD or even serum creatinine doubling are reached. Also, the use of a quantitative outcome rather than a dichotomous endpoint will maximize power, making the study more cost-efficient. The plasma iohexol clearance has been shown to provide accurate and reproducible GFR measurements82, 83. It is highly correlated with inulin clearance (the gold standard to measuring GFR84) and it is a safe, cost-effective method to test hundreds of patients enrolled in multicenter clinical trials26. Secondary outcomes will include: 1. The GFR at the end of the washout period (to test for effect durability); 2. Estimated GFR (eGFR) time trajectory estimated from quarterly serum creatinine and cystatin C measurements using the CKD-EPI SCr and the CKD-EPI SCr-SCysC equations 85,86; 3. Time to doubling of baseline serum creatinine value or ESRD; 4. Median urinary AER during the last three months of the intervention period, adjusted for the median urinary AER at baseline; 5. Time to fatal or non-fatal serious cardiovascular events.
f. Power
The study is powered to detect a difference in GFR loss between treatment arms equal to 1 ml/min/1.73 m2 per year. While this effect may seem clinically insignificant, it translates into large differences in GFR after 30 or 40 years of diabetes, which for T1D patients who develop diabetes in their first two decades of life may make the difference between developing ESRD in their 70s rather than their 50s.
Study implementation
A pilot of this study including 60 subjects recruited at two of the PERL center (Joslin and Steno) is currently underway with the support of the Juvenile Diabetes Research Foundation with the goal of establishing the feasibility of the trial and piloting all of its clinical research procedures and data flow and management (NCT01575379). The PERL Consortium has also been awarded an R34 trial planning grant by the NIDDK and a decision concerning the funding of the pivotal trial will be made in 2013.
Summary and conclusions
DN and ESRD continue to be major obstacles to the goal of reducing morbidity and mortality among people with T1D. With glycemic and BP control and the use of RAAS blockade failing to adequately curb this problem, there is a major need for novel approaches. The multiple observations linking serum uric acid levels to kidney disease development and progression in T1D strongly argue that uric acid lowering with allopurinol needs to be tested as one such novel intervention and a pilot of such trial is currently being conducted by the PERL Consortium. Of note, while this trial is focused on T1D individuals who are at highest risk of losing renal function, the use of allopurinol as a renoprotective agent may also be relevant to a much larger segment of the T1D population. As shown by the JKS, the association between serum uric acid and GFR loss is linear across the entire range of uric acid levels and can also be observed among individuals with normoalbuminuria9, and as shown in the Steno inception cohort, uric acid is a DN risk predictor shortly after T1D onset8. Thus, decreasing serum uric acid may be beneficial also for T1D patients who have not yet developed microalbuminuria and/or have completely normal uric acid levels. The fact that this drug is inexpensive and relatively safe could make its use cost-effective even for these low-risk subjects, following appropriate trials at these earlier stages.
Acknowledgments
Dr. Maahs was supported by a grant from NIDDK (DK075360). Dr. Caramori is supported by a Career Development Award from the Juvenile Diabetes Research Foundation. This project was supported by NIH grants R03 DK094484 and R34 DK097808, and by grant 17-2012-377 from the Juvenile Diabetes Research Foundation (JDRF). Its contents are the authors’ sole responsibility and do not necessarily represent official NIH or JDRF views.
Footnotes
Other PERL Consortium members:
University of Colorado: Marian Rewers, Richard Johnson, Satish Garg.
University of Michigan: Frank Brosius.
Steno Diabetes Center: Maria Lajer, Morten Kofod Lindhardt, and Bernt Johan Illum Horten von Scholten.
University of Minnesota: John Eckfeldt, Trudy Strand.
Joslin Diabetes Center: Andrzej Krolewski, Robert Stanton, Allison Goldfine.
The authors have no conflicts of interest to disclose.
Disclosure
David M. Maahs declares that he has no conflict of interest.
M. Luiza Caramori declares that she has no conflict of interest.
David Z.I. Cherney declares that he has no conflict of interest.
Andrzej T. Galecki declares that he has no conflict of interest.
Chuanyun Gao declares that she has no conflict of interest.
Diana Jalal has received ASN honoraria for speaking on the role of uric acid in kidney and cardiac disease in the elderly.
Bruce A. Perkins is a Senior Advisory Board Member for Neurometrix Inc.; and has been a Site investigator for a sponsored clinical trial for Medtronic Inc.; a Co-PI for a sponsored clinical trial by Boehringer Ingelheim; and has received speaker honoraria from Medtronic Inc., Roche, GlaxoSmithKline, Johnson & Johnson, Novo Nordisk, and Eli Lilly.
Rodica Pop-Busui declares that she has no conflict of interest.
Peter Rossing serves on the board for Astra Zeneca/BMS, Eli Lilly, Janssen, Novo Nordisk, and Astellas; has received grant support from Novo Nordisk, Novartis, and Abbott; has received payment for lectures including service on speakers bureaus from Astra Zeneca/BMS, Novartis, and Sanofi-Aventis; and has stock/stock options with Novo Nordisk.
Michael Mauer declares that he has no conflict of interest.
Alessandro Doria has received research grant support from Sanofi-Aventis; has received travel/accommodations expenses covered or reimbursed from the American Society of Nephrology and the Italian Society of Diabetology.
References
- 1.Marshall SM. Diabetic nephropathy in type 1 diabetes: has the outlook improved since the 1980s? Diabetologia. 2012;55(9):2301–6. doi: 10.1007/s00125-012-2606-1. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005) Arch Intern Med. 2009;169(14):1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Krolewski AS, Bonventre JV. High risk of ESRD in type 1 diabetes: new strategies are needed to retard progressive renal function decline. Semin Nephrol. 2012;32(5):407–414. doi: 10.1016/j.semnephrol.2012.07.002. Review highlighting the need for new strategies and novel therapeutics to prevent renal function decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus--progress made, more to be done. J Clin Endocrinol Metab. 2006;91(10):3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- 5*.de Boer I, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. Overview of prevalence of DKD in the US and demonstrates continued burden of diabetic kidney disease on individuals and its public health importance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Rosolowsky ET, Skupien J, Smiles AM, et al. Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol. 2011;22(3):545–553. doi: 10.1681/ASN.2010040354. Demonstrates persistance of ESRD risk despite advances in diabetes care in past decades. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: Findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25(6):1865–9. doi: 10.1093/ndt/gfp740. One of three epidemiologic studies from the PERL Consortium in which uric acid is associated with development of albuminuria over 6 years in young adults with type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58(7):1668–1671. doi: 10.2337/db09-0014. One of three epidemiologic studies from the PERL Consortium in which uric acid is associated with development of diabetic nephropathy over 18 years in an inception cohort of adults with type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33(6):1337–1343. doi: 10.2337/dc10-0227. One of three epidemiologic studies from the PERL Consortium in which uric acid is associated with progressive renal function loss over 6 years in adults with type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13(12):2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 11.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Segal MS, Srinivas T, et al. Essential hypertension, progressive renal disease, and uric acid: a pathogenetic link? J Am Soc Nephrol. 2005;16(7):1909–1919. doi: 10.1681/ASN.2005010063. [DOI] [PubMed] [Google Scholar]
- 13.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282(6):F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 14.Desco MC, Asensi M, Marquez R, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002;51(4):1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 15.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58(1):87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 17*.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393. doi: 10.2215/CJN.01580210. Clinical trial using allopurinol to lower uric acid to slow kidney and cardiovascular disease progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krolewski AS, Warram JH. Epidemiology of late complications of diabetes: A basis for the development and evaluation of preventive program. In: Kahn CR, Weir GC, King GL, Jacobson AM, Moses AC, Smith RJ, editors. Joslin’s Diabetes Mellitus. New York: Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- 19*.Groop PH, Thomas MC, Moran JL, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58(7):1651–1658. doi: 10.2337/db08-1543. Large epidemiologic cohort study from Finland in which people with type 1 diabetes without evidence of diabetic kidney disease have similar standardized mortality rates compared to the general Finnish population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–2319. doi: 10.1007/s00125-010-1860-3. Extends the findings from the FinnDiane study (19) over 20 years in a US cohort with type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.de Boer I, Sun W, Cleary PA, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365(25):2366–2376. doi: 10.1056/NEJMoa1111732. Demonstrates that intensive diabetes therapy reduces the risk to develop GFR < 60 ml/min/1.73m2 in the DCCT-EDIC study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 23.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31(1):81–86. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 24.Wood J, Miller K, Maahs D, et al. Most youth with type 1 diabetes in the T1D Exchange clinic registry do not meet ADA or ISPAD clinical guidelines. Diab care. In press. [Google Scholar]
- 25.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 26.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bilous R, Chaturvedi N, Sjolie AK, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med. 2009;151(1):11–14. doi: 10.7326/0003-4819-151-1-200907070-00120. [DOI] [PubMed] [Google Scholar]
- 28.Mathiesen ER, Hommel E, Giese J, Parving HH. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ. 1991;303(6794):81–87. doi: 10.1136/bmj.303.6794.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal End Points in a Trial of Aliskiren for Type 2 Diabetes. N Engl J Med. 2012 doi: 10.1056/NEJMoa1208799. In Press Recent clinical trial highlighting the need for novel therapeutics to improve cardiorenal health in people with diabetes. [DOI] [PubMed] [Google Scholar]
- 30.Mancia G, Schumacher H, Redon J, et al. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) Circulation. 2011;124(16):1727–1736. doi: 10.1161/CIRCULATIONAHA.110.008870. [DOI] [PubMed] [Google Scholar]
- 31.Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25(6):1865–1869. doi: 10.1093/ndt/gfp740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Rodrigues TC, Maahs DM, Johnson RJ, et al. Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care. 2010;33(11):2471–2473. doi: 10.2337/dc10-1007. Epidemiologic study in which uric acid is associated with progression of subclinical coronary atherosclerosis in young adults with type 1 diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libman IM, Pietropaolo M, Arslanian SA, LaPorte RE, Becker DJ. Changing prevalence of overweight children and adolescents at onset of insulin-treated diabetes. Diabetes Care. 2003;26(10):2871–2875. doi: 10.2337/diacare.26.10.2871. [DOI] [PubMed] [Google Scholar]
- 34.McGill M, Molyneaux L, Twigg SM, Yue DK. The metabolic syndrome in type 1 diabetes: does it exist and does it matter? J Diabetes Complications. 2008;22(1):18–23. doi: 10.1016/j.jdiacomp.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Thorn LM, Forsblom C, Fagerudd J, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study) Diabetes Care. 2005;28(8):2019–2024. doi: 10.2337/diacare.28.8.2019. [DOI] [PubMed] [Google Scholar]
- 36.Tsouli SG, Liberopoulos EN, Mikhailidis DP, Athyros VG, Elisaf MS. Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metabolism. 2006;55(10):1293–1301. doi: 10.1016/j.metabol.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Godsland IF, Johnston DG. Co-associations between insulin sensitivity and measures of liver function, subclinical inflammation, and hematology. Meatabolism. 2008;57(9):1190–1197. doi: 10.1016/j.metabol.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Cirillo P, Sato W, Reungjui S, et al. Uric acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol. 2006;17(12 Suppl 3):S165–S168. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- 39.Jalal DI, Maahs DM, Hovind P, Nakagawa T. Uric acid as a mediator of diabetic nephropathy. Semin Nephrol. 2011;31(5):459–465. doi: 10.1016/j.semnephrol.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards NL. The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med. 2008;75 (Suppl 5):S13–S16. doi: 10.3949/ccjm.75.suppl_5.s13. [DOI] [PubMed] [Google Scholar]
- 41.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoppini G, Targher G, Chonchol M, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35(1):99–104. doi: 10.2337/dc11-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58(1):2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. Post-hoc analysis suggesting losartan lowers uric acid as a mechanism of improving renal outcomes. [DOI] [PubMed] [Google Scholar]
- 44.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642–650. [PubMed] [Google Scholar]
- 45.Domrongkitchaiporn S, Sritara P, Kitiyakara C, et al. Risk factors for development of decreased kidney function in a southeast Asian population: a 12-year cohort study. J Am Soc Nephrol. 2005;16(3):791–799. doi: 10.1681/ASN.2004030208. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Zuo L, Xu G, et al. Community-based screening for chronic kidney disease among populations older than 40 years in Beijing. Nephrol Dial Transplant. 2007;22(4):1093–1099. doi: 10.1093/ndt/gfl763. [DOI] [PubMed] [Google Scholar]
- 47.Kuo CF, Luo SF, See LC, et al. Hyperuricaemia and accelerated reduction in renal function. Scand J Rheumatol. 2011;40(2):116–121. doi: 10.3109/03009742.2010.507218. [DOI] [PubMed] [Google Scholar]
- 48.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–1242. [PubMed] [Google Scholar]
- 49.Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295(5):C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 51.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27(8):967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanbay M, Yilmaz MI, Sonmez A, et al. Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol. 2011;33(4):298–304. doi: 10.1159/000324847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 54.Perlstein TS, Gumieniak O, Hopkins PN, et al. Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int. 2004;66(4):1465–1470. doi: 10.1111/j.1523-1755.2004.00909.x. [DOI] [PubMed] [Google Scholar]
- 55.Myllymaki J, Honkanen T, Syrjanen J, et al. Uric acid correlates with the severity of histopathological parameters in IgA nephropathy. Nephrol Dial Transplant. 2005;20(1):89–95. doi: 10.1093/ndt/gfh584. [DOI] [PubMed] [Google Scholar]
- 56.Netea MG, Kullberg BJ, Blok WL, Netea RT, Van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood. 1997;89(2):577–582. [PubMed] [Google Scholar]
- 57.Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27(5):435–440. doi: 10.1159/000105142. [DOI] [PubMed] [Google Scholar]
- 58.Roncal CA, Mu W, Croker B, et al. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2007;292(1):F116–F122. doi: 10.1152/ajprenal.00160.2006. [DOI] [PubMed] [Google Scholar]
- 59.Doria A, Niewczas MA, Fiorina P. Can existing drugs approved for other indications retard renal function decline in patients with type 1 diabetes and nephropathy? Semin Nephrol. 2012;32(5):437–444. doi: 10.1016/j.semnephrol.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker MA, Schumacher HR, Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 62.Schumacher HR, Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59(11):1540–1548. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 63.Noman A, Ang DS, Ogston S, Lang CC, Struthers AD. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet. 2010;375(9732):2161–2167. doi: 10.1016/S0140-6736(10)60391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dogan A, Yarlioglues M, Kaya MG, et al. Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011;20(3):182–187. doi: 10.3109/08037051.2010.538977. [DOI] [PubMed] [Google Scholar]
- 65.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35(3):746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe S, Kang DH, Feng L, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40(3):355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 67.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333(24):1600–1607. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 69.Jung JW, Song WJ, Kim YS, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011;26(11):3567–3572. doi: 10.1093/ndt/gfr060. [DOI] [PubMed] [Google Scholar]
- 70.Lonjou C, Borot N, Sekula P, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18(2):99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 71*.Tassaneeyakul W, Jantararoungtong T, Chen P, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19(9):704–709. doi: 10.1097/FPC.0b013e328330a3b8. Pharmacogenetic data in which the association of HLA-B*5801 is identified as an important risk factor for SJS . [DOI] [PubMed] [Google Scholar]
- 72.Chohan S, Becker MA, MacDonald PA, Chefo S, Jackson RL. Women with gout: efficacy and safety of urate-lowering with febuxostat and allopurinol. Arthritis Care Res (Hoboken) 2012;64(2):256–261. doi: 10.1002/acr.20680. [DOI] [PubMed] [Google Scholar]
- 73.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) Am J Kidney Dis. 2003;42(4):617–622. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 74.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52(4):1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 75.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 76.Premaratne E, Macisaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care. 2008;31(5):971–973. doi: 10.2337/dc07-1588. [DOI] [PubMed] [Google Scholar]
- 77.Molitch ME, Steffes M, Sun W, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33(7):1536–1543. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 79.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39(12):1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 80.Mauer M, Drummond K. The early natural history of nephropathy in type 1 diabetes: I. Study design and baseline characteristics of the study participants. Diabetes. 2002;51(5):1572–1579. doi: 10.2337/diabetes.51.5.1572. [DOI] [PubMed] [Google Scholar]
- 81.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18(4):1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 82.Gaspari F, Perico N, Matalone M, et al. Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol. 1998;9(2):310–313. doi: 10.1681/ASN.V92310. [DOI] [PubMed] [Google Scholar]
- 83.O’Reilly PH, Brooman PJ, Martin PJ, Pollard AJ, Farah NB, Mason GC. Accuracy and reproducibility of a new contrast clearance method for the determination of glomerular filtration rate. Br Med J (Clin Res Ed) 1986;293(6541):234–236. doi: 10.1136/bmj.293.6541.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaspari F, Perico N, Remuzzi G. Measurement of glomerular filtration rate. Kidney Int Suppl. 1997;63:S151–S154. [PubMed] [Google Scholar]
- 85.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


