Abstract
Acetaminophen (APAP) overdose is a classical model of hepatocellular necrosis; however, the involvement of the Fas receptor in the pathophysiology remains controversial. Fas receptor-deficient (lpr) and C57BL/6 mice were treated with APAP to compare the mechanisms of hepatotoxicity. Lpr mice were partially protected against APAP hepatotoxicity as indicated by reduced plasma ALT and GDH levels and liver necrosis. Hepatic Cyp2e1 protein, adduct formation and hepatic glutathione (GSH) depletion were similar, demonstrating equivalent reactive metabolite generation. There was no difference in cytokine formation or hepatic neutrophil recruitment. Interestingly, hepatic GSH recovered faster in lpr mice than in wild type animals resulting in enhanced detoxification of reactive oxygen species. Driving the increased GSH levels, mRNA induction and protein expression of glutamate-cysteine ligase (gclc) were higher in lpr mice. Inducible nitric oxide synthase (iNOS) mRNA and protein levels at 6h were significantly lower in lpr mice, which correlated with reduced nitrotyrosine staining. Heat shock protein 70 (Hsp70) mRNA levels were substantially higher in lpr mice after APAP. Conclusion: Our data suggest that the faster recovery of hepatic GSH levels during oxidant stress and peroxynitrite formation, reduced iNOS expression and enhanced induction of Hsp70 attenuated the susceptibility to APAP-induced cell death in lpr mice.
Keywords: Acetaminophen, drug hepatotoxicity, Fas receptor, oxidant stress, protein adducts, heat shock protein70
1. INTRODUCTION
Acetaminophen (APAP) overdose is a major clinical problem in the US and Europe, often resulting in severe liver injury and potentially acute liver failure (Lee, 2004; Larson et al., 2005). Many studies have described the mechanisms of injury which are initiated by the metabolism of APAP to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) resulting in glutathione (GSH) depletion and adduction of cellular proteins (Cohen et al., 1997). In particular, it is thought that the binding of NAPQI to mitochondrial proteins results in mitochondrial dysfunction (Nelson, 1990), which propagates injury through a mitochondrial oxidant stress and peroxynitrite formation (Cover et al., 2005) resulting ultimately in the opening of the mitochondrial membrane permeability transition (MPT) pore with collapse of the membrane potential and ATP depletion (Kon et al., 2004; Masubuchi et al., 2005; Ramachandran et al., 2011). Early mitochondrial bax translocation and the later MPT lead to permeabilization of the outer membrane with release of the intermembrane proteins endonuclease G and apoptosis-inducing factor (AIF), which translocate to the nucleus and initiate DNA fragmentation (Bajt et al., 2006, 2008, 2011). Together, these events lead to hepatocyte necrosis (Gujral et al., 2002) and the release of damage associated molecular patterns (DAMPs), which trigger a sterile inflammatory response (Jaeschke et al., 2012b).
Although APAP-induced cell death has some overlap with features of apoptosis, such as mitochondrial bax translocation, cytochrome c release, and DNA fragmentation, the overwhelming evidence suggests a necrotic cell death. Most importantly, morphological characteristics include cell swelling, karyorrhexis and vacuolation (Gujral et al., 2002). In addition, there is no relevant caspase activation (Lawson et al., 1999; Adams et al., 2001, El-Hassan et al., 2003), and pancaspase inhibitors neither protect against cell death nor prevent DNA fragmentation (Lawson et al., 1999; Cover et al., 2005; Jaeschke et al., 2006; Williams et al., 2010b, 2011b). However, in the past decade several studies have associated the Fas receptor, which can trigger apoptosis, with APAP hepatotoxicity. First, it has been shown that serum levels of soluble Fas receptor are increased in patients with APAP-induced liver failure (Tagami et al., 2003). An experimental study used a small-interfering-RNA (siRNA) construct to target Fas and showed that knocking it down resulted in reduced APAP-induced injury (Zhang et al., 2000). Another study demonstrated that protection against APAP induced injury could be observed in lpr (Fas receptor-deficient) mice (Liu et al., 2004). However, it was unclear in these reports why preventing Fas receptor signaling could reduce APAP hepatotoxicity. The Fas receptor is expressed on many cell types, including hepatocytes, but the highest expression of Fas can be seen on immature lymphocytes (Nisihara et al., 2001); this is part of an essential mechanism to eliminate self-reactive lymphocytes and is the cause of the autoimmune phenotype observed in lpr mice. This phenotype includes high lymphocyte counts, greatly enlarged lymph nodes and spleen, circulating rheumatoid factor, and an overall autoimmune phenotype (Hutcheson et al., 2008). Interestingly, lpr mice were protected against bile duct ligation-induced liver injury due to the reduced inflammatory response in the absence of apoptosis (Gujral et al., 2004). This suggested that lpr mice can show reduced liver injury independent of Fas receptor-induced apoptosis. Thus, the objective of this investigation was to evaluate the mechanisms of protection against APAP hepatotoxicity in Fas receptor-deficient lpr mice by comparing APAP-induced toxicity between C57BL/6 and lpr mice in vivo.
2. MATERIALS AND METHODS
2.1 Animals
Eight to twelve week old male C57BL/6J and age-matched B6.MRL-Faslpr/J (lpr) mice were purchased from Jackson Labs (Bar Harbor, ME) with an average weight of 19 to 24 g and maintained at the University of Kansas Medical Center. All animals were housed in environmentally controlled rooms with 12 h light/dark cycle and allowed free access to food and water. Experiments followed the criteria of the National Research Council for the care and use of laboratory animals in research and were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
2.2 Experimental design
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated. Mice were intraperitoneally (i.p.) injected with 300 mg/kg APAP (dissolved in warm saline) or with an equivalent volume of saline after overnight fasting (~15 h). Animals were terminated at 0 h (n=4 per strain), 0.5 h (n=4 per strain), 6 h (n=7 per strain) or 24 h (n=7 per strain) after APAP. Blood was drawn into heparinized syringes for measurement of plasma alanine aminotransferase (ALT) activity (Pointe Scientific, Canton, MI) and glutamate dehydrogenase (GDH) activity as described (McGill et al., 2012). The liver was removed and was rinsed in cold saline; liver sections were fixed in 10% phosphate buffered formalin for histological analyses. The remaining liver lobes were snap-frozen in liquid nitrogen and stored at −80 °C.
2.3 Histology
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) for blinded evaluation of the areas of necrosis by the pathologist. The percent of necrosis was estimated by evaluating the number of microscopic fields with necrosis compared to the total cross sectional area. Additional sections were stained for neutrophils using the anti-mouse neutrophil allotypic marker antibody (AbD Serotec, Raleigh, NC) as previously described (Williams et al., 2010a). Positively stained neutrophils consistent with cellular morphology were quantified in 15 high power fields (HPF). Sections were also stained using an anti-nitrotyrosine antibody (Invitrogen, Carlsbad, CA) as previously described (Knight et al., 2002).
2.4 Glutathione quantification
Glutathione (GSH) and glutathione disulfide (GSSG) were measured from liver homogenate using the modified Tietze method as previously described in detail (Jaeschke and Mitchell, 1990). Briefly, frozen tissue was homogenized in sulfosalicylic acid/EDTA. For total GSH determination samples were assayed using dithionitrobenzoic acid. Similarly, measurement of GSSG was performed using the same method after trapping and removal of GSH with N-ethylmaleimide.
2.5 APAP-protein adducts
Liver APAP protein adducts were measured based on a method developed by Muldrew et al. (2002) with minor modifications (Ni et al., 2012) and normalized to total protein (MicroBCA kit, Thermo Scientific, Rockford, IL).
2.6 Western Blotting
Liver tissue was homogenized in 25 mM HEPES buffer (containing 5 mM EDTA, 2 mM DTT and 0.1% CHAPS) and diluted to uniform protein concentration. Tissue homogenate was used for β-actin (Santa Cruz Biotech, Santa Cruz, CA), cyp2e1 (Abcam, Cambridge, MA), iNOS (BD, Franklin Lakes, NJ) and gclc (GeneTex, Irvine, CA) western blotting as described in detail (Bajt et al., 2000).
2.7 Real-time PCR for mRNA quantification
mRNA expression of several genes was performed by real-time PCR (RT-PCR) analysis as previously described (Gujral et al., 2004). cDNA was generated by reverse transcription of total RNA by M-MLV reverse transcriptase with random primers (Invitrogen, Carlsbad, CA). Forward and reverse primers for the genes were designed using Primer Express software (Applied Biosystems, Foster City, CA) and listed in supplemental table 1. After normalization of cDNA concentration, SYBR green PCR Master Mix (Applied Biosystems) was used for analysis. The relative differences in expression between groups were expressed us ing cycle time (Ct) values generated by the ABI 7900 instrument (Applied Biosystems). All genes evaluated were first normalized to GAPDH and then expressed as a fold increase relative to control (arbitrarily set as 1.0). Calculations are made by assuming one cycle is equivalent to a two-fold difference in copy number which is the 2^(-ddCt) formula.
2.8 Statistics
All results were expressed as mean ± SE. Comparisons between multiple groups were performed with one-way ANOVA or, where appropriate, by two-way ANOVA, followed by a post hoc Bonferroni test. If the data were not normally distributed, the Kruskal-Wallis test (nonparametric ANOVA) followed by Dunn’s Multiple Comparisons Test was used. P < 0.05 was considered significant. All statistics were evaluated using SigmaStat (Systat Software, San Jose, CA).
3. RESULTS
3.1 Liver injury in C57BL/6 and lpr mice
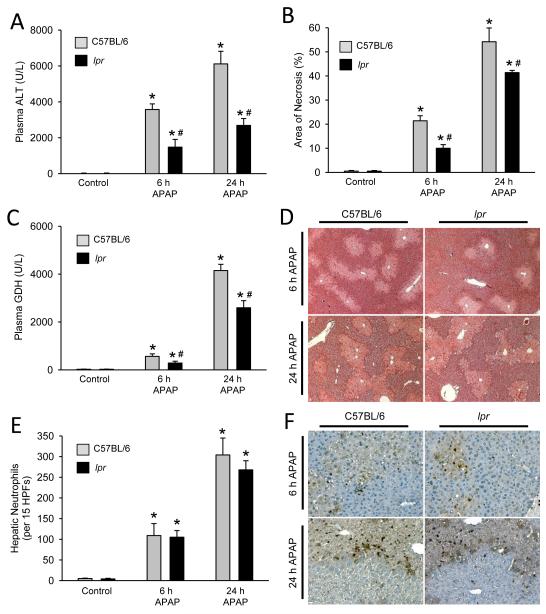
To assess the differences in liver injury, lpr and wildtype C57BL/6 mice were treated with 300 mg/kg APAP for 6 or 24 h. APAP caused severe liver injury in wildtype animals as indicated by the massive increase in plasma ALT activities and the development of severe centrilobular necrosis (Fig. 1A,B,D; Supplemental Figure 1). To evaluate the degree of mitochondrial injury, the mitochondrial-specific enzyme glutamate dehydrogenase (GDH) was measured in plasma. GDH activities also increased substantially at 6 and especially at 24 h after APAP administration (Fig. 1C). All parameters indicated that APAP overdose caused significantly less injury in lpr mice at both time points (Fig. 1A-D), however the difference in injury appeared to be most pronounced at 6 h.
Figure 1. Acetaminophen-induced liver injury and hepatic neutrophil recruitment in lpr and C57BL/6 mice.
Animals were treated with 300 mg/kg APAP and plasma ALT was measured at 0 h, 6 h and 24 h (A). The area of necrosis was quantified from H&E stained liver sections by the pathologist in a blinded manner (B). Plasma glutamate dehydrogenase (GDH) was measured as a marker of mitochondrial injury (C). Representative H&E sections are shown (50x magnification) (D). Quantification of hepatic neutrophil recruitment was performed via immunohistochemistry (E). Representative histology for neutrophil staining is shown (200x magnification) (F). Data represent means ± SE of n = 4-7 animals per group. *P<0.05 (compared to genotype control). #P<0.05 (compared to equivalent C57BL/6 time point).
3.2 Inflammation and neutrophil recruitment during APAP overdose
It was hypothesized that altered inflammatory response and neutrophil recruitment into the liver potentiates APAP induced injury in lpr mice (Liu et al. 2004). To further evaluate this hypothesis, hepatic cytokine and chemokine induction (Table 1) and hepatic neutrophil recruitment were quantified in lpr and C57BL/6 mice (Fig. 1E,F). Some variation in cytokine and chemokine induction was observed between genotypes with C57BL/6 mice having higher IL-6 and IL-10 induction at 6h while CXCL1 induction was higher in lpr mice at 6 and 24 h. Despite these differences, the overall pattern of cytokine and chemokine induction was similar between genotypes. To determine if these slight differences altered neutrophil recruitment, immunohistochemistry was performed (Fig. 1E,F). When quantified, the distribution and total number of hepatic neutrophils was equivalent between genotypes despite a reduced injury in lpr mice.
Table 1. Hepatic cytokine and chemokine induction after acetaminophen overdose in C57BL/6 and LPR mice.
| Liver mRNA expression (fold change) | ||||
|---|---|---|---|---|
| C57BL/6 6 h APAP |
LPR 6 h APAP |
C57BL/6 24 h APAP |
LPR 24 h APAP |
|
| IL-10 | 22 ± 4 | 4.2 ± 0.4 * | 2.0 ± 0.8 | 1.6 ± 0.5 |
| IL-6 | 4.7 ± 1.0 | 1.8 ± 0.6 * | 4.4 ± 1.5 | 5.7 ± 1.7 |
| CXCL1 | 41 ± 12 | 90 ± 20 * | 52 ± 10 | 90 ± 14 * |
| CCL2 | 29 ± 5.1 | 34 ± 5.2 | 47 ± 13 | 87 ± 18 * |
| CCL3 | 3.7 ± 0.8 | 3.7 ± 0.6 | 16 ± 3.1 | 21 ± 8.1 |
| CCL4 | 2.3 ± 0.5 | 4.1 ± 0.5 | 11 ± 5.0 | 18 ± 7.3 |
Hepatic mRNA levels 6 and 24 h after administration of 300 mg/kg APAP in C57BL/6 and LPR mice were measured. mRNA levels were calculated as the cytokine mRNA-to-β-actin mRNA ratio. The values of untreated controls were set as 1 and the fold change of the APAP-treated animals is shown relative to untreated, genotype-matched controls. Basal expression of all genes measured was not significantly different between genotypes for any gene measured. Data represent means ± SE (n=7 animals per group).
Indicates a significant difference from APAP-treated C57BL/6 mice at the same time point (P < 0.05).
3.3 Metabolism of APAP in C57BL/6 and lpr mice
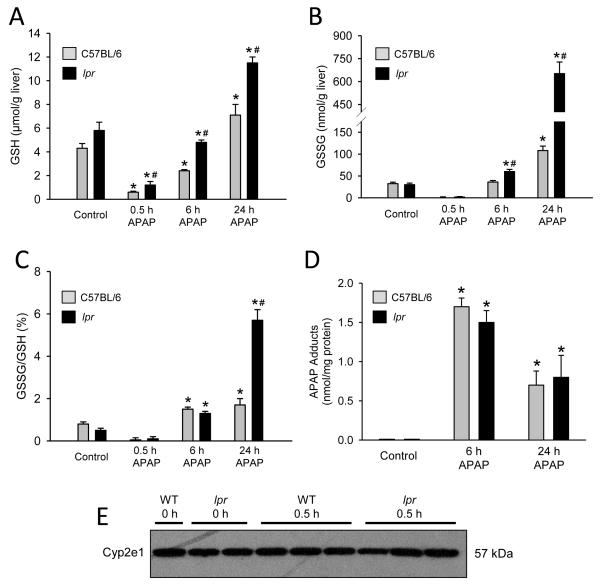
To determine if the protection seen in lpr mice was due to inhibition of reactive metabolite formation, we measured the time course of GSH depletion and recovery and APAP-protein adducts. Fasted control lpr mice had 26% more GSH than fasted control C57BL/6 mice (Fig. 2A). Thirty minutes after APAP injection liver GSH levels were depleted >80% in both genotypes (Fig. 2A). In addition, APAP-protein adducts were similar at 6 h and at 24 h (Fig. 2D). Furthermore, western blot analysis of cyp2e1, the dominant Cyp responsible for metabolism of APAP, showed no significant difference between wild type and lpr mice (Fig. 2E). Together, these data show that the metabolic activation of APAP is similar in both genotypes. Interestingly, during the recovery phase of the hepatic GSH levels, i.e. after APAP was metabolized, the liver GSH content recovered faster in lpr mice compared to the wildtype animals (Fig. 2A).
Figure 2. Glutathione and metabolic activation of APAP in lpr and C57BL/6 mice.
C57BL/6 and lpr mice were treated with 300 mg/kg APAP (0 h, 0.5, 6 h and 24 h) and liver tissue was homogenized. Total hepatic glutathione (GSH) (A) glutathione disulfide (GSSG) (B) and GSSG:GSH ratio (C) were measured. Metabolic activation of APAP was determined by hepatic APAP-protein adduction (D) and hepatic levels of cyp2e1 (E). Data represent means ± SE of n = 4-7 mice per group. *P<0.05 (compared to genotype control). #P<0.05 (compared to equivalent C57BL/6 time point).
During APAP overdose, GSH is critical for detoxifying reactive oxygen species (ROS), particularly in the mitochondria, generating glutathione disulfide (GSSG) selectively in mitochondria as a result (Jaeschke, 1990). Quantification of GSSG showed increased detoxification of ROS in lpr mice compared to C57BL/6 at 6 h and an even more dramatic increase at 24 h (Fig. 2B). When GSSG is normalized to total GSH the difference between genotypes is lost at 6 h, but at 24 h lpr mice had a 3-fold higher GSSG-to-GSH ratio compared to C57BL/6 (Fig. 2C). These data show that lpr mice have higher liver GSH and enhanced capacity to detoxify reactive oxygen generated during mitochondrial dysfunction.
3.4 Protection in lpr mice due in part to enhanced glutathione recovery
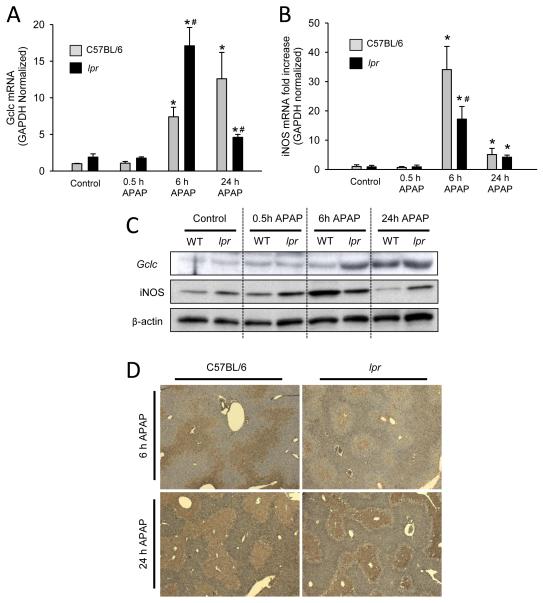
The rate limiting step in GSH synthesis is catalyzed by glutamate-cysteine ligase which is composed of the catalytic (gclc) and modulatory (gclm) subunits. To investigate why lpr mice have higher basal GSH levels and enhanced GSH recovery after APAP, we measured gclc mRNA and protein levels in the liver. Basal levels of gclc mRNA were slightly higher in lpr mice, however, the difference did not reach statistical significance. The same trend was observed 30 minutes after APAP (Fig. 3A). Western blotting showed no difference in gclc protein levels between genotypes in control or 30 minute APAP-treated mice (Fig. 3C). Six hours post-APAP lpr mice had substantially higher mRNA induction of gclc (Fig. 3A) and more liver gclc protein (Fig. 3C). By 24 h post-APAP, gclc mRNA levels declined in lpr mice but further increased in wild type animals (Fig. 3A). Protein levels of gclc were equivalent between genotypes at 24 h (Fig. 3C). These data show no quantifiable difference in gclc protein in untreated animals, however, the induction and translation of gclc occurs sooner in lpr mice allowing for increased GSH levels in these mice, and improving the scavenging capacity for reactive oxygen species.
Figure 3. Gclc expression, iNOS induction and nitrotyrosine staining in lpr and C57BL/6 mice after APAP overdose.
C57BL/6 and lpr mice were treated with 300 mg/kg APAP (0 h, 0.5, 6 h and 24 h), liver tissue was homogenized in Trizol and q-PCR was used to determine glutamate-cysteine ligase (gclc) (A) and inducible nitric oxide synthase (iNOS) (B) mRNA induction. Western blot analysis of representati ve mice from each group are shown for gclc and iNOS hepatic protein levels (C). Representative nitrotyrosine immunohistochemistry sections from each group are shown (D). Data represent means ± SE of n = 4-7 mice per group. *P<0.05 (compared to genotype control). #P<0.05 (compared to equivalent C57BL/6 time point).
3.5 Attenuated injury in lpr mice correlates with decreased peroxynitrite formation
One of the most powerful oxidants in vivo is peroxynitrite, which can be formed when superoxide generated from mitochondrial dysfunction reacts with nitric oxide produced in part by nitric oxide synthases (Pryor and Squadrito, 1995). During APAP overdose, the inflammatory response activates the inducible form of nitric oxide synthase (iNOS) thereby generating nitric oxide which is freely diffusible across cell membranes. Although lpr mice showed slightly higher baseline values of iNOS protein expression compared to wild type animals, there was only a mild induction after APAP treatment (Fig. 3C). In contrast, extensive iNOS induction was observed in wild type mice with peak levels at 6 h, and returned to baseline by 24 h (Fig. 3C). Consistent with the stronger iNOS expression at 6 h, wild type animals stained more extensively for nitrotyrosine adducts, which did not significantly change at 24 h (Fig. 3D). In contrast, there was very limited nitrotyrosine staining in the livers of lpr mice at 6 h but more extensive staining at 24 h (Fig. 3D). Together these data suggest that the more robust induction of iNOS, together with the ROS formation and slow recovery of GSH in wild type animals, contributes to the more severe liver injury in wild type compared to lpr mice.
3.6 Increased expression of hepatoprotective genes in lpr mice
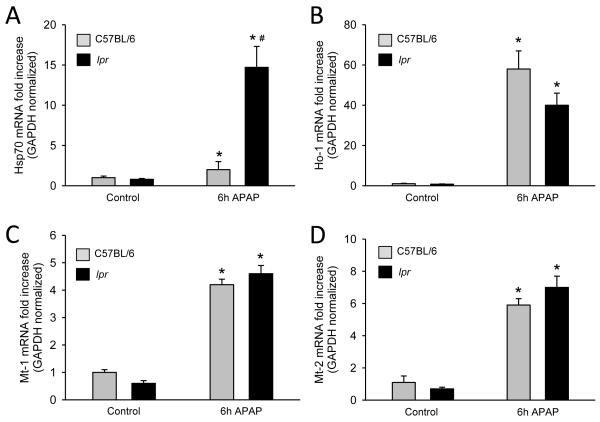
The induction of a number of endogenous genes has been shown to limit APAP-induced liver injury. These include metallothionein (Mt) (Saito et al., 2010a), heme oxygenase-1 (Ho-1) (Chiu et al., 2002), and heat shock protein 70 (Hsp70) (Salminen et al., 1998). Hsp70 mRNA induction was substantially higher in lpr mice compared to wild type animals (Figure 4A). Although Mt-1, Mt-2 and Ho-1 mRNAs were upregulated after APAP treatment, there was no significant difference between wild type and lpr mice (Fig. 4B-D).
Figure 4. Induction of cytoprotective genes in lpr and C57BL/6 mice after APAP overdose.
C57BL/6 and lpr mice were treated with 300 mg/kg APAP (0 h and 6 h), liver tissue was homogenized in Trizol and q-PCR was used to determine inducible heat shock protein 70 (Hsp70) (A), heme oxygenase-1 (Ho-1) (B), metallothionein-1 (Mt-1) (C), and metallothionein-2 (Mt-2) (D) mRNA induction. Data represent means ± SE of n = 4-7 mice per group. *P<0.05 (compared to genotype control). #P<0.05 (compared to equivalent C57BL/6 time point).
4. DISCUSSION
Lpr mice are a common model to study autoimmunity, in particular systemic lupus erythematosus (SLE) (Hutcheson et al., 2008). The observed autoimmunity of lpr mice results in various reported abnormal phenotypes which alone or in combination have the potential to modulate stress responses and influence drug toxicity testing. A recent example in which a gene knockout was shown to alter important off-target functions is the chronic stress observed in conditional Atg5-deficient mice. The long-term inhibition of autophagy triggers apoptotic cell death, regeneration and Nrf-2 activation (Ni et al., 2012). This compensatory response makes the animals resistant to APAP overdose (Ni et al., 2012). For these reasons it was important to evaluate the mechanism of protection against APAP-induced liver injury in lpr mice.
4.1 Inhibition of metabolic activation
The first concern regarding any intervention that reduces APAP hepatotoxicity has to be whether formation of reactive metabolites and protein adducts is affected. Both wild type and lpr mice experienced a similar hepatic GSH depletion during the first 30 min, which is a sensitive indirect measure of NAPQI formation (Jaeschke et al., 2011). In addition, cysteine protein adducts, which represent >90% of all APAP protein adducts (Streeter et al., 1984; Muldrew et al., 2002), were quantitatively not different between the different mouse genotypes. Thus, it can be concluded that there was no significant difference in reactive metabolite formation between wild type and lpr mice.
4.2 Inhibition of Fas receptor-mediated apoptosis
It was previously concluded that APAP hepatotoxicity is a “mixed necrotic and apoptotic event” and for this reason lpr mice have attenuated progression of injury (Liu et al., 2004). However, overwhelming evidence in animals (Gujral et al., 2002) and humans (McGill et al., 2012; Antoine et al., 2012) after APAP overdose, and in murine hepatocytes (Bajt et al., 2004; Kon et al., 2004) and human HepaRG cells (McGill et al., 2011) indicates that APAP-induced cell death occurs by oncotic necrosis not apoptosis. APAP does not cause relevant caspase activation (Lawson et al., 1999; Adams et al., 2001; El-Hassan et al., 2003; Jaeschke et al., 2006; Williams et al., 2010b, 2011b) and pancaspase inhibitors do not protect (Lawson et al., 1999; Jaeschke et al., 2006; Williams et al., 2010b/2011b; Antoine et al., 2010). As a consequence, nuclear DNA fragmentation, a well-established phenomenon after APAP overdose (Ray et al., 1990; Lawson et al., 1999; Jahr et al., 2001) cannot be prevented by caspase inhibitors but only by inhibiting mitochondrial dysfunction (Cover et al., 2005). Nuclear DNA fragmentation occurs through mitochondria-derived endonuclease G and AIF (Bajt et al., 2006; 2008; 2011). Some of the confusion comes from the overlap in signaling mechanisms between apoptosis and necrosis and the fact that some assays, e.g. the TUNEL assay, DNA ladders, mitochondrial bax translocation and cytochrome c release, are not specific for apoptosis (Jaeschke and Lemasters, 2003). Nevertheless, if multiple parameters are measured and if a positive control for apoptosis, e.g. Fas receptor or TNF receptor-induced apoptosis, is used, there is little doubt that APAP-induced cell death is caused by necrosis and thus, the protection in lpr mice cannot be caused by inhibition of Fas receptor-mediated apoptosis.
4.3 Attenuation of an inflammatory response
Previous studies have shown a reduced inflammatory injury after bile duct ligation in lpr mice (Gujral et al., 2004). This opens up the possibility that a reduced inflammatory response might explain the protection against APAP toxicity in lpr mice. The extensive necrosis induced by APAP overdose leads to extensive release of damage associated molecular patterns, including high mobility group box-1 protein and DNA fragments in mice and humans (Martin-Murphy et al., 2010; Antoine et al., 2009, 2012; McGill et al., 2012). As a result, there is cytokine formation and hepatic neutrophil and monocyte recruitment (Lawson et al., 2000; James et al., 2005; Holt et al., 2008). However, whether or not these inflammatory cells actually aggravate the injury is controversial (Jaeschke et al., 2012b). Although there are papers that suggested that neutrophils may cause additional injury (Liu et al., 2006, Ishida et al., 2006), the effect can be explained by off-target effects of the neutropenia-inducing antibody (Jaeschke and Liu, 2007). In contrast, numerous interventions that prevent neutrophil cytotoxicity such as antibodies against CD18, inhibitors of NADPH oxidase as well as deficiency of various adhesion molecules or NADPH oxidase are all ineffective in attenuating APAP hepatotoxicity (Lawson et al., 2000; James et al., 2003a; Cover et al., 2006, Williams et al., 2010a,b; 2011a). Since pro-inflammatory cytokine formation and hepatic neutrophil recruitment was not significantly different between wild type and lpr mice, and given the extensive evidence against an aggravation of injury by neutrophils in this model, it is unlikely that the reduced injury in lpr mice was caused by a reduced inflammatory response.
4.4 Induction of GSH synthesis and hepatoprotective genes
Mitochondrial oxidant stress and peroxynitrite formation are critical for APAP-induced cell death (Jaeschke et al., 2012a). The extensive depletion of GSH, particularly in the mitochondria, severely impairs this endogenous defense mechanism and makes the cell highly susceptible to oxidant stress. Therefore, replenishing mitochondrial GSH levels by supply of sulfhydryl reagents is highly effective for scavenging ROS and peroxynitrite and attenuating APAP-induced liver injury, which promotes regeneration and recovery (Knight et al., 2002; Bajt et al., 2003; James et al., 2003b; Saito et al., 2010b). Our results demonstrate that hepatic GSH levels recover faster in lpr mice and this correlates with increased GSSG formation and less nitrotyrosine adducts. This effect may have been triggered in part by the more extensive induction of gclc, the rate-limiting enzyme of the GSH synthesis pathway (Lu, 2009). This suggest that the accelerated recovery of GSH concentrations in the hepatocytes, which translates in a faster uptake of GSH into mitochondria (Saito et al., 2010b), detoxified more effectively the oxidant stress and peroxynitrite resulting in less injury in lpr mice. A similar mechanism, i.e. enhanced GSH recovery, has been proposed to make female mice less susceptible to APAP-induced liver injury (Masubuchi et al., 2011). In addition to the effects on gclc, the attenuated induction of iNOS in lpr mice may have contributed to reduced peroxynitrite formation. Thus, at least during the early phase of APAP-induced injury, reduced peroxynitrite formation together with improved detoxification of oxidant stress appear to be the dominant mechanisms of protection in lpr mice. Similar to effects observed with GSH treatment (Bajt et al., 2003), the initial protection fades somewhat during prolonged oxidant stress.
In addition to GSH recovery, other enzymes, e.g. Ho-1, are known to generate antioxidants and protect against APAP overdose (Chiu et al., 2002). In addition, Mt-1/2 can scavenge NAPQI (Saito et al., 2010a) and heat shock proteins, e.g. Hsp70, can protect against APAP toxicity (Tolson et al., 2006). However, neither Mt1/2 nor Ho-1 mRNA was induced differently between wild type and lpr mice. In contrast, induction of Hsp70 mRNA was dramatically higher in lpr mice compared to wild type animals which may contribute to the reduced injury in lpr mice.
4.5 Summary
Our data confirmed the reduced susceptibility of Fas receptor-deficient lpr mice against APAP hepatotoxicity. There was no evidence that the protection was related to inhibition of metabolic activation, Fas receptor-mediated apoptosis or modulation of the hepatic inflammatory response. In contrast, the faster recovery of hepatic GSH levels during the mitochondrial oxidant stress and peroxynitrite formation, reduced iNOS induction and enhanced expression of Hsp70 attenuated the detrimental effects of the oxidant stress and thus reduced the susceptibility to APAP-induced cell death. Thus, when working with gene-deficient animals it is critical to consider and mechanistically evaluate potential off-target effects as the reason for the modified susceptibility against hepatotoxic agents. Often these gene deficient animals have multiple pathologies and compensatory responses that are not sufficiently considered in the interpretation of the data. This can lead to unjustified conclusions about therapeutic targets that are not correct for the experimental model and could not be translated into clinical practice.
Supplementary Material
Highlights.
Fas receptor defective (lpr) mice are protected from APAP-induced liver injury
Lpr mice show no evidence of changes in metabolic activation of APAP
Lpr mice have reduced iNOS induction and delayed nitrotyrosine staining
Lpr mice show increased Gclc and enhanced GSH recovery for better ROS scavenging
Multiple factors account for the APAP resistant phenotype of lpr mice
ACKNOWLEDMENTS
This investigation was supported in part by the National Institutes of Health grants R01 DK070195 and R01 AA12916 to H.J., and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM12345. CD Williams and MR McGill were supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
Abbreviations
- ALT
alanine aminotransferase
- APAP
acetaminophen
- GDH
Glutamate dehydrogenase
- GSH
glutathione
- GSSG
glutathione disulfide
- gclc
Glutamate-cysteine ligase, catalytic subunit
- Hsp
heat shock protein
- Ho-1
heme oxygenase 1
- iNOS
inducible nitric oxide synthase
- lpr
lymphoproliferation, recessive (Fas defective mouse)
- Mt
metallothionein
- NAPQI
N-acetyl-p-benzoquinone imine
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol. Pharmacol. 2001;60:907–915. doi: 10.1124/mol.60.5.907. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J. Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Regan SL, Sathish JG, Kitteringham NR, Park BK. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol. Sci. 2009;112:521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol. Med. 2010;16:479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Farhood A, Jaeschke H. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J. Pharmacol. Exp. Ther. 2003;307:67–73. doi: 10.1124/jpet.103.052506. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol. Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol. Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Brittingham JA, Laskin DL. Differential induction of heme oxygenase-1 in macrophages and hepatocytes during acetaminophen-induced hepatotoxicity in the rat: effects of hemin and biliverdin. Toxicol. Appl. Pharmacol. 2002;181:106–115. doi: 10.1006/taap.2002.9409. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Cover C, Liu J, Farhood A, Malle E, Waalkes MP, Bajt ML, Jaeschke H. Pathophysiological role of the acute inflammatory response during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2006;216:98–107. doi: 10.1016/j.taap.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol. Appl. Pharmacol. 2003;191:118–129. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson J, Scatizzi J, Siddiqui A, Haines G, Wu T, Li Q, Davis L, Mohan C, Perlman H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur. J. Immunol. 2006;36:1028–1038. doi: 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990;255:935–41. [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology. 2007;45:1588–1589. doi: 10.1002/hep.21549. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012a;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity--a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012b;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–65. [PubMed] [Google Scholar]
- James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic. Res. 2003a;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol. Sci. 2003b;75:458–467. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- James LP, Simpson PM, Farrar HC, Kearns GL, Wasserman GS, Blumer JL, Reed MD, Sullivan JE, Hinson JA. Cytokines and toxicity in acetaminophen overdose. J. Clin. Pharmacol. 2005;45:1165–1171. doi: 10.1177/0091270005280296. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J. Pharmacol. Exp. Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM, Acute Liver Failure Study Group Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol. Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol. Appl. Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- Lee W. Acetaminophen and the US acute liver failure study group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- Liu Z, Govindarajan S, Kaplowitz N. I nnate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Mol. Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi Y, Nakayama J, Watanabe Y. Sex difference in susceptibility to acetaminophen hepatotoxicity is reversed by buthionine sulfoximine. Toxicology. 2011;287:54–60. doi: 10.1016/j.tox.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J. Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol. Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab. Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol. Sci. 2012;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisihara T, Ushio Y, Higuchi H, Kayagaki N, Yamaguchi N, Soejima K, Matsuo S, Maeda H, Eda Y, Okumura K, Yagita H. Humanization and epitope mapping of neutralizing anti-human fas ligand monoclonal antibodies: Structural insights into fas/fas ligand interactions. J. Immunol. 2001;167:3266–3275. doi: 10.4049/jimmunol.167.6.3266. [DOI] [PubMed] [Google Scholar]
- Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic. Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SD, Sorge CL, Raucy JL, Corcoran GB. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol. Appl. Pharmacol. 1990;106:346–351. doi: 10.1016/0041-008x(90)90254-r. [DOI] [PubMed] [Google Scholar]
- Saito C, Yan HM, Artigues A, Villar MT, Farhood A, Jaeschke H. Mechanism of protection by metallothionein against acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010a;242:182–190. doi: 10.1016/j.taap.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010b;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen WF, Jr, Voellmy R, Roberts SM. Effect of N-acetylcysteine on heat shock protein induction by acetaminophen in mouse liver. J. Pharmacol. Exp. Ther. 1998;286:519–524. [PubMed] [Google Scholar]
- Streeter AJ, Dahlin DC, Nelson SD, Baillie TA. The covalent binding of acetaminophen to protein. Evidence for cysteine residues as major sites of arylation in vitro. Chem. Biol. Interact. 1984;48:349–66. doi: 10.1016/0009-2797(84)90145-5. [DOI] [PubMed] [Google Scholar]
- Tagami A, Ohnishi H, Hughes RD. Increased serum soluble Fas in patients with acute liver failure due to paracetamol overdose. Hepatogastroenterology. 2003;50:742–745. [PubMed] [Google Scholar]
- Tolson JK, Dix DJ, Voellmy RW, Roberts SM. Increased hepatotoxicity of acetaminophen in Hsp70i knockout mice. Toxicol. Appl. Pharmacol. 2006;210:157–162. doi: 10.1016/j.taap.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol. Appl. Pharmacol. 2011a;252:289–97. doi: 10.1016/j.taap.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010a;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol. Appl. Pharmacol. 2010b;247:169–78. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, Koerner MR, Lampe JN, Farhood A, Jaeschke H. Mouse strain-dependent caspase activation during acetaminophen hepatotoxicity does not result in apoptosis or modulation of inflammation. Toxicol. Appl. Pharmacol. 2011b;257:449–458. doi: 10.1016/j.taap.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cook J, Nickel J, Yu R, Stecker K, Myers K, Dean NM. Reduction of liver Fas expression by an antisense oligonucleotide protects mice from fulminant hepatitis. Nat. Biotechnol. 2000;18:862–867. doi: 10.1038/78475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.